Abstract
Previous studies have demonstrated that early surgery in Crohn disease (CD) can result in a better clinical course than late surgery. The aim of this study was to compare the clinical course of CD following bowel resection performed at the time of diagnosis (early surgery) and during the course of the disease (late surgery).
We reviewed medical records from a hospital-based cohort database that includes Korean CD patients diagnosed before 2009. Patients who underwent bowel resection were included. Age, sex, disease phenotype, time of surgery, medication history including use of corticosteroids, immunomodulators, and biologics, and further surgical history were assessed.
In all, 243 CD patients who had undergone bowel resection were included, and 120 patients underwent surgery at the time of diagnosis, while 123 underwent surgery after diagnosis (median 105 months, range 2–277). The use of biologics was significantly higher in the late surgery group than in the early surgery group (P = .020). The use of immunomodulators and reoperation rates did not differ between the groups. Early surgery was associated with less use of biologics (Kaplan–Meier curve analysis P = .015). Multivariate analysis indicated that early surgery and old age at surgery were independent variables associated with less use of biologics.
CD patients who underwent bowel resection at the time of diagnosis have a more favorable disease course, represented by less use of biologics. Early surgery might be a treatment option in a subset of CD patients.
Keywords: bowel resection, clinical course, Crohn disease, early surgery
1. Introduction
Crohn disease (CD) is a chronic and idiopathic inflammatory disease that can involve any part of the digestive tract. The incidence and prevalence rates of CD in Korea (0.53 cases per 105 person-years, and 11.24 cases per 105 persons, respectively, from 1986 to 2005) are still lower than those of Western countries.[1] However, the number of patients with CD has been rapidly increasing in recent years and CD is an emerging disease in Korea.
The behavior of CD can change during the course of the disease, and complications may emerge despite proper treatment. Strictures or fistulas can occur, presenting as acute abdominal concerns. These complications can require surgical intervention, such as bowel resection. A study conducted by researchers from the Cleveland Clinic reported that 91.5% of ileocolic CD patients underwent surgery during a 13-year follow-up period.[2] Other population-based studies in the United States have found that 41% to 57% of patients with CD had at least 1 surgery in a lifetime.[3,4] Although the surgical rate seems to have decreased recently because of evolving medical treatments such as biologics,[5] surgery is still an important treatment option for refractory and complicated CD.
In addition, early surgery in ileocolonic CD patients appears to be associated with more favorable long-term outcomes. European studies have revealed that early surgery led to a stable clinical course compared to late surgery.[6,7] However, it is generally accepted that there are notable differences in the epidemiology, genetics, and clinical characteristics of CD between Westerners and Asians.[8] Thus, larger, multicenter studies of other ethnic groups are needed. Recently, a nationwide hospital-based cohort, the Crohn Disease Clinical Network and Cohort (CONNECT), was established in Korea.[9] It is composed of 2 cohorts – retrospective and prospective – with the retrospective unit containing multicenter clinical data of patients who were diagnosed with CD before 2009. Since data from the retrospective cohort is now open to researchers, we have analyzed the data to evaluate the postoperative course of CD in Korea. This study was designed to compare the postoperative course of CD following bowel resection performed at the time of diagnosis (early surgery) and during the course of the disease (late surgery) in Korean CD patients.
2. Methods
2.1. Patients
We reviewed 1337 medical records in the hospital-based cohort database that includes CD patients diagnosed from July 1982 to December 2008 in Korea. Patients who underwent bowel resection were included in the analysis, and patients treated with other surgical interventions such as strictureplasty or fistulotomy were excluded.
