Abstract
To compare long-term overall survival (OS) in patients with G1 and G2 grade Ta bladder cancer after transurethral resection of bladder tumors (TURBTs). Secondary aim was to investigate clinical and pathologic prognostic factors for OS of Ta patients, except G3/high grade (HG).
A total of 243 patients, retrospectively selected, with Ta nonmuscle invasive bladder cancer (NMIBC) underwent TURBT between January 2006 and December 2008 (median follow-up 109 months). Inclusion criteria were: Ta at first manifestation, G1 or G2 grade with no associated carcinoma in situ (CIS). Seventy-nine patients were excluded due to concomitant CIS (1), G3/HG tumors (47), and lost to follow-up (31). Ethical approval was obtained from the Ethical Committee of the Mures County Hospital. Statistical analysis was performed using STATA 11.0.
Following inclusion criteria, 164 patients with primary G1 or G2 Ta tumors, were enrolled. Recurrence was observed in 26 (15.8%) and progression in 5 (3%) patients. Ten-year survival in G1 patients was 67.8% (CI 54.3–78.1) and in G2 patients 59% (CI 49–67.3) (P = .31). Univariable and multivariable logistic regression analysis underlined that advanced age at diagnosis (hazard ratio [HR] 1.10) and no Bacillus Calmette–Guerin (BCG) treatment (HR 0.24 and 0.29) were independent predictors for death at 10 years after diagnosis.
Long-term analysis confirms that patients with well differentiated (G1) and moderately well differentiated (G2) Ta tumors have similar OS. A longer OS was even reported in those who underwent BCG adjuvant therapy.
Keywords: G1 and G2 grade nonmuscle invasive bladder cancer, long-term, overall survival, progression, recurrence
1. Introduction
Bladder cancer (BC) is one of the most common of the urinary tract, occupying the 2nd place after prostate cancer.[1] Approximately half of these newly diagnosed tumors are low grade (LG)[2] and >70% are nonmuscle invasive bladder cancer (NMIBC).[3] The main procedure for the diagnosis and treatment of NMIBC is the transurethral resection of bladder tumor (TURBT) and according to the latest version of the European Association of Urology guidelines, “an immediate chemotherapy instillation is recommended in tumors presumed to be at low or intermediate risk.”[2] Low-risk tumors are considered primary, solitary, TaG1 (papillary urothelial neoplasm of low malignant potential, LG), and <3 cm, with no carcinoma in situ (CIS). Intermediate risk tumors are those that do not fit to low category or in any of the following: T1 tumor, G3 (high grade [HG]) tumor, and CIS; or recurrent and large (>3 cm) TaG1G2/LG tumors (all features must be present).[3]
Although NMIBC is a nonmuscle invasive tumor, it is well known for its high risk of recurrence and progression. The European Organization for Research and Treatment of Cancer (EORTC) introduced a scoring system for calculating the probability of recurrence and progression of these patients.[4] To our knowledge, in terms of overall survival (OS), it has not been yet published any long-term comparison between well differentiated (G1) and moderately well differentiated (G2) (according to 1973 World Health Organization [WHO] system) Ta tumors. Many large cohorts studies analyzing the long-term survival of NMIBC patients in general after TURBT and intravesical chemotherapy,[5,6] survival range varies between studies but are mostly higher than in muscle-invasive bladder cancer (MIBC).[7]
The main aim of the study was to compare the long-term OS in patients with well-differentiated (G1) and moderately well differentiated (G2) grade Ta BC after TURBT. The secondary aim was to investigate clinic and pathologic prognostic factors for OS of these patients.
2. Methods
All patients with Ta NMIBC that underwent TURBT in the urology department from Tirgu Mures – Romania, within 3 years between January 2006 and December 2008, have been enrolled. Ethical approval was obtained from the Ethical Committee of the Mures County Hospital. Inclusion criteria were: primary Ta, G1, and G2 with nonassociated in situ carcinoma are classified as low and intermediate tumors according to European Association of Urology guidelines. Exclusion criteria were: any recurrent or progressed cancer, any T1 and/or G3/HG tumor, any concomitant CIS, and all cases of lost follow-up. EORTC risk scores were calculated for each patient; EORTC risk tables allow to estimate the probability of recurrence and progression based on a number of tumors, tumor size, prior recurrence rate, T category, concomitant CIS, and grade.[8]
Histological classification was done according to 1973 WHO classification (Fig. 1). Recurrence was defined as disease recurrence at more than 3 months postoperatively, and progression was considered the evolution of HG T1 tumors or MIBC. According to European Association of Urology guidelines, intravesical immunotherapy with Bacillus Calmette–Guerin (BCG) was administrated for intermediate risk Ta tumors, following the Lamm scheme: 1 weekly instillation for 6 weeks, followed by 3 years of maintenance instillation with 3 weekly instillation every 3 or 6 months.[9–11] Follow-up was made by clinical examination and cystoscopy at 3 months in the first 2 years and another cystoscopy at 6 months in the next 3 years and then annually after that. Firstly, end-point was set at the time until the first recurrence; secondly, end-point was timed until progression, and thirdly end-point was the death of any cause (from National Health Insurance Registry).
