Abstract
Background:
The non-steroidal mineralocorticoid receptor antagonist finerenone (BAY 94–8862) has been used to treat chronic heart failure (CHF) with reduced ejection fraction (HFrEF). However, conflicting results were reported for its efficacy and safety. The study aimed to compare the efficacy and safety of finerenone versus spironolactone or eplerenone in patients with chronic heart failure.
Methods:
Electronic databases including MEDLINE, EMBASE, and CENTRAL were searched from inception to December 2017 for randomized controlled trials assessing finerenone treatment in patients with chronic heart failure. Data concerning the study's design, patients’ characteristics, and outcomes were extracted. Risk ratio (RR) and mean differences (MD) were calculated using either fixed or random effects models.
Results:
Three trials with 1520 CHF patients were included in the systematic review. In terms of anti-ventricular remodeling, we calculated the effective number of cases with a 30% reduction in NT-proBNP. Finerenone was equivalent to the existing steroidal mineralocorticoid antagonist (P < .05). However, the efficacy of finerenone appeared to be dose-dependent. At a dose of 10 mg/d finerenone was found to be marginally better than that of steroidal mineralocorticoid receptor antagonists (MRAs) (RR = 1.18, 95% confidence interval [CI] 0.88, 1.57, P > .05). The incidence of treatment-related adverse events (TEAEs) of finerenone at 10 mg/d was significantly lower than 25 to 50 mg/d of steroidal MRAs (RR = 0.81, 95% CI = 0.66–0.99, P = .04). Moreover, the serum potassium levels in the finerenone 10 mg/d group were lower than those in the 25 to 50 mg/d steroidal MRAs group (MD = –0.14, 95% CI –0.30–0.02, P = .09), whereas the estimated glomerular filtration rate (eGFR) was higher in finerenone versus steroidal MRAs treated patients (MD = 2.07, 95% CI –0.04–4.17, P = .05).
Conclusions:
Finerenone reduced NT-proBNP level, urinary albumin/creatinine ratio (UACR), and other biochemical indicators, in a dose-dependent manner. In terms of anti-ventricular remodeling in patient with chronic heart failure, finerenone at 10 mg/d is as effective as 20 to 50 mg/d of steroidal MRAs. However, finerenone is much safer to patients with chronic kidney disease.
Keywords: BAY 94–8862, efficacy, finerenone, heart failure, safety
1. Introduction
Chronic heart failure (CHF) has become a global epidemic in the 21st century, a great impact on the quality of life of patients, to the health care system has brought a heavy burden.[1] The patients with chronic heart failure is characterized by a progressive decline in health-related quality of life and functional, as well as a high risk of hospitalization and mortality. One of the most promising strategies to reduce cardiovascular risk in chronic heart failure patients with worsening renal function are mineralocorticoid receptor antagonists (MRAs)-based treatment regimens. The classic steroidal MRAs (spironolactone and eplerenone) have a Class 1A recommendation for chronic heart failure (CHF) patients with heart failure of reduced ejection fraction (HFrEF) and LVEF ≤35%, regardless of whether they have been therapy with an ACE inhibitor (or angiotensin receptor blocker) and a beta-blocker.[2–4] MRAs have been used to reduce mortality and hospitalizations for patients with chronic heart failure with HFrEF.[5] However, the classic steroidal MRAs uses are limited due to potential risks of hyperkalemia, renal function deterioration, male breast development, and menstrual disorders.[6,7]
In order to overcome these inherent limitations of the steroidal MRAs, a novel non-steroidal selective MRA, finerenone (previous nomenclature BAY 94–8862), as a candidate for clinical treatment. The preclinical studies showed that the novel non-steroidal mineralocorticoid receptor antagonist finerenone had a higher selectivity towards mineralocorticoid receptor (MR) similar to spironolactone, and low affinity for androgen, glucocorticoid, and progesterone receptors, similar to eplerenone.[8] It has a better selectivity than spironolactone and a better affinity than eplerenone. Finerenone has a more favorable balance between and renal side effects, especially in populations prone to hyperkalemia such as patients with chronic kidney disease or diabetes. Unlike spironolactone and eplerenone, which have a higher tendency to focus on the kidney rather than the heart, finerenone has the same tendency in heart and kidney tissues.[9]
Finerenone is currently the most advanced third generation non-steroidal MRAs drug. In recent years, more and more studies have focused on the clinical effectiveness and safety of finerenone.[10–12] However, the application dose of finerenone has not been unified. The current clinical research had to be divided into many dose groups ranging from 1.25 to 20 mg/d. Meanwhile, its clinical efficacy, renal protection, and safety is still not clear. Therefore, we decide to perform a systematic review and meta-analysis to compare the efficacy and safety of finerenone versus steroidal MRAs in patients with chronic heart failure.
