Abstract
Rationale:
Splenic artery embolization (SAE) is a common procedure in trauma patients with blunt splenic injuries. We report a case of acute ischemic stroke following orthopedic surgery in a patient with post-SAE reactive thrombocytosis.
Patient concerns:
A 37-year-old woman with idiopathic thrombocytopenic purpura (ITP) suffered from multiple trauma scheduled for open reduction and internal fixation for right tibial and left radius fracture five days after SAE. The patient did not have any thromboembolic complications, although the platelet counts increased from 43 × 109/L to 568 × 109/L within two days after SAE. Surgery was completed under general anesthesia with tracheal intubation without complications. The patient complained of visual loss followed by limb weakness on the fourth and eighth hour postoperatively.
Diagnoses:
Magnetic resonance imaging (MRI) of head demonstrated ischemic change over bilateral basal ganglia, and occipital areas, suggesting the diagnosis of cortical blindness.
Interventions:
To suppress platelet count and avoid platelet hyper-aggregation, anti-platelet drug (i.e., oral aspirin 100 mg daily), hydration, and hydroxyurea (i.e., 20 mg/kg daily) were used for the treatment of reactive thrombocytosis.
Outcomes:
Although right-sided hemiparesis persisted, the patient reported mild visual recovery. She was discharged four months after SAE with active rehabilitation.
Lessons:
Our report highlights an increased risk of acute arterial thromboembolic events in patients with reactive thrombocytosis, especially those undergoing surgery.
Keywords: reactive thrombocytosis, splenic artery embolization, stroke, visual loss
1. Introduction
Splenic artery embolization (SAE), which has been demonstrated to decrease the need for operative intervention by 16%,[1] is a common procedure in hemodynamically stable trauma patients with blunt splenic injuries.[2] An isolated case report demonstrated that SAE may be safe and effective for blunt splenic injury in patients with idiopathic thrombocytopenic purpura (ITP).[3] Reactive thrombocytosis, which is defined as a platelet count of over 450 × 109/L,[4] is common after splenectomy or SAE with a reported overall incidence of 41%.[5] It has been proposed that patients with reactive thrombocytosis do not require cytoreductive medications or antiplatelet treatment because thromboembolic events are often restricted to the venous system and occur only in the presence of other prothrombotic risk factors.[6,7] Herein, we report a case of acute ischemic stroke in a patient with ITP experiencing reactive thrombocytosis following SAE for blunt splenic injuries. Written consent was obtained from the patient.
2. Case report
A 37-year-old woman (height: 155 cm; weight: 49 kg) with a history of ITP and seizure was transferred to our medical institute (ie, a tertiary referral center) after traffic accident with the diagnoses of traumatic spleen rupture, right tibial fracture, and left radius fracture. On arrival at the emergency department, it was noted that the patient had received tracheal intubation at the referring hospital because of hemorrhagic shock and loss of consciousness. According to the transfer record of the referring hospital, her hemodynamic profile was stabilized after blood transfusion with 8 units of packed red blood cells and intravenous epinephrine of 1 mg before transferal. She had no history of cardiovascular disease, thromboembolic events, or prothrombotic risk factors. On physical examination in our emergency department, her abdomen was mildly distended with ecchymosis on 4 limbs. Laboratory studies revealed hemoglobin: 12.6 g/dL (range, 12.0–16.0 g/dL), Hct: 39.2% (range, 37–47%), platelet count: 43 × 109/L (range, 150–400 × 109 /L), partial thromboplastin time: 20.7 seconds (range, 9–12 seconds), international normalized ratio: 2.03 (range, 0.9–1.2), and activated partial thromboplastin time: 91.2 seconds (range, <35 seconds). As a precaution against continuous bleeding and coagulopathy, the patient was transfused with 4 units of packed red blood cells, 24 units of platelets, and 4 units of fresh frozen plasma in the emergency department. The platelet count after blood transfusion was 111 × 109/L. After successful SAE for bleeding caused by the blunt splenic injuries, she was sent to intensive care unit for further care without complications.