Figure 1.
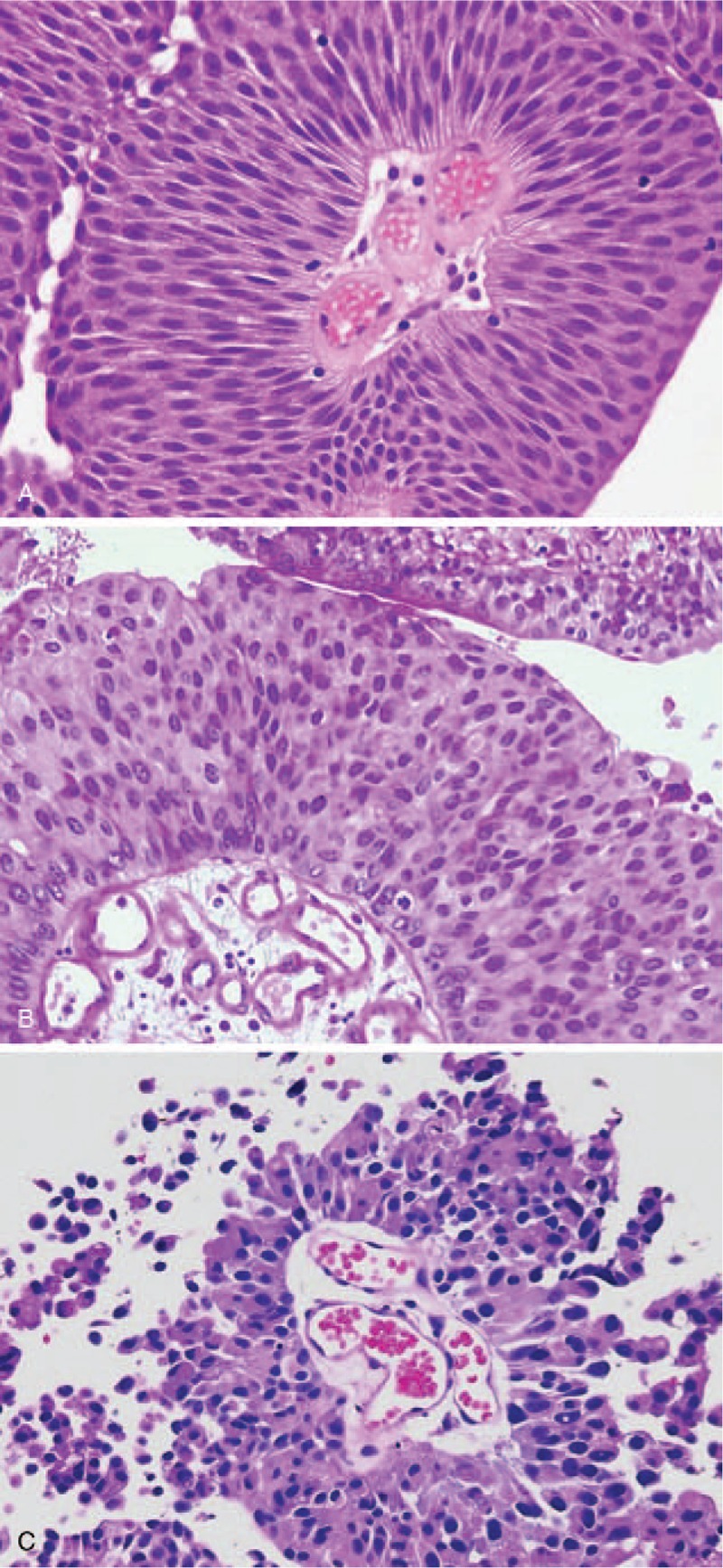
Histological grading according to World Health Organization (WHO) 1973 grading system: (A) G1: transverse section of a papilla; thickened urothelium with slender fibrovascular core; uniform elongated nuclei with preserved nuclear polarity and differentiation to the surface, no mitosis; (B) G2: moderately altered polarity, larger nuclei, variable in size, dense or irregular nuclear chromatin; visible nucleoli; some mitosis can be identified; (C) G3: deep architectural disorganization and severe nuclear atypia; coarse chromatin; massive cellular desquamation.
