Abstract
Early allograft dysfunction (EAD) is considered a precursor to graft loss in liver transplantation. To date, the use of preoperative serum cytokine profiles to predict EAD development has not been systematically investigated in living donor liver transplantation (LDLT). Here, we investigated the association between preoperative serum cytokine profiles and EAD development in LDLT patients.
Serum cytokine profiles collected preoperatively and on postoperative day 7 were retrospectively reviewed. The specific serum cytokines analyzed included interleukin (IL)-2, IL-6, IL-10, IL-12, IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. The cytokine levels of patients with EAD were compared with those of patients without EAD and the impact of cytokine levels on the occurrence of EAD was evaluated.
Preoperatively, the serum levels of IL-6, 10, 17, and TNF-α were significantly higher in the EAD group than in the non-EAD group. In univariate logistic analysis, the preoperative levels of IL-6, IL-10, IL-17, IFN-γ, and TNF-α were potentially associated with EAD development. After multivariate logistic analysis, higher preoperative serum levels of IL-6 and 17 were significantly associated with EAD development. In addition, the incidence of EAD increased as the preoperative serum levels of IL-6 and IL-17 increased.
Preoperative serum levels of IL-6 and IL-17 were significantly associated with EAD development in LDLT.
Keywords: cytokines, interleukin-17, interleukin-6, liver transplantation, living donors, primary graft dysfunction
1. Introduction
Predictive indicators for graft outcomes are important in liver transplantation (LT), and previous studies have suggested that early allograft dysfunction (EAD) is a precursor step in the pathway to eventual graft loss.[1,2] Recently, a multicenter study by Olthoff et al[3] in patients undergoing deceased donor liver transplantation (DDLT) suggested a definition of EAD that included cholestasis, coagulopathy, and graft injury. DDLT patients with EAD more often suffered from graft loss and had worse overall survival rates than those without EAD. In one study conducted in a living donor liver transplantation (LDLT) population, patients diagnosed with EAD based on the definition proposed above[3] showed an approximately fivefold higher incidence of graft failure within 90 days than those without EAD.[4] Several other studies have also accepted this definition of EAD as a valid postoperative outcome in LDLT patients.[5,6]
Donor age and model for end-stage liver disease (MELD) score were significantly associated with EAD development in patients who underwent DDLT.[3] In LDLT patients, several factors including left-lobe and small size grafts, high levels of recipient preoperative bilirubin, high recipient portal reperfusion pressure, an older aged donor, and a donor with higher body mass index (BMI) have been implicated in the development of EAD.[4] The inflammatory status of patients also plays an important role in outcomes following transplantation. In patients with end-stage liver disease (ESLD), systemic inflammatory response syndrome was closely related to poor outcomes including complications from hepatic decompensation and mortality.[7] In a previous study on DDLT recipients, postoperative levels of C-reactive protein (CRP) were higher in patients with EAD than in those without EAD. In addition, in patients with EAD, higher postoperative CRP levels were associated with increased mortality.[8] However, no studies have adopted serum cytokine levels as a marker of systemic inflammation to evaluate EAD development in LT patients.
Serum cytokines are important inflammatory mediators in DDLT patients, and many studies have suggested a relationship between serum cytokine levels and post-transplant complications. Peak serum concentrations of interleukin (IL)-1β and IL-6 are correlated with the presence of preoperative cholestasis and the intraoperative transfusion of blood products.[9]. Preoperative serum cytokine and chemokine profiles, specifically those related to T-cell immunity and the nuclear factor-κB pathway, are also associated with EAD development.[10]
The predictive role of preoperative serum cytokine profiles on EAD development has not been systematically investigated in patients who underwent LDLT. Therefore, we studied the association between preoperative serum cytokine profiles including IL-2, IL-6, IL-10, IL-12, IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, and EAD development in the LDLT patient population. Pre- and postoperative serum cytokine levels were compared between patients with and without EAD.
2. Patients and methods
2.1. Study population
In total, 260 adult patients (age ≥ 19 years) underwent LDLT for ESLD between January 2010 and December 2014 at St. Mary's Hospital (Seoul, Korea). The perioperative data of both recipients and donors were retrospectively reviewed using the hospital electronic medical records system. Patients who required hemodialysis before and during surgery, including those on continuous renal replacement therapy, were excluded from analysis, as the hemodialysis removed cytokines from the systemic circulation.[11–14] Recipients and donors with inaccurate data were not included in the analysis. This study was approved by the St. Mary's Hospital Ethics Committee (KC17RISI0001). Informed consent was waived because of the retrospective study design.