Clinical and demographic data were assessed, including age, sex, disease location, disease behavior, time of surgery, additional surgeries, and medication history (eg, use of immunomodulators, and biologics). Location and behavior of disease were categorized according to the Montreal classification.[10] Location of disease was defined as the maximum extent of disease involvement at the time of diagnosis, as follows: terminal ileum, possibly involving the cecum (L1), colon (L2), or ileocolon (L3). Disease phenotype was defined as: “non-stricturing, nonpenetrating” disease (B1) in the presence of inflammatory disease without evidence of structuring or penetrating complications; “stricturing” disease (B2) in the presence of luminal narrowing combined with prestenotic dilatation and/or obstructive signs and symptoms; or “penetrating” disease (B3) in the presence of intraabdominal fistula, inflammatory masses, and/or abscesses. Eligible patients were divided into 2 groups according to the time of surgery. The “Early surgery” group was defined as patients who underwent bowel resection within 1 month prior to or after diagnosis of CD. The “Late surgery” group included patients who underwent surgery during the course of the disease (at least 1 month after diagnosis). The study was reviewed and approved by Institutional Review Board of the Catholic University of Korea (VC09RIMI0013).
2.2. Postoperative clinical courses
To compare the postoperative clinical course of the 2 groups, we evaluated medication use and reoperation rates. The use of immunomodulators or biologics and starting dates of each treatment were assessed. Patients treated with immunomodulators or biologics before surgery were excluded. The used immunomodulators were azathioprine or 6-mercaptopurine. The used biologics were infliximab or adalimumab. Since our cohort data did not contain information about when steroid therapy was initiated, it was impossible to evaluate whether steroids were used postoperatively.
2.3. Statistical analysis
Continuous variables are presented as the mean ± standard deviation, and categorical variables are expressed as totals and percentages. We used the t test and Wilcoxon rank sum test for continuous variables, and the chi-square test for univariate analyses. The chi-square test was used to calculate odds ratios and 95% confidence intervals (CIs) associated with the relation between time of surgery, use of drugs, and reoperation. Kaplan–Meier curve analysis was used to estimate the cumulative probability of immunomodulators and biologics during postoperative treatment. The Cox proportional hazards regression test was used to determine the hazard ratios (HRs) and 95% CIs of independent risk factors, especially time of surgery that affected the use of biologics. All P-values < .05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics 20 (IBM Corp, Armonk, NY).
3. Results
A total of 243 CD patients who had undergone bowel resection were included in the analysis (Fig. 1). A total of 120 patients were in the “early surgery” group and 123 patients were classified as having “late surgery.” The median follow-up period after surgery in the “early” and “late” groups was 99 months (range, 1–323) and 105 months (range, 2–277), respectively. The median interval between onset of symptoms and bowel resection was 2 months (range, 0–153) and 39 months (range, 0–312), respectively. Patient characteristics are summarized in Table 1.
Figure 1.
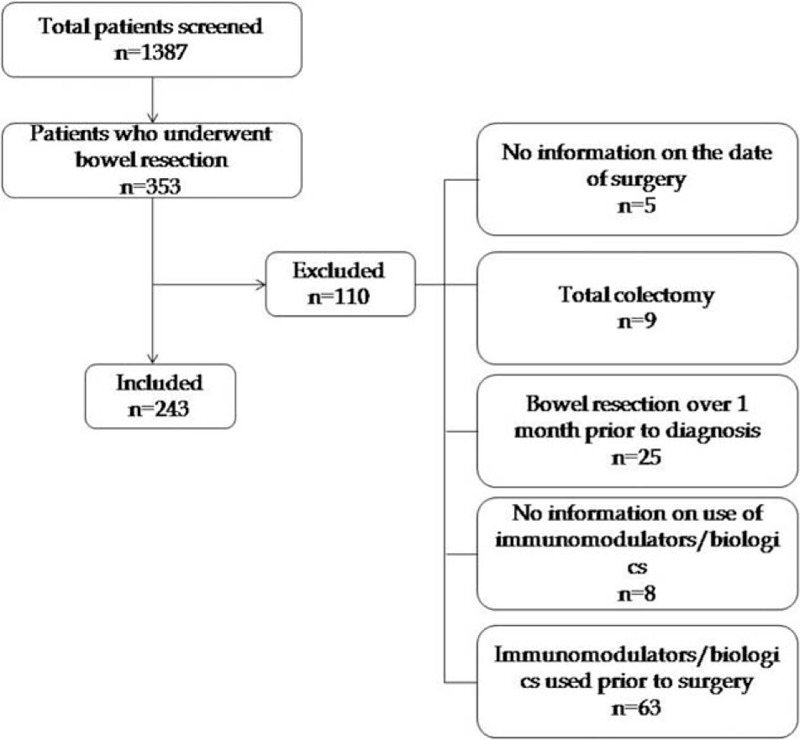
Flowchart of data selection.