2.1. Statistical analysis
Data were labeled as nominal or quantitative variables. Nominal variables were characterized using frequencies. Quantitative variables were tested for normality of distribution by applying the Kolmogorov–Smirnov test and were described by mean ± standard deviation or median and quartiles. The frequencies of nominal variables were compared with the chi-square test and Student t test was used to assess the differences between means of continuous variables (expressed as mean ± SD), while differences between nonparametric variables (expressed as median, range) were compared using the Mann–Whitney U test. Survival analysis was performed using the Kaplan–Meyer method, and the log-rank test was used for univariate comparisons. Univariable and multivariable Cox regression models addressed the association of prognostic factors with OS after TURBT. Logistic regression analyses were performed to identify predictors for 10-year OS. All P values were 2-sided, and statistical significance was defined as a P < .05. Statistical analyses were performed using Stata 11.0 statistical software (Stata Corp., College Station, TX).
3. Results
A total of 243 patients, with G1 and G2 Ta tumors, have been included and 164 met inclusion criteria. Seventy-nine patients were excluded due to concomitant CIS,[1] G3/HG tumors (47), lost of follow-up, and no data about 10-year survival.[31] Patients were followed in average 109 months (IQR 70–121 months). The mean age at diagnosis was 63.3 years (range 21–89) and 135 (82.3%) patients were males. Multiple tumors were observed in 78 (47.6%) cases, in 86 (52.4%) cases the diameter of tumor was >3 cm, G2 tumors were observed in 105 (64%) patients. Intravesical immunotherapy with BCG was administrated in 32 (19.5%) patients according to guidelines recommendations at that time (intermediate risk Ta tumors) as was the only available adjuvant therapy following TURBT. Most patients had EORTC recurrence score of 1 to 4: 99 (60.4%). Progression EORTC score of 1 to 6 was observed in 107 (65.2%) patients. Recurrence was observed in 26 (15.8%) and progression in 5 (3%) patients. At 10 years after diagnosis, a total of 102 patients (62.2%) were survivors (Table 1).
Table 1.
Characteristics of 164 patients with low and intermediate grade Ta bladder tumors at diagnosis.
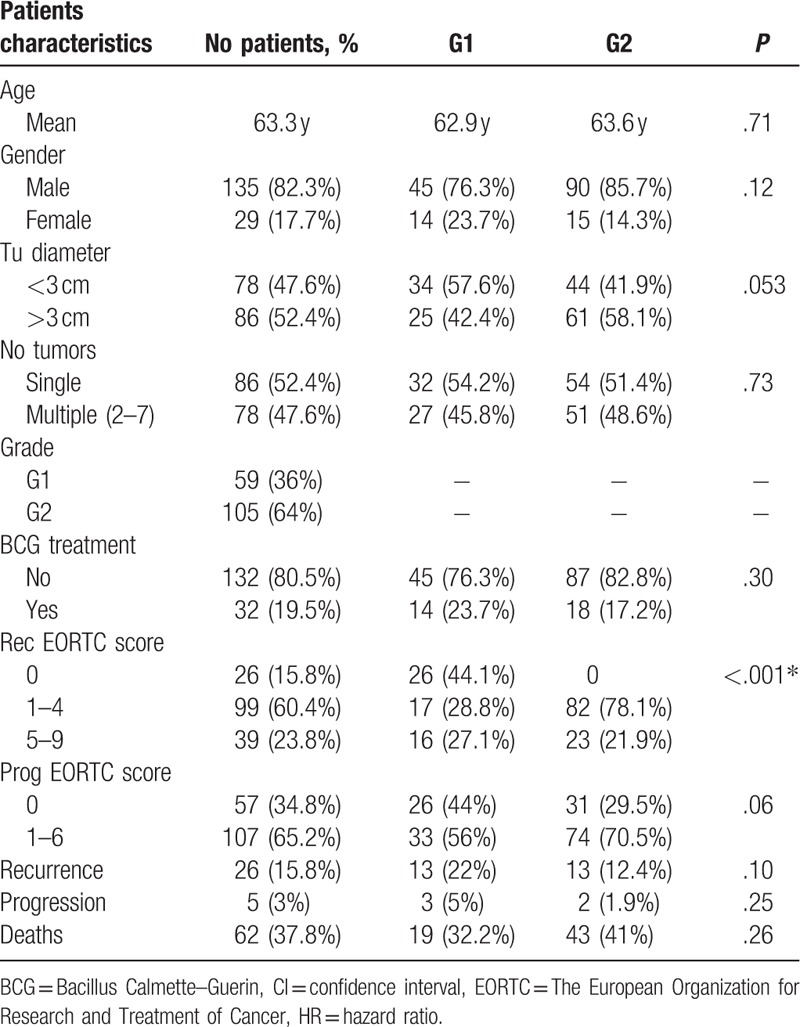
Table 2 presents the association between clinicopathological features and OS in the studied cohort. Looking at deaths, advanced age at diagnosis was associated with lower OS: 69 versus 59.8 years mean age, respectively, in survivals (P < .001). Mortality was higher in males (38.5%) than in females (34.3%) but not statistically significant (P = .68). Even if not significant, the survival rate was higher in G1 (67.8%) compared to G2 (59%) patients (P = .26). BCG treatment was associated with higher survival 84.4% versus 56.8%, P = .004.