2.2. Patient management
LDLT was performed by an experienced transplant team using the piggyback transplantation technique. Operations were performed with balanced anesthesia and under invasive hemodynamic monitoring, according to the institutional LDLT protocol.[15] All of the patients who underwent LDLT were given immunosuppression such as tacrolimus, mycophenolate mofetil, prednisolone, and basiliximab based on the institutional immunosuppression schedule.[16]
2.3. Definition of early allograft dysfunction
In successful LDLT operations with no surgical complications, EAD was defined as: total serum levels of bilirubin ≥ 10 mg/dL or international normalized ratio (INR) ≥ 1.6 on postoperative day (POD) 7; and an alanine (ALT) or aspartate (AST) aminotransferase level > 2000 U/L within the first 7 postoperative days.[3] Based on this definition, patients were classified into EAD and non-EAD groups.
2.4. Serum cytokine measurement
Serum cytokine profiles were collected from LDTL patients preoperatively and on POD 7. The specific serum cytokines analyzed included IL-2, IL-6, IL-10, IL-12, IL-17, IFN-γ, and TNF-α. Blood samples were collected in a sterile manner for immunoassay (BD Vacutainer, K2EDTA; Becton, Dickinson and Company, Franklin Lakes, NJ), and the samples were sent to the laboratory in an iced container. Then samples underwent centrifugation (1500 rpm for 10 minutes at 4°C) and freezing at –70°C for storage until the analyses were complete. Serum cytokine levels were measured with an enzyme-linked immunosorbent assay using a human 25-plex antibody bead kit (Invitrogen Corp., Camarillo, CA). Quantitative analyses were done using the Luminex detection system (200; Luminex Corp., Austin, TX).
2.5. Clinical data
The preoperative recipient data collected for analysis included age; sex; BMI; etiology of liver disease; MELD score; complications from hepatic decompensation; and laboratory values such as hematocrit, sodium, white blood cell (WBC) count, neutrophil to lymphocyte ratio (NLR), platelet count, CRP level, total bilirubin level, and INR. The donor-graft data collected for analysis included age, sex, BMI, estimated graft volume to standard liver volume ratio at the time of surgery, the presence and degree of steatosis, total graft ischemic time, and average of hepatic circulation (portal venous flow and hepatic arterial resistive index) on PODs 1, 3, and 5.
2.6. Statistical analysis
Continuous data are expressed as the median and interquartile range and were compared using the Mann–Whitney U test. Categorical data were presented as the number and proportion and evaluated using the χ2 test or Fisher's exact test, as appropriate. The test for trends was performed using linear-by-linear association. Changes in serum cytokine levels between the preoperative samples and those taken on POD 7 were analyzed using the Wilcoxon signed rank test with the Bonferroni post-hoc test. The association between preoperative serum cytokine profiles, divided into high and low levels at the median, and EAD development were investigated using univariate logistic regression analyses. Factors with potential significance were entered into the multivariate logistic regression analyses. The accuracy of the predictive model for the development of EAD was analyzed using the area under the receiver operating characteristics curve (AUC). All of the tests were two sided, and a P-value < .05 was considered statistically significant. Statistical analyses were conducted using SPSS version 24.0 for Windows (SPSS Inc., Chicago, IL) and MEDCALC for Windows software (version 11.0; MedCalc Software, Mariakerke, Belgium).
3. Results
Sixteen patients were excluded because they underwent hemodialysis before and during surgery. Eighteen patients were excluded due to missing serum cytokine profile data. In total, 226 patients satisfied the criteria for analysis and were included in this study. Transplant recipients were a mean age of 53 ± 8 years, and were predominantly male (69.7%). The mean preoperative WBC count was 5.0 ± 3.4 × 109/L; the mean NLR was 4.1 ± 5.5, and mean CRP was 1.0 ± 1.8 mg/dL. The most common cause for LDLT was hepatitis B virus infection (61.1%). The average recipient MELD score was 17 ± 9 points and the incidence of EAD was 12.4%. Preoperatively, WBC count, NLR, and CRP were higher in the EAD group than in the non-EAD group. Other recipient and donor-graft factors did not differ between groups (Table 1).