Her platelet count increased from 163 to 568 × 109/L on the 2nd day after SAE. The patient was extubated in a stable condition with no thromboembolic events. Four days after SAE, a decision was made to treat the fractures by open reduction and internal fixation with plates and screws. Preoperative physical examination of the patient showed clear consciousness without respiratory distress, evidence of arterial occlusive disease, or signs of fat embolism such as axillary or subconjunctival petechiae. Vital signs included a blood pressure (BP) of 113/83 mm Hg, heart rate of 121 beats/minute, and respiratory rate of 12 breaths/minute. The results of electrocardiography, chest radiography, and coagulation test were unremarkable. After the application of standard monitoring, anesthesia was induced with propofol and rocuronium. Following successful endotracheal intubation, general anesthesia was maintained with desflurane in oxygen. During surgery, the systolic BP was maintained between 100 and 140 mm Hg, the arterial oxygen saturation was 100%, and the end-tidal partial pressure of carbon dioxide was 32 to 35 mm Hg. During surgery, there were no significant changes in BP or heart rate. The surgical time was 3 hours 15 minutes with minimal blood loss. After surgery, she was sent to intensive care unit for further care.
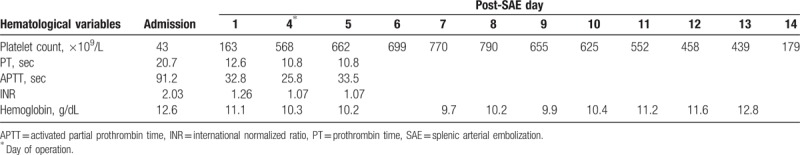
During the immediate postoperative period, the patient was neurologically intact. Because of uneventful recovery from anesthesia, tracheal extubation was performed 1 hour after surgery. Four hours after surgery, however, the patient complained of visual loss. Ophthalmological examination showed severely impaired visual acuity in both eyes. The semiquantitative “hand motion” (HM) test[8] demonstrated that the farthest distance at which the patient could see hand motion was 1 m (ie, HM at 1 m). Despite sustained effort to identify the possible causes, she developed right side limb weakness 12 hours after surgery with a National Institutes of Health Stroke Scale (NIHSS) score of 8. She remained hemodynamically stable without respiratory distress. Physical examination did not demonstrate axillary or subconjunctival petechiae. Immediate computed tomographic scan revealed no intracranial hemorrhage, and magnetic resonance imaging of the head demonstrated hypoxic-ischemic encephalopathy over bilateral basal ganglia as well as right occipitoparietal and left occipital areas (Fig. 1). No aneurysm, arteriovenous malformation, or vascular stenosis of the main cerebral arteries were found. Besides, chest radiograph did not demonstrate notable pulmonary infiltrates. The changes of hemoglobin concentration, platelet count, and other coagulation profiles during hospitalization are shown in Table 1. To suppress platelet count and avoid platelet hyper-aggregation, antiplatelet drug (ie, oral aspirin 100 mg daily), hydration, and hydroxyurea (ie, 20 mg/kg daily) were used for the treatment of reactive thrombocytosis. The treatment was initiated on postoperative day 2 and lasted for 7 days. Although right-sided hemiparesis persisted, the patient reported mild visual recovery. She was discharged 4 months after SAE with active rehabilitation.
Figure 1.
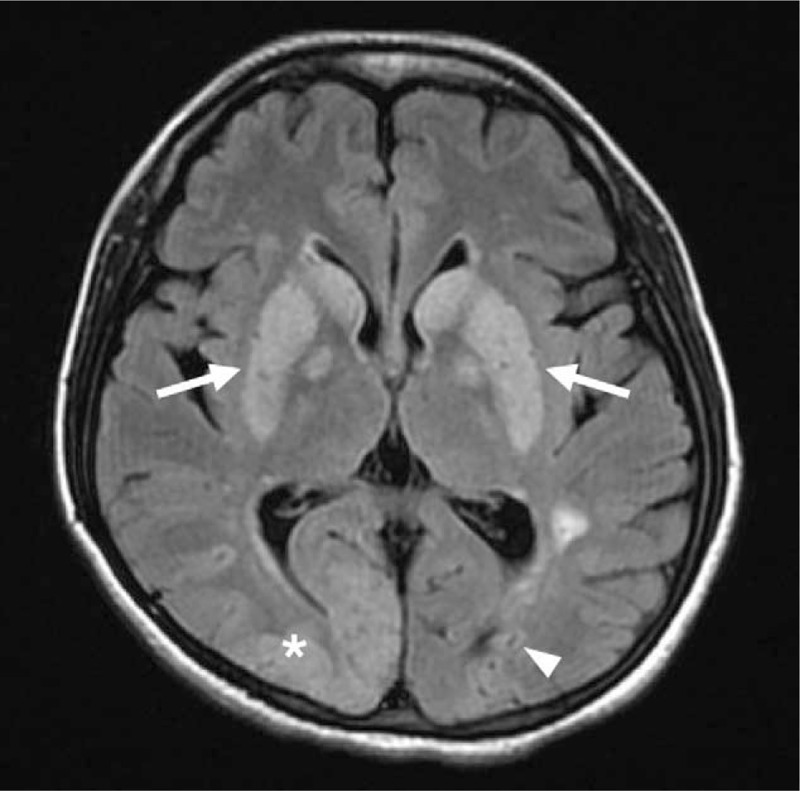
Magnetic resonance imaging (MRI) of the head demonstrating hypoxic-ischemic encephalopathy over bilateral basal ganglia (white arrows), right occipitoparietal (asterisk), and left occipital areas (white arrowhead) without evidence of aneurysm, arteriovenous malformation, or vascular stenosis of the main cerebral arteries.