Table 2.
Association of clinico-pathologic factors with overall survival.
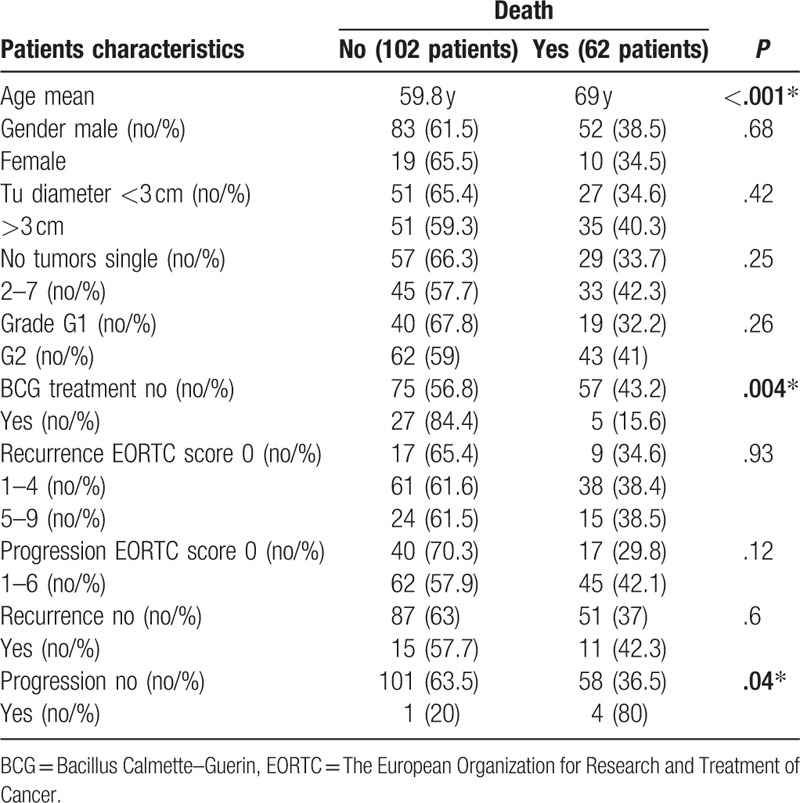
Univariable Cox analysis showed that predicting factors for OS were the advanced age with a hazard ratio (HR) of 1.07; no BCG treatment HR 0.30 and progression during follow-up HR 5.25. Multivariable Cox analysis showed that independent predictors for OS were: age (HR 1.07); EORTC recurrence scores 1 to 4 (HR 0.23); EORTC recurrence scores 5 to 9 (HR 0.17); and progression (HR 5.18) (Table 3).
Table 3.
Univariable and multivariable Cox regression analyses predicting overall survival of 164 patients with low and intermediate Ta bladder cancer.
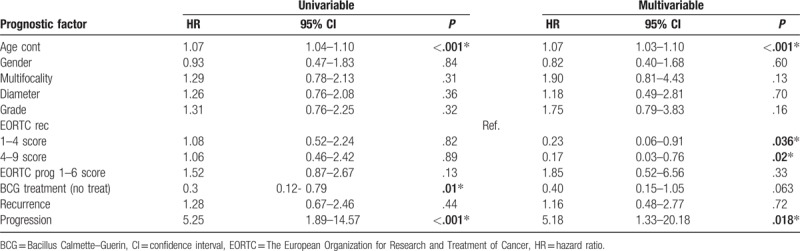
Univariable and multivariable logistic regression analysis underlined that the advanced age at diagnosis (HR 1.10) and no BCG treatments (HR 0.24 and 0.29) were independent predictors for death at 10 years after diagnosis (Table 4).
Table 4.
Univariable and multivariable logistic regression predicting overall survival of 164 patients with low and intermediate Ta bladder cancer.