Table 1.
Patient demographics.
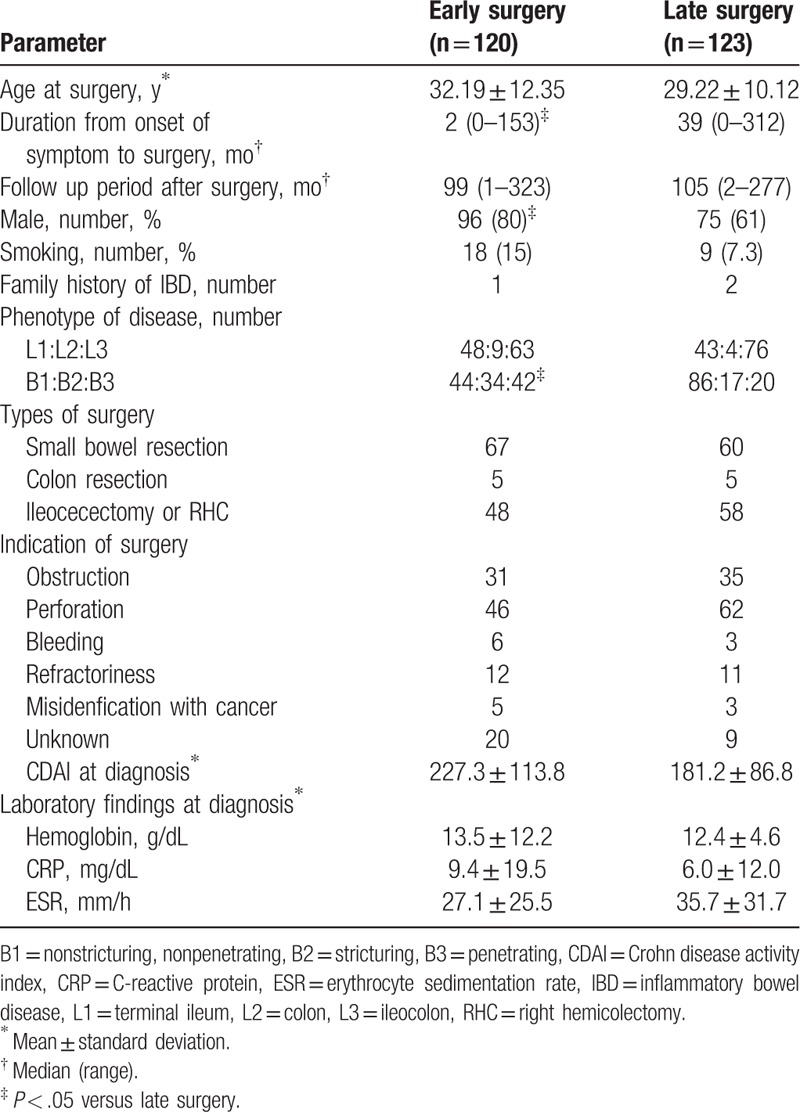
No statistical differences were observed between the groups for age at surgery, smoking history, family history of inflammatory bowel disease, and location of disease. Duration from onset of symptoms to surgery was shorter in the early surgery group compared to the late surgery group. B2 and B3 phenotypes were found more frequently in the early surgery group, while the B1 phenotype was present more often in the late surgery group.