Table 1.
Comparison of recipient and donor-graft clinical characteristics between patients with and without early allograft dysfunction in living donor liver transplantation.
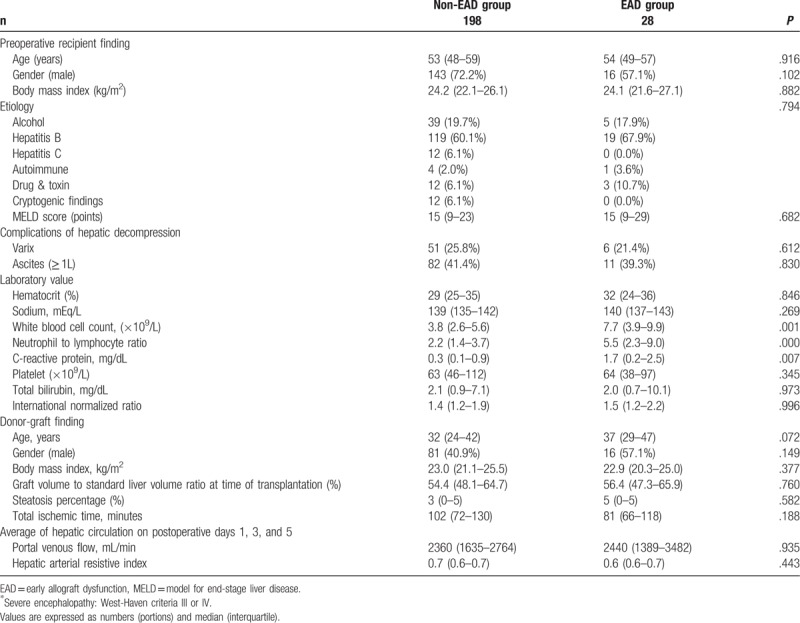
Patients with EAD had higher preoperative serum levels of IL-6, IL-10, IL-17, and TNF-α than those without EAD (Table 2). One week after LDLT, the serum levels of IL-2, IL-6, IL-10, IL-12, IL-17, IFN-γ, and TNF-α, were higher in the EAD group than in the non-EAD group. In patients with EAD, samples taken on POD 7 showed an increase in serum levels of IL-2 and a decrease in serum levels of TNF-α from preoperative baseline samples. In patients without EAD, IL-10 levels increased, and IL-6, IL-17, and IFN γ decreased during the same period.
Table 2.
Comparison of serum cytokine levels on preoperative day and postoperative day 7 between patients with and without early allograft dysfunction in living donor liver transplantation.
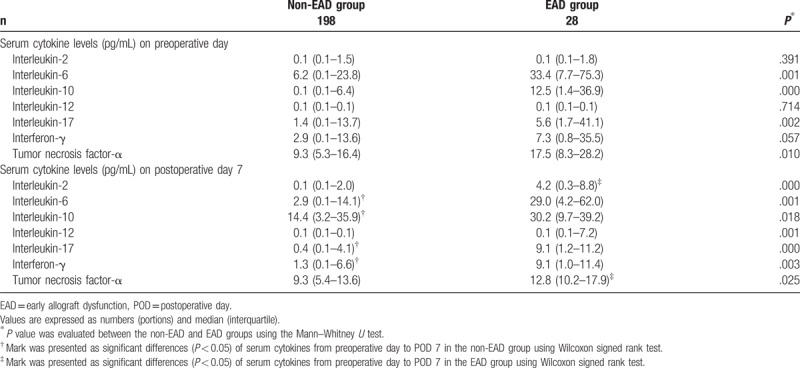
The association between preoperative serum levels of IL-6, IL-10, IL-17, IFN-γ, and TNF-α and postoperative EAD development (Table 3) was analyzed with a univariate predictive model. Pre-operative levels of IL-6 and IL-17 were independently and significantly associated with EAD development after LDLT. The accuracy of the predictive model was as follows: AUC = 0.717 (95% confidence interval = 0.653–0.775; sensitivity = 64.3%; specificity = 74.2%; P < .001. When preoperative serum levels of IL-6 and IL-17 levels were divided into quartiles, the incidence of EAD significantly increased as serum levels increased (Table 4).