Kaplan–Meier survival analysis showed that there is 8% difference in the survival between G1 and G2 grade, but with no statistical significance. Five years survival was 86.4% (CI 74.7–93) in G1 patients and 84.7% (CI 76.3–90.3) in G2 patients; 10 years survival was 67.8% (CI 54.3–78.1) in G1 patients and 59% (CI 49–67.3) in G2 patients (Fig. 2A, P = .31). BCG treatment had a benefit on the OS; 5 years OS was 82.6% (CI 74.9–88) in non-BCG treated patients, and 96.8% (CI 79.8–99) in BCG treated patients, respectively. Ten years OS was 56.8% (CI 47.9–64.7) in non-BCG treated patients, and 84.4% (CI 66.4–93.1) in BCG treated patients, P = .006 (Fig. 2B).
Figure 2.
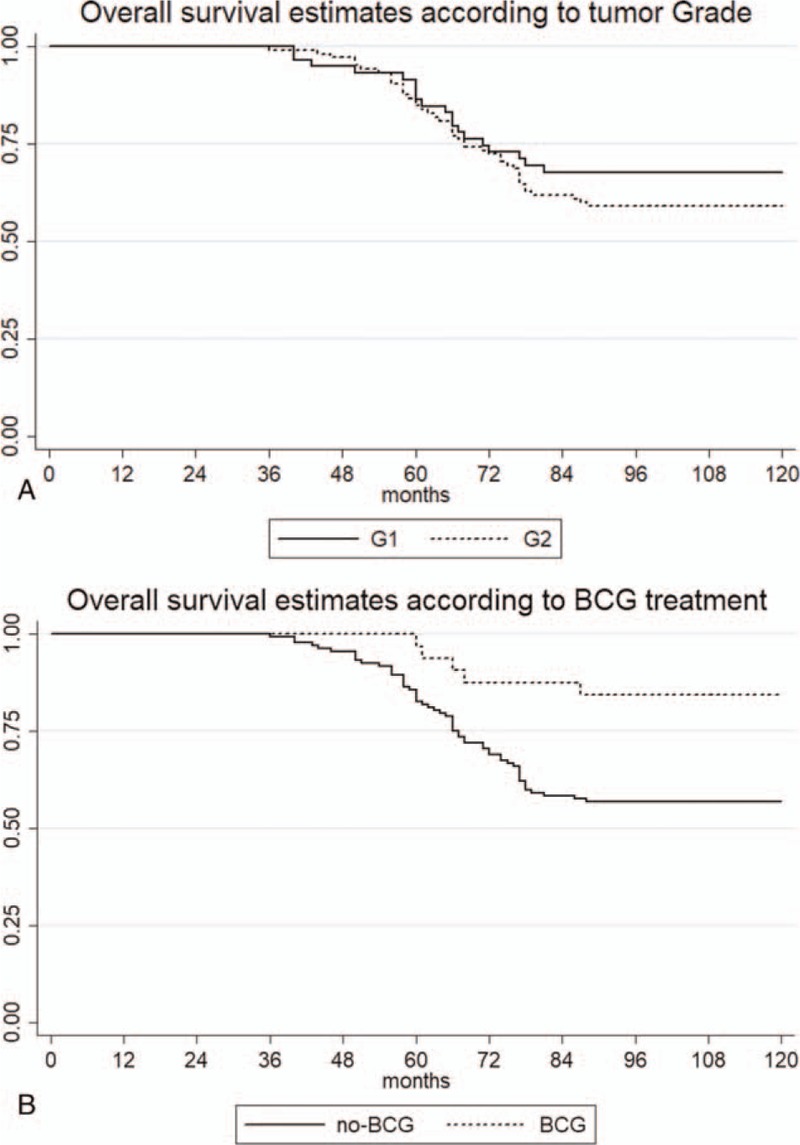
Kaplan–Meier survival estimates: (A) according to grade; (B) according to intravesical treatment.
4. Discussion
We evaluated the long-term survival of 164 Ta G1-G2 NMIBC patients in a single center with a median follow-up of 109 months. At 10 years after diagnosis, 62% of patients were survivors. To make it easy to understand, we demonstrated that advanced age at diagnosis was associated with worse OS and that there is no statistical significance regarding 10-year OS between patients with TaG1 compared with TaG2 patients. More interestingly was the statistically significant relationship that we found between BCG treatment and longer OS.
In 2009 Truls Gårdmark et al[12] published the Sweden data regarding NMIBC. Several similarities could be observed in our study. They showed that Ta cancer is more common in men, but suggesting that this data may change because, in Sweden, cigarette smoking is decreasing among men and is now equally prevalent among women. They analyzed the data from 6 health care institutions, stating that tumor grading significantly differed between institutions. This information suggests that tumor grading highly depends on the local histopathologist.