Types of surgery were segmental resection including small bowel resection, colon resection, ilieocecectomy, or right hemicolectomy. Indications of surgery were obstruction, perforation including fistula and intraabdominal abscess, bleeding, cases which were refractory to the previous medical treatments, misidentification with cancer, and unknown. Most common indication of surgery was a perforation in both groups. CD activity index and laboratory data such as hemoglobin, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) at the time of diagnosis were not significantly different between 2 groups.
3.1. Use of drugs and reoperation according to time of surgery
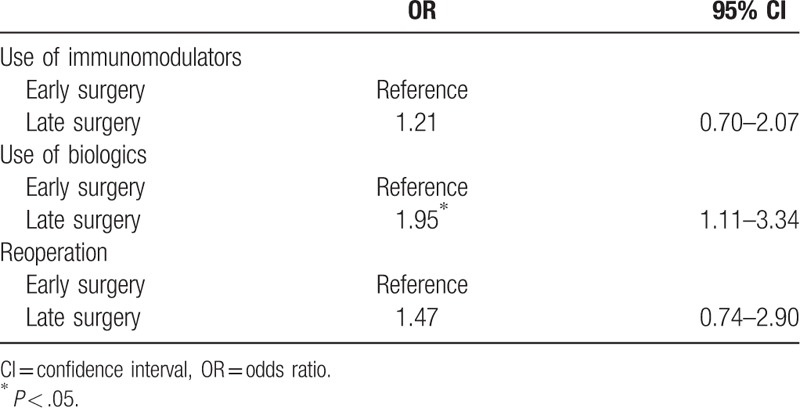
In crosstab analysis, the use of biologics was significantly higher in the late surgery group (odds ratio = 1.47, 95% CI 1.11–3.34, P = .020). There was no significant difference by time of surgery in the use of immunomodulators and reoperations (Table 2).
Table 2.
Use of drugs and reoperation according to time of surgery in postoperation (ORs obtained using the Chi-square test).
3.2. Cumulative probability of use of drugs and reoperation
The cumulative probability of a course free from the use of biologics in all enrolled patients was 91.1%, 81.7%, 71.3%, 62.2%, and 29.5% after 50, 100, 150, 200, and 250 months. The cumulative probability of a course free from use of immunomodulators in all patients was 81.0%, 61.5%, 47.0%, and 31.8% after 50, 100, 150, and 200 months. The cumulative probability of a course free from the use of reoperation in all patients was 89.1%, 85.6%, 82.6%, and 78.5% after 50, 100, 150, and 200 months.
Early surgery was associated with lower clinical recurrence, represented by use of biologics (P = .015; Fig. 2). The cumulative probability of a course free from use of biologics in the early surgery group was 94.7%, 80.8%, 70.7%, 60.7%, and 45.5%, after 50, 100, 150, 200, and 250 months. In the late surgery group, the probability was 90.4%, 71.4%, 54.3%, 42.7%, and 14.2%, respectively. No significant difference was observed between groups, based on the probability of use of immunomodulators or reoperations (P = .379, .245; Figs. 3 and 4).
Figure 2.