Table 1.
Patient hemoglobin concentration and coagulation profile after splenic arterial embolization.
3. Discussion
Thrombocytosis can be classified as primary or secondary (reactive).[4] Primary thrombocytosis (eg, essential thrombocythemia and polycythemia vera) is characterized by abnormalities in both platelet structure and function, leading to an increased risk of bleeding as well as arterial and venous thromboembolism.[4] In contrast, secondary thrombocytosis is a relatively benign condition without overt symptoms. Reactive thrombocytosis, the commonest secondary cause of thrombocytosis,[9] has been reported to be triggered by infection, inflammation, tissue damage from surgery or trauma, iron deficiency, hemolysis, malignancy, hyposplenism, and other causes of an acute phase response.[4] As the spleen plays a major role in platelet homeostasis, reactive thrombocytosis is common after splenectomy or SAE.[5] The platelet count after splenectomy usually peaks at 1 to 3 weeks and returns to normal levels within weeks, months, and rarely, years.[10] Our patient had a rapid and significant rise in platelet count on the 4th days after SAE. Das et al[11] reported an ITP patient in whom the platelet count increased rapidly from 9 × 109 to 1623 × 109 /L on the 7th postoperative day after splenectomy. It remained unclear whether concomitant multiple trauma contributed to the steep increase in platelet count in our case.
Reactive thrombocytosis is often believed to be a relatively benign condition with thromboembolic events often being restricted to the venous system in the presence of other prothrombotic risk factors.[6,7] Our patient had no previous history of cardiovascular disease, thromboembolic events, or notable prothrombotic risk factors. Her perioperative hemodynamic profile, a predictor of perioperative stroke,[12] was also unremarkable. We suggest that the steep increase in platelet count may have led to the development of platelet hyper-aggregation, acute stroke, as well as cortical blindness in our case. Although the relationship between stroke and reactive thrombocytosis remains unclear, previous population-based studies had reported an association between splenectomy and an increased long-term risk of stroke.[13,14]
Acute arterial thromboembolic events from reactive thrombocytosis are rare, and most of these case reports in the current literature are related to the coronary artery.[15,16] Khan et al[15] reported the occurrence of myocardial infarction on the 7th day after splenectomy in a 61-year-old hypertensive man with concomitant thrombocytosis. Ghaffari and Pourafkari[16] reported the occurrence of myocardial infarction on the 12th day after splenectomy in a 34-year-old smoking man with concomitant thrombocytosis. In contrast to the presence of cardiovascular risk factors (ie, hypertension and smoking) in the previous reports,[15,16] there was no notable risk factors in our patient. It has been reported that tissue damage from surgery could trigger reactive thrombocytosis[4] and operation could also increase perioperative thrombotic risk.[4,6,17] Both causes, therefore, may further amplify the arterial thromboembolic risk in our case.
To prevent thrombocytosis-associated complications, preoperative precautions should be taken for patients who have had thrombotic events and cardiovascular risk factors. Avoidance of acute blood loss during surgery and anemia, which are known to induce reactive thrombocytosis,[4] may reduce the risk of perioperative thrombocytosis. In addition, antiplatelet, cytoreductive therapy, or therapeutic thrombocytapheresis may be used preoperatively, but the anticipated reduction in risk of thrombosis should outweigh the risk of drug-induced bleeding. Regular monitoring of platelet count after splenectomy is also important. Elective surgery may be performed until the platelet count returns to normal range. Furthermore, preoperative identification and control of existing thrombotic risk factors may reduce the risk of perioperative thromboembolic events for patients with reactive thrombocytosis.