In 2016 Hernández et al[13] published an interesting paper on active surveillance in case of well selected NMIBC patients (especially TaG1), but still, experts and guidelines consider that active surveillance should be definitively abandoned for BC and banished from daily practice without further investigation.[14]
Several studies have highlighted the importance of adjuvant therapy in improving long-term oncologic outcomes of NMIBC,[15–20] resulting in higher recurrence-free survival (RFS) with the usage of BCG compared to Epirubicin[16] but no statistical difference between BCG and Mitomycin-C was reported.[21]
Gupta and Parr examined a large cohort of NMIBC and concluded that large size of tumors is not a negative prognostic factor for patients with NMIBC and that pT1G2 should be considered high-risk tumors,[22] with a median follow-up of 60 months, however, the study lacks data regarding OS.
Multiple tumors are considered as a risk factor for tumor recurrence and worsening progression according to Kobayashi et al[23]; lung cancer, hematuria, and >60 pack-years smoking history according to Starke et al.[24] In line with Herr et al[24] in our study, advanced age, lack of adjuvant therapy, and progression proved to be statistically independent prognostic factors. We also proved that advanced age is an independent prognostic factor. At 5 years, 27% of patients older than 70 years were cancer-free compared to 37% who were younger than 70 years, including high-risk NMIBC patients.[25]
Thinking about the future predictive models, to better understand urothelial neoplasm behavior and to better understand recurrence and progression multiple biomarkers have been studied as predictors for NMIBC patients outcomes,[26] the closest to be embraced in clinical practice is neutrophil-to-lymphocyte ratio, which is accessible and a less costly biomarker.[27] Elevated neutrophil-to-lymphocyte ratio was demonstrated to be an independent predictor for poor OS, cancer-specific survival, recurrence, and progression in BC patients.[28]
Data regarding long-term (at least 10 years) OS after initial diagnosis of Ta BCa lack in the literature. In a UK study, OS for Ta patients was 54% at 10 years,[29] on the other hand, OS in high-risk NMIBC patients that received BCG or Mytomicin C was 44% at 10 years.[21] Librenjak et al[18] showed, in a cohort of NMIBC, that there is no significant difference in OS between patients with BCG treatment (56% 10 years OS) and patients without BCG treatment (44% OS at 10 years). In a large multicenter cohort of TaG1 patients, the estimated OS at 5 years was 86%,[30] (similar to our study, where OS of TaG1 at 5 years was 86.4%) but more than half of the patients received immediate postoperative instillation of chemotherapy.
Our study has several limitations. First, the retrospective design requires further confirmation in prospective cohorts. Second, patients did not receive immediate postoperative instillation of chemotherapy, although those at intermediate risk received adjuvant treatment. Third, we did not perform a central pathology review on the specimens and did not reassign the specimens to the latest WHO grading.[31] Also, we did not have any information on the smoking status of patients, which is a well-known prognostic factor associated with outcomes in urothelial carcinomas.[32] Furthermore cancer-specific survival should have been a primary endpoint, but unfortunately, we did not have access to death certificates of the patients. Despite these limitations, this study fulfilled its aim to compare the OS of patients with newly diagnosed G1 and G2 Ta BC at 10 years after diagnosis.
5. Conclusion
Patients with well differentiated (G1) and moderately well differentiated (G2) Ta tumors have similar long-term OS after diagnosis. BCG treatment, even if administered to intermediate risk cancers, is related to a longer OS.
Acknowledgments
The authors thank EUSP lab/clinical fellowship awarded by EAU (European Association of Urology) and an Ernst Mach Grant awarded by OeAD, Austria to VMD.
Author contributions
Conceptualization: Orsolya Martha, Mihai Dorin Vartolomei, Ettore De Berardinis, Angela Borda, Matteo Ferro, Akos Pytel, Daniel Porav-Hodade.
Data curation: Daniel Balan, Calin Bogdan Chibelean, Mihai Dorin Vartolomei, Francesco Del Giudice.
Formal analysis: Daniel Balan, Calin Bogdan Chibelean, Septimiu Voidezan, Mihai Dorin Vartolomei, Angela Borda, Gian Maria Busetto.
Investigation: Daniel Balan, Sabin Tataru, Anca Sin, Mihai Dorin Vartolomei, Antonio Cioffi, Matteo Ferro, Daniel Porav-Hodade.
Methodology: Orsolya Martha, Sabin Tataru, Septimiu Voidezan, Anca Sin, Mihai Dorin Vartolomei, Antonio Cioffi, Gian Maria Busetto, Matteo Ferro, Akos Pytel, Daniel Porav-Hodade.
Project administration: Mihai Dorin Vartolomei, Daniel Porav-Hodade.