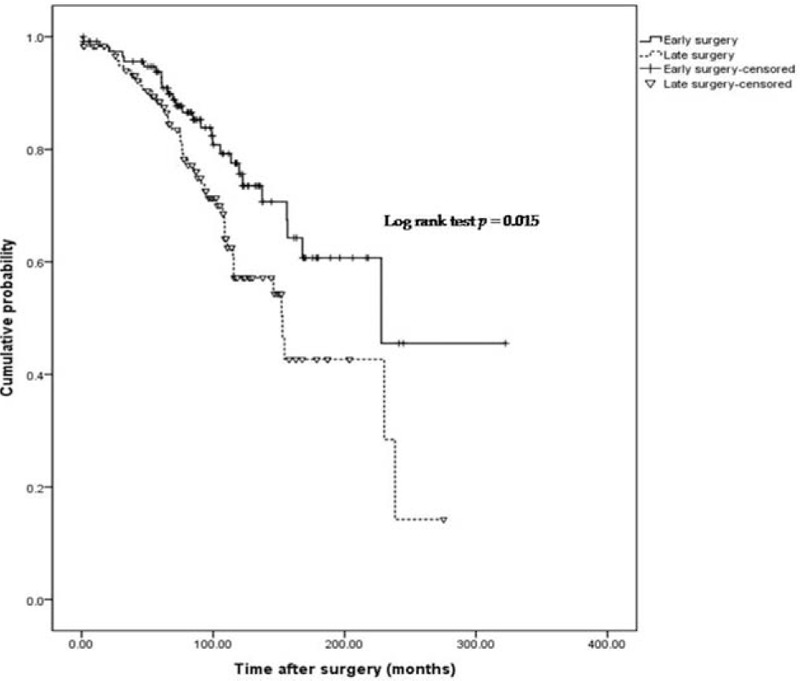
Cumulative probability of postoperative course without the use of biologics.
Figure 3.
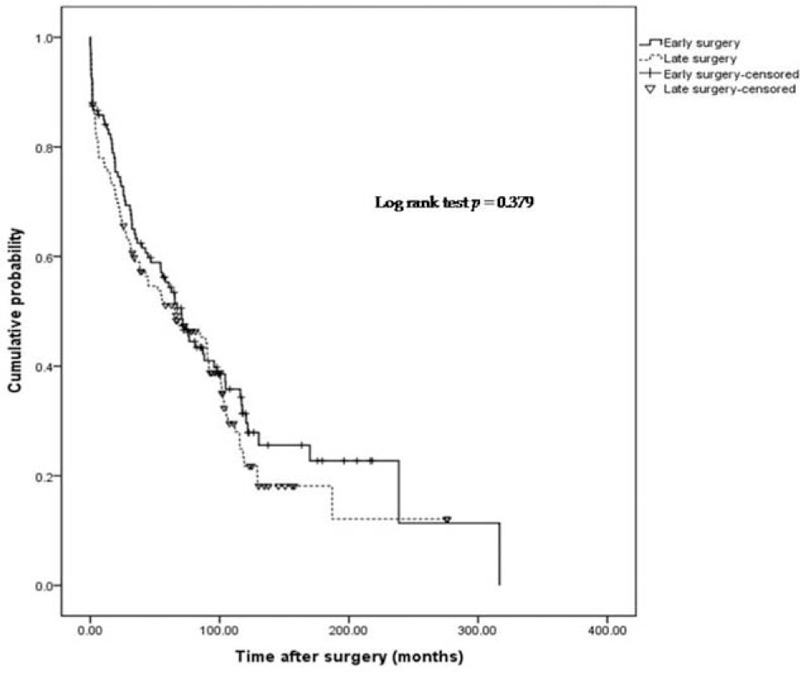
Cumulative probability of postoperative course without use of immunomodulators.
Figure 4.
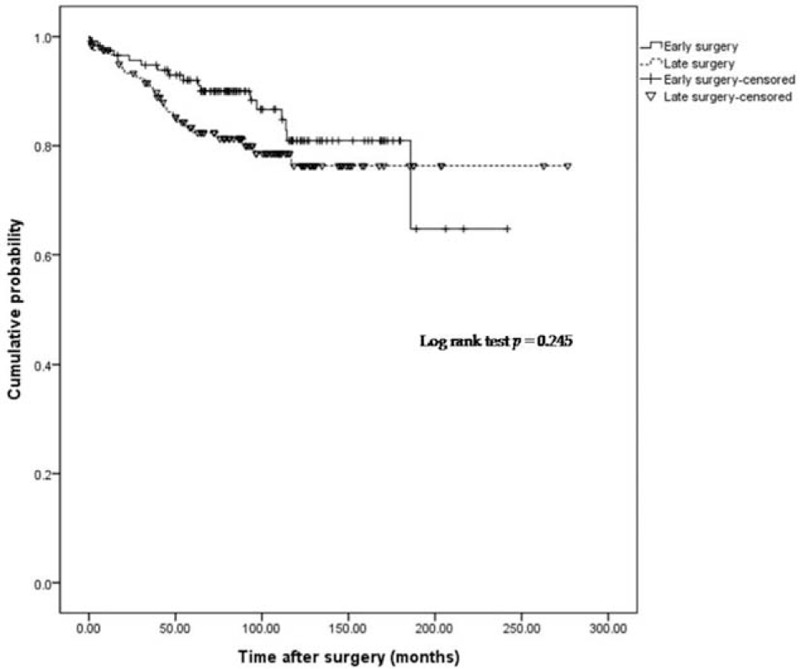
Cumulative probability of postoperative course in cases not requiring reoperation.