Table 3.
Association between preoperative serum cytokine profiles and early allograft dysfunction development after living donor liver transplantation.
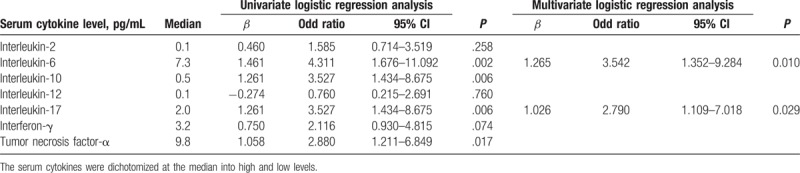
Table 4.
Comparison of incidence of EAD by preoperative serum IL-6 and IL-17 quartiles in living donor liver transplantation.
4. Discussion
We found that preoperative serum levels of IL-6 and IL-17 were associated with the development of EAD after LDLT. Patients with relatively high preoperative levels of serum IL-6 and IL-17 were significantly more likely to develop EAD than those with lower preoperative levels. In patients with EAD, serum levels of IL-6 and IL-17 remained elevated in the first week following surgery. However, in patients without EAD, there was a distinct decline in IL-6 and IL-17 levels during this period.
Liver inflammatory processes are essential in maintaining organ and systemic homeostasis when challenged by infectious pathogens, cancerous cells, or tissue injuries. Dysregulated inflammation can result in severe parenchymal and systemic pathology including cirrhosis, neoplasia, autoimmune disease, and sepsis.[17] IL-6 is a regulatory cytokine in the inflammatory response of various immune cells including T-cells, B-cells, and macrophages.[18] In patients with ESLD, increased serum levels of IL-6 are related to increased mortality. The predictive value of serum IL-6 level for 90-day mortality is higher than that of other inflammatory markers such CRP level and WBC count, and its predictive value for 1-year mortality is better than that of hepatic function markers including INR and total bilirubin.[19] Animal studies have shown that increased serum levels of IL-6 are associated with acute cellular rejection after orthotopic LT.[20] Recipients with acute histopathological rejection showed continuously higher serum levels of IL-6 than those without rejection after orthotopic LT.[21] However, IL-6 may have some favorable effects on graft function. One study of patients following DDLT showed that lower preoperative serum levels of IL-6 were significantly associated with EAD development.[10] In addition, a previous animal study suggested that IL-6 may protect the liver from ischemia/reperfusion injury, and may play a role in liver cell regeneration.[22] In this study of patients undergoing LDLT, preoperative serum levels of IL-6 were significantly higher in patients with EAD than in those without. In addition, the incidence of EAD development increased proportionally to increased preoperative serum levels of IL-6 in these patients. This finding contradicts a previous study by Friedman et al[10] that could not explain why relatively low levels of IL-6 before LT were associated with the development of EAD. They speculated that the hepatoprotective role of IL-6 reduced the incidence of EAD after DDLT. In the present study, relatively high levels of IL-6 were related to more frequent development of EAD after surgery. We presume that IL-6 may have adverse effects on graft homeostasis. In addition, the effects of IL-6 on the development of EAD may differ between LDLT and DDLT, as the size of the grafts differs. The relationship between serum IL-6 level and graft function recovery, specifically according to graft size, has not been fully investigated in human LT. Thus, further studies are required to fully characterize the effects of serum IL-6 levels on regenerating liver grafts following LDLT.
In various inflammatory and autoimmune diseases, T helper 17 (TH17) cells, characterized by the production of IL-17, have emerged as important mediators of the pathological process. IL-17 has pro-inflammatory features and plays a crucial role in the activation and maintenance of the immune response.[23] In previous studies on the association between liver injury and IL-17, hepatic ischemia/reperfusion injury activated TH17 cells and upregulated the secretion of IL-17.[24] Increased expression of IL-17 was related to hepatic fatty change, and drove the progression from steatosis to steatohepatitis in a mouse model of non-alcoholic fatty liver disease.[25] In solid organ transplantation settings, studies on IL-17 have focused on acute graft rejection, because of the high pro-inflammatory nature of this process. A study by Fabrega et al[26] showed that IL-17 levels were higher in patients with acute cellular rejection than in those without, in the setting of DDLT. Research by Kim et al[27] demonstrated that serum IL-17 was a major predictive biomarker of acute rejection after LT. Finally, a study by Friedman et al[10] found that preoperative serum levels of IL-17 were not a factor in the development of EAD, because they were below the limit of measurement. Here, in the context of LDLT, preoperative IL-17 levels were significantly higher in patients who developed EAD than in those that did not. Although the mechanism by which IL-17 mediates inflammation post-transplant is unknown, the serum levels of IL-17 may serve as an important predictive biomarker for post-LDLT graft function.