Resources: Daniel Porav-Hodade.
Software: Septimiu Voidezan.
Supervision: Orsolya Martha, Victor Deliu Matei, Mihai Dorin Vartolomei, Giuseppe Lucarelli, Francesco Del Giudice, Angela Borda, Matteo Ferro, Akos Pytel, Daniel Porav-Hodade.
Validation: Victor Deliu Matei, Antonio Cioffi, Giuseppe Lucarelli, Daniel Porav-Hodade.
Visualization: Sabin Tataru, Francesco Del Giudice, Ettore De Berardinis, Gian Maria Busetto, Matteo Ferro.
Writing – original draft: Daniel Balan, Orsolya Martha, Calin Bogdan Chibelean, Sabin Tataru, Septimiu Voidezan, Anca Sin, Victor Deliu Matei, Antonio Cioffi, Giuseppe Lucarelli, Francesco Del Giudice, Ettore De Berardinis, Angela Borda, Gian Maria Busetto, Matteo Ferro, Akos Pytel, Daniel Porav-Hodade.
Writing – review & editing: Orsolya Martha, Calin Bogdan Chibelean, Sabin Tataru, Anca Sin, Victor Deliu Matei, Mihai Dorin Vartolomei, Antonio Cioffi, Giuseppe Lucarelli, Francesco Del Giudice, Ettore De Berardinis, Angela Borda, Gian Maria Busetto, Matteo Ferro, Akos Pytel, Daniel Porav-Hodade.
Footnotes
Abbreviations: BC = bladder cancer, BCG = Bacillus Calmette–Guerin, CIS = carcinoma in situ, EORTC = The European Organization for Research and Treatment of Cancer, HG = high grade, HR = hazard ratio, LG = low grade, MIBC = muscle invasive bladder cancer, NMIBC = nonmuscle invasive bladder cancer, OS = overall survival, TURBT = transurethral resection of bladder tumor, WHO = World Health Organization.
VDM, GL, GMB, AP, and DP-H equally contributed as senior coauthors.
Funding/support: This study was supported by EUSP lab/clinical fellowship awarded by EAU (European Association of Urology) and an Ernst Mach Grant awarded by OeAD, Austria to VMD.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Babjuk M, Bohle A, Burger M, et al. Guidelines on non-muscle-invasive Bladder Cancer (Ta,T1 and CIS). In: EAU Guidelines. Eur Assoc Urol 2015. [Google Scholar]
- [3].Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 2017;71:447–61. [DOI] [PubMed] [Google Scholar]
- [4].Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466–5. discussion 475–477. [DOI] [PubMed] [Google Scholar]
- [5].Holmäng S, Ströck V. Should follow-up cystoscopy in bacillus Calmette-Guérin-treated patients continue after five tumour-free years? Eur Urol 2012;61:503–7. [DOI] [PubMed] [Google Scholar]
- [6].Matsumoto K, Kikuchi E, Horiguchi Y, et al. Late recurrence and progression in non-muscle-invasive bladder cancers after 5-year tumor-free periods. Urology 2010;75:1385–90. [DOI] [PubMed] [Google Scholar]
- [7].Vartolomei MD, Kiss B, Vidal A, et al. Long-term results of a prospective randomised trial assessing the impact of readaptation of the dorsolateral peritoneal layer following extended pelvic lymph node dissection and cystectomy. BJU Int 2015;n/a. [DOI] [PubMed] [Google Scholar]
- [8].Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466–77. [DOI] [PubMed] [Google Scholar]
- [9].Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9. [PubMed] [Google Scholar]
- [10].Oosterlinck W, Lobel B, Jakse G, et al. Guidelines on bladder cancer. Eur Urol 2002;41:105–12. [DOI] [PubMed] [Google Scholar]
- [11].De Berardinis E, Busetto GM, Antonini G, et al. T1G3 high-risk NMIBC (non-muscle invasive bladder cancer): conservative treatment versus immediate cystectomy. Int Urol Nephrol 2011;43:1047–57. [DOI] [PubMed] [Google Scholar]
- [12].Gårdmark T, Bladström A, Hellsten S, et al. Members Of The Swedish National Bladder Cancer Registry. Analysis of clinical characteristics, management and survival of patients with Ta T1 bladder tumours in Sweden between 1997 and 2001. Scand J Urol Nephrol 2006;40:276–82. [DOI] [PubMed] [Google Scholar]
- [13].Hernández V, Llorente C, de la Peña E, et al. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol 2016;34:165.e19-23. [DOI] [PubMed] [Google Scholar]
- [14].Cotte J, Rouprêt M. Re: long-term oncological outcomes of an active surveillance program in recurrent low grade ta bladder cancer. Eur Urol 2017;72:152. [DOI] [PubMed] [Google Scholar]