3.3. Risk factors for use of biologics after surgery
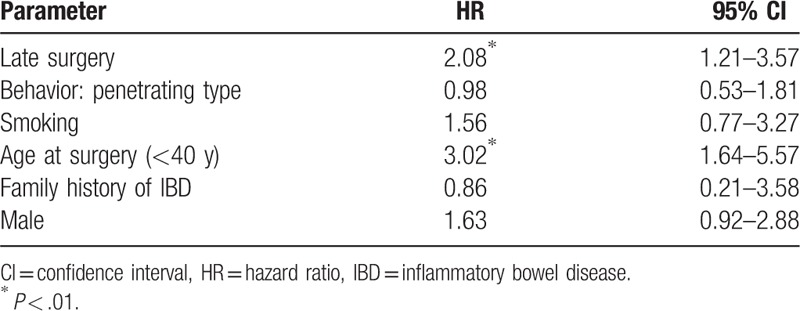
Upon multivariate analysis, late surgery (HR = 2.08, 95% CI 1.21–3.57, P = .008) and young age at surgery (HR = 3.02, 95% CI 1.64–5.57, P = .001) were independently associated with a higher probability of use of biologics (Table 3).
Table 3.
Risk factors for the use of biologics (HRs obtained using the Cox proportional hazard regression model).
4. Discussion
In this study, early surgery was associated with less use of biologics compared to late surgery, indicating better postoperative clinical outcomes in the early surgery group. This result is consistent with other studies in Caucasian populations, in terms of showing a better clinical course in early surgery. A single center Italian study reported that early surgery was the only independent variable associated with a reduced need for corticosteroids, but not with the need for immunosuppressants.[6] Another Italian study showed that initial diagnosis of CD during surgery was associated with a lower risk of reoperation, and less use of steroids and immunosuppressants, compared to CD diagnosed clinically and initially managed with medical treatment.[7] A Hungarian study suggested that early limited resective surgery was associated with a lower risk for reoperation and overall exposure to steroids and biologics.[11] Our study, however, is the first large cohort study to investigate the postoperative course of CD according to the timing of bowel resection in Asian patients.
All subjects included in this analysis have undergone bowel resection and, therefore, the cohort is suitable for estimating the rate of and predictive factors for postoperative recurrence. Since our retrospective data do not include information about clinical activity (such as the CD activity index) and endoscopic activity, we can only identify “postoperative recurrence” indirectly, based on the use of new medications and reoperation.
In this study, early surgery resulted in less use of biologics represented as good clinical outcome. As previous mentioned, patients treated with immunomodulators or biologics before surgery were excluded. And, prophylactic use of biologics is not covered by health insurance in Korea so that it is practically impossible to use biologics as prophylactic purpose after surgery. Thus, the use of biologics in the study could represent postoperative “clinical” recurrence, and the rate of 29.2% is similar to previously reported data.[6]
The result of our study, demonstrating differences between early and late surgical patients in the use of biologics, possibly representing postoperative recurrence, might be explained by followed concept. Lémann et al have proposed the concept of “cumulative structural bowel damage” to describe ongoing subclinical inflammation and disease progression that continues during clinical remission in CD patients.[12] We believe that the patients in the early surgery group had less cumulative bowel damage prior to surgery, because they had a shorter duration of disease and a limited course of medications. Similarly, the PRECiSE 2 trial showed that extended disease duration caused lower efficacy of certolizumab during the maintenance therapy phase.[13] This may also result from cumulative bowel damage.