This study had several limitations. First, inflammation is a complicated biological process that involves many mediators. Thus, it is difficult to adequately characterize the inflammatory status of the liver graft using only cytokine profiles, in the setting of LT. However, serum cytokine levels differed between patients with and without EAD. Second, there are currently no widely accepted and validated normal ranges for serum levels of IL-6 and IL-17 in clinical practice. Here, we simply showed that patients with EAD had higher serum levels of IL-6 and IL-17 than those without EAD. Further studies are required to develop preoperative serum IL-6 and IL-17 cut-off values that most likely lead to EAD development. This area of inquiry may significantly improve clinical outcomes for patients undergoing LDLT. Finally, because of the retrospective study design, it was inevitable that a small number of patients would have deficient or missing data.
In conclusion, we suggest that preoperative serum levels of IL-6 and IL-17 may serve as important mediators of the development of EAD in the setting of LDLT. Considering that the incidence of EAD increased in proportion to the preoperative levels of serum IL-6 and IL-17, it is possible that patients with a higher degree of preoperative inflammation are at a higher risk for developing EAD after LDLT. However, these data should be interpreted cautiously because of diverse effects of inflammatory cytokines on graft homeostasis. Further prospective studies are required to determine the appropriate cut-off values for preoperative serum levels of IL-6 and IL-17 and their utility in predicting the development of EAD after LDLT.
Author contributions
Conceptualization: Min Suk Chae, Sang Hyun Hong.
Data curation: Min Suk Chae, Jong-Woan Kim, Hyun Sik Chung, Chul Soo Park, Jaemin Lee, Jong Ho Choi, Sang Hyun Hong.
Formal analysis: Min Suk Chae, Jong-Woan Kim, Hyun Sik Chung, Chul Soo Park, Jaemin Lee, Jong Ho Choi, Sang Hyun Hong.
Investigation: Min Suk Chae, Sang Hyun Hong.
Methodology: Min Suk Chae, Sang Hyun Hong.
Project administration: Min Suk Chae, Sang Hyun Hong.
Resources: Min Suk Chae, Sang Hyun Hong.
Software: Min Suk Chae, Sang Hyun Hong.
Supervision: Sang Hyun Hong.
Validation: Min Suk Chae, Sang Hyun Hong.
Visualization: Min Suk Chae.
Writing – original draft: Min Suk Chae.
Writing – review & editing: Min Suk Chae.
Footnotes
Abbreviations: CRP = C-reactive protein, DDLT = deceased donor liver transplantation, EAD = early allograft dysfunction, ESLD = end-stage liver disease, IFN = interferon, IL = interleukin, LDLT = living donor liver transplantation, MELD = model for end-stage liver disease, TNF = tumor necrosis factor.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ben-Ari Z, Weiss-Schmilovitz H, Sulkes J, et al. Serum cholestasis markers as predictors of early outcome after liver transplantation. Clin Transplant 2004;18:130–6. [DOI] [PubMed] [Google Scholar]
- [2].Tekin K, Imber CJ, Atli M, et al. A simple scoring system to evaluate the effects of cold ischemia on marginal liver donors. Transplantation 2004;77:411–6. [DOI] [PubMed] [Google Scholar]
- [3].Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010;16:943–9. [DOI] [PubMed] [Google Scholar]
- [4].Pomposelli JJ, Goodrich NP, Emond JC, et al. Patterns of early allograft dysfunction in adult live donor liver transplantation: the A2ALL experience. Transplantation 2016;100:1490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li L, Wang H, Yang J, et al. Immediate postoperative low platelet counts after living donor liver transplantation predict early allograft dysfunction. Medicine (Baltimore) 2015;94:e1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hong SH, Kwak JA, Chon JY, et al. Prediction of early allograft dysfunction using serum phosphorus level in living donor liver transplantation. Transpl Int 2013;26:402–10. [DOI] [PubMed] [Google Scholar]