- [15].Yoo KH, Lim TJ, Chang S-G. Monthly intravesical bacillus Calmette-Guérin maintenance therapy for non-muscle-invasive bladder cancer: 10-year experience in a single institute. Exp Ther Med 2012;3:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheng CW, Chan SFP, Chan LW, et al. Twelve-year follow up of a randomized prospective trial comparing bacillus Calmette-Guerin and epirubicin as adjuvant therapy in superficial bladder cancer. Int J Urol 2005;12:449–55. [DOI] [PubMed] [Google Scholar]
- [17].Davis JW, Sheth SI, Doviak MJ, et al. Superficial bladder carcinoma treated with bacillus Calmette-Guerin: progression-free and disease specific survival with minimum 10-year follow-up. J Urol 2002;167:494–500. [DOI] [PubMed] [Google Scholar]
- [18].Librenjak D, Situm M, Vrdoljak E, et al. Results of long-term follow-up of patients with superficial bladder carcinoma treated with intravesically applied bacillus Calmette-Guerin vaccine according to the schedule of 6 weekly plus 6 monthly instillations. Urol Oncol-Semin Orig Invest 2012;30:259–65. [DOI] [PubMed] [Google Scholar]
- [19].Weizer AZ, Tallman C, Montgomery JS. Long-term outcomes of intravesical therapy for non-muscle invasive bladder cancer. World J Urol 2011;29:59–71. [DOI] [PubMed] [Google Scholar]
- [20].Koie T, Ohyama C, Hosogoe S, et al. Oncological outcomes of a single but extensive transurethral resection followed by appropriate intra-vesical instillation therapy for newly diagnosed non-muscle-invasive bladder cancer. Int Urol Nephrol 2015;47:1509–14. [DOI] [PubMed] [Google Scholar]
- [21].Gardmark T, Jahnson S, Wahlquist R, et al. Analysis of progression and survival after 10 years of a randomized prospective study comparing mitomycin-C and bacillus Calmette-Guerin in patients with high-risk bladder cancer. BJU Int 2007;99:817–20. [DOI] [PubMed] [Google Scholar]
- [22].Gupta SK, Parr NJ. Outcome of very large superficial bladder tumours: a 10-year experience. Scand J Urol Nephrol 2008;42:243–8. [DOI] [PubMed] [Google Scholar]
- [23].Kobayashi H, Kikuchi E, Mikami S, et al. Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: tumor recurrence and worsening progression. BMC Urol 2014;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Starke N, Singla N, Haddad A, et al. Long-term outcomes in a high-risk bladder cancer screening cohort. BJU Int 2016;117:611–7. [DOI] [PubMed] [Google Scholar]
- [25].Herr HW. Age and outcome of superficial bladder cancer treated with bacille calmette-guerin therapy. Urology 2007;70:65–8. [DOI] [PubMed] [Google Scholar]
- [26].Alvarez A, Lokeshwar VB. Bladder cancer biomarkers: current developments and future implementation. Curr Opin Urol 2007;17:341–6. [DOI] [PubMed] [Google Scholar]
- [27].Martha O, Porav-Hodade D, Bălan D, et al. Easily available blood test neutrophil-to-lymphocyte ratio predicts progression in high-risk non-muscle invasive bladder cancer. Rev Romana Med Lab 2017;25:181–9. [Google Scholar]
- [28].Tang X, Du P, Yang Y. The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: a systematic review and meta-analysis. Int J Clin Oncol 2017;22:817–25. [DOI] [PubMed] [Google Scholar]
- [29].Low VJ, Wang D, Abel PD. Survival of patients with bladder cancer from a UK hospital: a 10-year follow-up study. BJU Int 2010;105:1667–71. [DOI] [PubMed] [Google Scholar]
- [30].Rieken M, Xylinas E, Kluth L, et al. Long-term cancer-specific outcomes of TaG1 urothelial carcinoma of the bladder. Eur Urol 2014;65:201–9. [DOI] [PubMed] [Google Scholar]
- [31].Soukup V, Čapoun O, Cohen D, et al. Prognostic Performance and reproducibility of the 1973 and 2004/2016 World Health Organization Grading Classification Systems in Non-muscle-invasive Bladder Cancer: A European Association of Urology Non-muscle Invasive Bladder Cancer Guidelines Panel Systematic Review. Eur Urol 2017. [DOI] [PubMed] [Google Scholar]
- [32].Crivelli JJ, Xylinas E, Kluth LA, et al. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. Eur Urol 2014;65:742–54. [DOI] [PubMed] [Google Scholar]