There was no statistically significant difference in the use of immunomodulators between groups, although there was a trend of lower use of immunomodulators in the early surgery patients, as time progressed. Immunomodulators are known to be effective in maintenance of remission in CD, and to have steroid-sparing effects.[14,15] In addition, they are effective for preventing postoperative recurrence of CD.[16] For these reasons, immunomodulators may have been used with similar frequency in both groups in our study. Guidelines for the management of CD in Korea recommend immunomodulators for the prevention of postoperative recurrence,[17] and prophylactic use of immunomodulators was the only predictor of clinical recurrence in postoperative Korean CD patients.[18]
The use of steroids can also be an important surrogate marker representing postoperative clinical recurrence. Aratari et al[6] showed that the cumulative probability of using steroids was significantly lower in an early surgery group. However, the cohort data we used did not have information about initiation of steroids, so we could not analyze the association between steroids and timing of surgery.
Postoperative recurrence was observed in a considerable number of patients in our cohort, and the rates based on the use of immunomodulators, biologics, and reoperation were 67.9%, 29.2%, and 16.9%, respectively. Western studies have found clinical recurrence rates of postoperative CD ranging from 10% to 35% at 1 year and 35% to 85% at 3 years,[19] and a recent Korean study reported cumulative clinical recurrence rates of 8.8% at 1 year, 12.5% at 2 years, and 33.5% at 4 years.[18] Considering the high postoperative recurrence rate, optimal preventive strategies are important for all ethnic groups. Recently, the randomized postoperative Crohn endoscopic recurrence (POCER) trial has reported that treatment with early colonoscopy, according to the clinical risk of recurrence, can effectively prevent postoperative recurrence.[20]
To evaluate the factors affecting the postoperative clinical course of CD (ie, assumed postoperative recurrence), we analyzed the risk factors for the use of biologics. As a result, late surgery and young age at surgery were independent variables associated with an increasing probability of biologics use.
There are some limitations of this study. First, we used a retrospective design, so some data may be inaccurate. In addition, we did not have information about clinical and endoscopic activity or the initiation of steroids, so we could not evaluate disease activity and clinical recurrence represented by steroid use. Second, the data were hospital-based, so there may have been referral bias. However, the results of this large cohort study are nevertheless meaningful. Prospective controlled studies are needed to address these limitations, but they are difficult to design because treatment options can differ based on individual clinical scenarios. Third, our registry did not have the data about detail residual lesions after surgery so that we could not analyze the clinical outcome according to residual disease. Therefore, it is difficult to differentiate clearly between postoperative recurrence and aggravation of residual disease. However, since indications and types of surgery were similar in both groups (Table 1), if present, the residual lesions would be similar between the 2 groups.
In conclusion, we investigated the postoperative course of CD according to the time point of surgery, and demonstrated that early surgery is associated with less use of biologics, thus suggesting a more favorable disease course. Early surgery might be a treatment option in a subset of CD patients.
Acknowledgments
The authors thank the Research Program funded by the Korea Centers for Disease Control and Prevention (2016-E63001–01).
Author contributions
Wrote the paper: Ji Min Lee.
Designed the research and analyzed the data: Ji Min Lee, Kang-Moon Lee.
Collected and interpreted the data: Joo Sung Kim, You Sun Kim, Jae Hee Cheon, Byong Duk Ye, Young-Ho Kim, Dong Soo Han, Chang Kyun Lee, Hyun-Ju Park.
All authors contributed to revising and finalizing the paper and agreed on taking responsibility for integrity to this work.
Conceptualization: Ji Min Lee, Kang-Moon Lee.