- [7].Cazzaniga M, Dionigi E, Gobbo G, et al. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol 2009;51:475–82. [DOI] [PubMed] [Google Scholar]
- [8].Oweira H, Lahdou I, Daniel V, et al. Early post-operative acute phase response in patients with early graft dysfunction is predictive of 6-month and 12-month mortality in liver transplant recipients. Hum Immunol 2016;77:952–60. [DOI] [PubMed] [Google Scholar]
- [9].Miki C, McMaster P, Mayer AD, et al. Factors predicting perioperative cytokine response in patients undergoing liver transplantation. Crit Care Med 2000;28:351–4. [DOI] [PubMed] [Google Scholar]
- [10].Friedman BH, Wolf JH, Wang L, et al. Serum cytokine profiles associated with early allograft dysfunction in patients undergoing liver transplantation. Liver Transpl 2012;18:166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bellomo R, Tipping P, Boyce N. Continuous veno-venous hemofiltration with dialysis removes cytokines from the circulation of septic patients. Crit Care Med 1993;21:522–6. [DOI] [PubMed] [Google Scholar]
- [12].Bellomo R, Tipping P, Boyce N. Interleukin-6 and interleukin-8 extraction during continuous venovenous hemodiafiltration in septic acute renal failure. Ren Fail 1995;17:457–66. [DOI] [PubMed] [Google Scholar]
- [13].Peng Y, Yuan Z, Li H. Removal of inflammatory cytokines and endotoxin by veno-venous continuous renal replacement therapy for burned patients with sepsis. Burns 2005;31:623–8. [DOI] [PubMed] [Google Scholar]
- [14].Tarakcioglu M, Erbagci AB, Usalan C, et al. Acute effect of hemodialysis on serum levels of the proinflammatory cytokines. Mediators Inflamm 2003;12:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chae MS, Park CS, Oh SA, et al. Predictive role of intraoperative plasma fibrinogen for postoperative portal venous flow in living donor liver transplantation. Ann Transplant 2017;22:83–95. [DOI] [PubMed] [Google Scholar]
- [16].Choi HJ, Kim DG, Na GH, et al. The clinical outcomes of patients with portal vein tumor thrombi after living donor liver transplantation. Liver Transpl 2017;23:1023–31. [DOI] [PubMed] [Google Scholar]
- [17].Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 2016;13:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011;1813:878–88. [DOI] [PubMed] [Google Scholar]
- [19].Remmler J, Schneider C, Treuner-Kaueroff T, et al. Increased level of interleukin 6 associates with increased 90-day and 1-year mortality in patients with end-stage liver disease. Clin Gastroenterol Hepatol 2017;S1542-3565:31106–10. [DOI] [PubMed] [Google Scholar]
- [20].Ohzato H, Monden M, Yoshizaki K, et al. Serum interleukin-6 levels as an indicator of acute rejection after liver transplantation in cynomologous monkeys. Surg Today 1993;23:521–7. [DOI] [PubMed] [Google Scholar]
- [21].Kita Y, Iwaki Y, Demetris AJ, et al. Evaluation of sequential serum interleukin-6 levels in liver allograft recipients. Transplantation 1994;57:1037–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Camargo CA, Jr, Madden JF, Gao W, et al. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 1997;26:1513–20. [DOI] [PubMed] [Google Scholar]
- [23].Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 2007;8:345–50. [DOI] [PubMed] [Google Scholar]
- [24].Caldwell CC, Okaya T, Martignoni A, et al. Divergent functions of CD4 + T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 2005;289:G969–76. [DOI] [PubMed] [Google Scholar]
- [25].Tang Y, Bian Z, Zhao L, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol 2011;166:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fabrega E, Lopez-Hoyos M, San Segundo D, et al. Changes in the serum levels of interleukin-17/interleukin-23 during acute rejection in liver transplantation. Liver Transpl 2009;15:629–33. [DOI] [PubMed] [Google Scholar]
- [27].Kim N, Yoon YI, Yoo HJ, et al. Combined detection of serum IL-10, IL-17, and CXCL10 predicts acute rejection following adult liver transplantation. Mol Cells 2016;39:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