In our patient, other possible causes of stroke should also be considered. Posttraumatic fat embolism syndrome (FES), a well-described complication of long bone fracture characterized by both pulmonary and systemic fat embolism, is a possible diagnosis for our patient. The most common sign of FES was hypoxia (96%), followed by mental status change (59%), and petechiae (33%).[18] FES remains a diagnosis of exclusion based on clinical criteria. Based on the diagnostic criteria proposed by Schonfeld et al,[19] a score of more than 5 is required for the diagnosis of FES (petechiae = 5; lung infiltration = 4; hypoxemia = 3; fever = 1; tachycardia = 1; tachypnea = 1; confusion = 1). Accordingly, FES is an unlikely cause of acute stroke in our case due to the lack of typical signs. Furthermore, the image findings of brain magnetic resonance imaging are also inconsistent with the diagnosis of brain fat embolism.
In addition, hypoxic-ischemic encephalopathy from hypovolemic shock during initial resuscitation may be another cause of stroke in our case. Delayed presentations of hypoxic-ischemic encephalopathy were reported in current literature. Limaye et al[20] described a case of hypoxic-ischemic encephalopathy-induced delayed cortical blindness that occurred 5 days after an initial hypoxic event of 10 minutes. Therefore, it is possible that the hypovolemic shock and loss of consciousness during the initial resuscitation may have contributed to the development of ischemic stroke and cortical blindness in our case.
In general, since reactive thrombocytosis is considered a benign condition without significant risk of thrombosis, cytoreductive medications, or antiplatelet treatment is not recommend for this patient population.[21] With this belief, orthopedic surgery was not delayed and antiplatelet agents were not given to our patient to reduce the risk of complications from multiple bone fractures. Accordingly, the occurrence of perioperative visual loss, which is an uncommon complication primarily associated with cardiac, spine, and head and neck surgery,[22] also did not raise the concern of thromboembolic event in our patient because the initial possible diagnosis of visual loss was considered to be related to hypovolemic shock[23,24] or FES. Not until the progression of neurological symptoms from visual loss to limb weakness did we realize that thromboembolism may be a possibility. Therefore, it is reasonable to believe that early medical intervention, including antiplatelet combined with cytoreductive therapy or therapeutic thrombocytapheresis, may avoid the deterioration of neurological functions in our case.
In conclusion, our report underscores an increased risk of thromboembolism in a patient with reactive thrombocytosis undergoing surgery, suggesting the importance of preoperative precautions, close perioperative monitoring of platelet count, and early realization of possible thromboembolic complications in this patient population.
Author contributions
Conceptualization: Wei-Ting Wang, Yu-Yu Li, Jen-Yin Chen, Kuo-Mao Lan, Cheuk-Kwan Sun, Kuo-Chuan Hung.
Data curation: Wei-Ting Wang, Yu-Yu Li, Wan-Ching Lin.
Formal analysis: Wan-Ching Lin, Jen-Yin Chen, Kuo-Chuan Hung.
Investigation: Wan-Ching Lin, Jen-Yin Chen, Kuo-Mao Lan, Cheuk-Kwan Sun.
Methodology: Yu-Yu Li.
Writing – original draft: Wei-Ting Wang, Yu-Yu Li, Wan-Ching Lin, Jen-Yin Chen, Kuo-Mao Lan, Cheuk-Kwan Sun, Kuo-Chuan Hung.
Writing – review & editing: Cheuk-Kwan Sun, Kuo-Chuan Hung.
Footnotes
Abbreviations: BP = blood pressure, FES = fat embolism syndrome, ITP = idiopathic thrombocytopenic purpura, SAE = splenic artery embolization.