Data curation: Ji Min Lee, Joo Sung Kim, You Sun Kim, Jae Hee Cheon, Byong Duk Ye, Young-Ho Kim, Dong Soo Han, Chang Kyun Lee, Hyun-Ju Park.
Formal analysis: Ji Min Lee, Kang-Moon Lee.
Investigation: Joo Sung Kim, You Sun Kim, Jae Hee Cheon, Byong Duk Ye, Young-Ho Kim, Dong Soo Han, Chang Kyun Lee, Hyun-Ju Park.
Methodology: Kang-Moon Lee.
Supervision: Kang-Moon Lee.
Writing – original draft: Ji Min Lee.
Footnotes
Abbreviations: CD = Crohn disease, CI = confidence interval, HR = hazard ratio.
Funding/support: This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2016-E63001-01).
The authors have no conflicts of interest to disclose.
References
- [1].Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis 2008;14:542–9. [DOI] [PubMed] [Google Scholar]
- [2].Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn's disease. Relationship between the clinical pattern and prognosis. Gastroenterology 1985;88:1818–25. [DOI] [PubMed] [Google Scholar]
- [3].Agrez MV, Valente RM, Pierce W, et al. Surgical history of Crohn's disease in a well-defined population. Mayo Clin Proc 1982;57:747–52. [PubMed] [Google Scholar]
- [4].Silverstein MD, Loftus EV, Sandborn WJ, et al. Clinical course and costs of care for Crohn's disease: Markov model analysis of a population-based cohort. Gastroenterology 1999;117:49–57. [DOI] [PubMed] [Google Scholar]
- [5].Niewiadomski O, Studd C, Hair C, et al. Prospective population-based cohort of inflammatory bowel disease in the biologics era: Disease course and predictors of severity. J Gastroenterol Hepatol 2015;30:1346–53. [DOI] [PubMed] [Google Scholar]
- [6].Aratari A, Papi C, Leandro G, et al. Early versus late surgery for ileo-caecal Crohn's disease. Aliment Pharmacol Ther 2007;26:1303–12. [DOI] [PubMed] [Google Scholar]
- [7].Latella G, Cocco A, Angelucci E, et al. Clinical course of Crohn's disease first diagnosed at surgery for acute abdomen. Dig Liver Dis 2009;41:269–76. [DOI] [PubMed] [Google Scholar]
- [8].Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis 2012;18:1164–76. [DOI] [PubMed] [Google Scholar]
- [9].Cheon JH, Kim YS, Ye BD, et al. Crohn's Disease Clinical Network and Cohort (CONNECT) Study: the first step toward nationwide multicenter research of Crohn's disease in Korea. Intest Res 2014;12:173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Golovics PA, Lakatos L, Nagy A, et al. Is early limited surgery associated with a more benign disease course in Crohn's disease? World J Gastroenterol 2013;19:7701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011;17:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn's disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010;105:1574–82. [DOI] [PubMed] [Google Scholar]
- [14].Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- [15].Lee KM, Kim YS, Seo GS, et al. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res 2015;13:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Cruz P, Kamm MA, Prideaux L, et al. Postoperative recurrent luminal Crohn's disease: a systematic review. Inflamm Bowel Dis 2012;18:758–77. [DOI] [PubMed] [Google Scholar]
- [17].Byong Duk Y, lt sup, et al. Guidelines for the management of Crohn's disease. Intest Res 2012;10:26–66. [Google Scholar]
- [18].Lee YW, Lee KM, Chung WC, et al. Clinical and endoscopic recurrence after surgical resection in patients with Crohn's disease. Intest Res 2014;12:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Buisson A, Chevaux JB, Allen PB, et al. Review article: the natural history of postoperative Crohn's disease recurrence. Aliment Pharmacol Ther 2012;35:625–33. [DOI] [PubMed] [Google Scholar]
- [20].De Cruz P, Kamm MA, Hamilton AL, et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet 2015;385:1406–17. [DOI] [PubMed] [Google Scholar]