C-KS and K-CH contributed equally as corresponding authors to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Wei B, Hemmila MR, Arbabi S, et al. Angioembolization reduces operative intervention for blunt splenic injury. J Trauma 2008;64:1472–7. [DOI] [PubMed] [Google Scholar]
- [2].Ekeh AP, McCarthy MC, Woods RJ, et al. Complications arising from splenic embolization after blunt splenic trauma. Am J Surg 2005;189:335–9. [DOI] [PubMed] [Google Scholar]
- [3].Memon S, Laing AD, Frye JN. Traumatic splenic injury managed with arterial embolisation in a patient with idiopathic thrombocytopenic pupura. Cardiovasc Intervent Radiol 2010;33:877–9. [DOI] [PubMed] [Google Scholar]
- [4].Harrison CN, Bareford D, Butt N, et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br J Haematol 2010;149:352–75. [DOI] [PubMed] [Google Scholar]
- [5].Chia TL, Chesney TR, Isa D, et al. Thrombocytosis in splenic trauma: in-hospital course and association with venous thromboembolism. Injury 2017;48:142–7. [DOI] [PubMed] [Google Scholar]
- [6].Griesshammer M, Bangerter M, Sauer T, et al. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med 1999;245:295–300. [DOI] [PubMed] [Google Scholar]
- [7].Ho KM, Yip CB, Duff O. Reactive thrombocytosis and risk of subsequent venous thromboembolism: a cohort study. J Thromb Haemost 2012;10:1768–74. [DOI] [PubMed] [Google Scholar]
- [8].Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci 2006;47:1236–40. [DOI] [PubMed] [Google Scholar]
- [9].Tefferi A, Ho TC, Ahmann GJ, et al. Plasma interleukin-6 and C-reactive protein levels in reactive versus clonal thrombocytosis. Am J Med 1994;97:374–8. [DOI] [PubMed] [Google Scholar]
- [10].Greer JP, Foerster J, Lukens JN, eds. Wintrobe's Clinical Hematology, 11th ed. Philadelphia: Lippincott Williams & Wilkins, 1981: 1128–34. [Google Scholar]
- [11].Das SS, Bhattacharya S, Sen S. Managing uncontrolled postsplenectomy reactive thrombocytosis in idiopathic thrombocytopenic purpura: role of thrombocytapheresis. Transfus Apher Sci 2013;49:171–3. [DOI] [PubMed] [Google Scholar]
- [12].Bijker JB, Persoon S, Peelen LM, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology 2012;116:658–64. [DOI] [PubMed] [Google Scholar]
- [13].Rørholt M, Ghanima W, Farkas DK, et al. Risk of cardiovascular events and pulmonary hypertension following splenectomy – a Danish population-based cohort study from 1996–2012. Haematologica 2017;102:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin JN, Lin CL, Lin MC, et al. Increased risk of hemorrhagic and ischemic strokes in patients with splenic injury and splenectomy: A Nationwide Cohort Study. Medicine (Baltimore) 2015;94:e1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khan PN, Nair RJ, Olivares J, et al. Postsplenectomy reactive thrombocytosis. Proc (Bayl Univ Med Cent) 2009;22:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ghaffari S, Pourafkari L. Acute myocardial infarction in a patient with post-splenectomy thrombocytosis: a case report and review of literature. Cardiol J 2010;17:79–82. [PubMed] [Google Scholar]
- [17].Schmuziger M, Christenson JT, Maurice J, et al. Reactive thrombocytosis after coronary bypass surgery. An important risk factor. Eur J Cardiothorac Surg 1995;9:393–7. [DOI] [PubMed] [Google Scholar]
- [18].Bulger EM, Smith DG, Maier RV, et al. Fat embolism syndrome. A 10-year review. Arch Surg 1997;132:435–9. [DOI] [PubMed] [Google Scholar]
- [19].Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med 1983;99:438–43. [DOI] [PubMed] [Google Scholar]
- [20].Limaye K, Jadhav AP. Delayed transient cortical blindness from hypoxic ischemic encephalopathy. Am J Med 2017;130:e391–2. [DOI] [PubMed] [Google Scholar]
- [21].Cheung MC, Hicks LK, Pendergrast J. Thrombocytosis. N Engl J Med 2004;350:2524–5. [DOI] [PubMed] [Google Scholar]
- [22].Lee LA. Perioperative visual loss and anesthetic management. Curr Opin Anaesthesiol 2013;26:375–81. [DOI] [PubMed] [Google Scholar]
- [23].Klewin KM, Appen RE, Kaufman PL. Amaurosis and blood loss. Am J Ophthalmol 1978;86:669–72. [DOI] [PubMed] [Google Scholar]
- [24].Presencia AC, Hernandez AM, Guia ED. Amaurosis following blood loss. Ophthalmologica 1985;191:119–21. [DOI] [PubMed] [Google Scholar]