2. Methods
2.1. Search strategy
Electronic databases including MEDLINE, EMBASE, and CENTRAL were searched from inception to December 2017, using items related to “heart failure,” “finerenone,” “BAY 94–8862,” “spironolactone,” and “eplerenone.” The search term combinations used in the literature search is (((((heart failure) AND finerenone) OR BAY 94–8862) AND spironolactone) OR eplerenone) AND randomized controlled trial. The study was approved by the institutional ethics committee of Shandong University.
2.2. Selection criteria
Drs. HP and ZZ reviewed all the literature of the citations and retrieved by titles or abstracts and subsequently by full texts. We selected the studies which met the following inclusion criteria: (1) the studies were randomized controlled trial (RCT) and published in the English language; (2) the study population was the patients with chronic HFrEF; (3) the studies compared the MRAs (spironolactone or eplerenone) versus standard CHF therapy (ACEIs and ARB or β-adrenoceptor blocker). We use the Cochrane Risk Bias Evaluation Table of Review Manager 5.3 to evaluate the quality of the included studies.
2.3. Data extraction
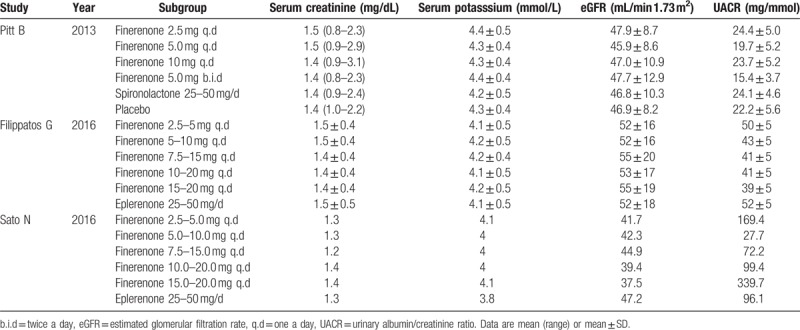
Following the guidelines of Cochrane's reviewer's handbook, data were independently extracted by 2 reviewers from each study according to the selection criteria. The following information was extracted from the studies including the first author, year of publication, male, age, body mass index (BMI), the types and doses of intervention agents, and follow-up duration. The effective case number with a >30% reduction in NT-proBNP was calculated. Treatment-emergent adverse events (TEAEs) includes: cardiac disorders (angina pectoris, sinus tachycardia), gastrointestinal disorders (constipation, flatulence, nausea), investigations needed (blood CPK level increased, blood glucose level increased), metabolism and nutrition disorders (diabetes mellitus, hyperkalemia), nervous system disorders (dizziness, headache), renal disorders, vascular disorders, hypotension, and so on. Indicators of renal tolerance include: serum potassium, estimated glomerular filtration rate (eGFR), urinary albumin/creatinine ratio (UACR).
2.4. Data synthesis and analysis
Dichotomous data were reported using a risk ratio (RR) with 95% confidence intervals (CIs), whereas continuous variables (changing from baseline to predefined follow-up time) were reported using mean differences (MD) with 95% CIs. Pooled analyses were calculated using fixed effect models.[13] Some studies heterogeneity I2 were slightly >50%. However, due to limited number of included trials, heterogeneity was not considered. Sensitivity analyses (excluding one study at a time) were performed to determine the stability of the overall treatment effects. The Cochrane Collaboration meta-analysis software Review Manager 5.3 was used for the meta-analysis. A two-tailed P value of <.05 was considered to be statistically significant.
3. Results
3.1. Description of included studies
Figure 1A shows the flow chart of study selection. After removal of duplicates, 126 citations were screened for potential eligibility and 4 articles[14–17] were reviewed in full text. A total of 3 RCTs[15–17] involving 1520 participants were included in this meta-analysis. Two studies[16,17] included patients who used eplerenone and were followed up for 3 months. One study[15] used spironolactone and patients were followed up for 1 month. The dose of finerenone was 2.5 to 20 mg once daily and spironolactone or eplerenone was 25 to 50 mg per day. The characteristics of component trials and study patients are shown in Tables 1 and 2, respectively. Figure 1B shows the risk of bias table of Review Manager 5.3 used to evaluate the overall quality of the articles.
Figure 1.
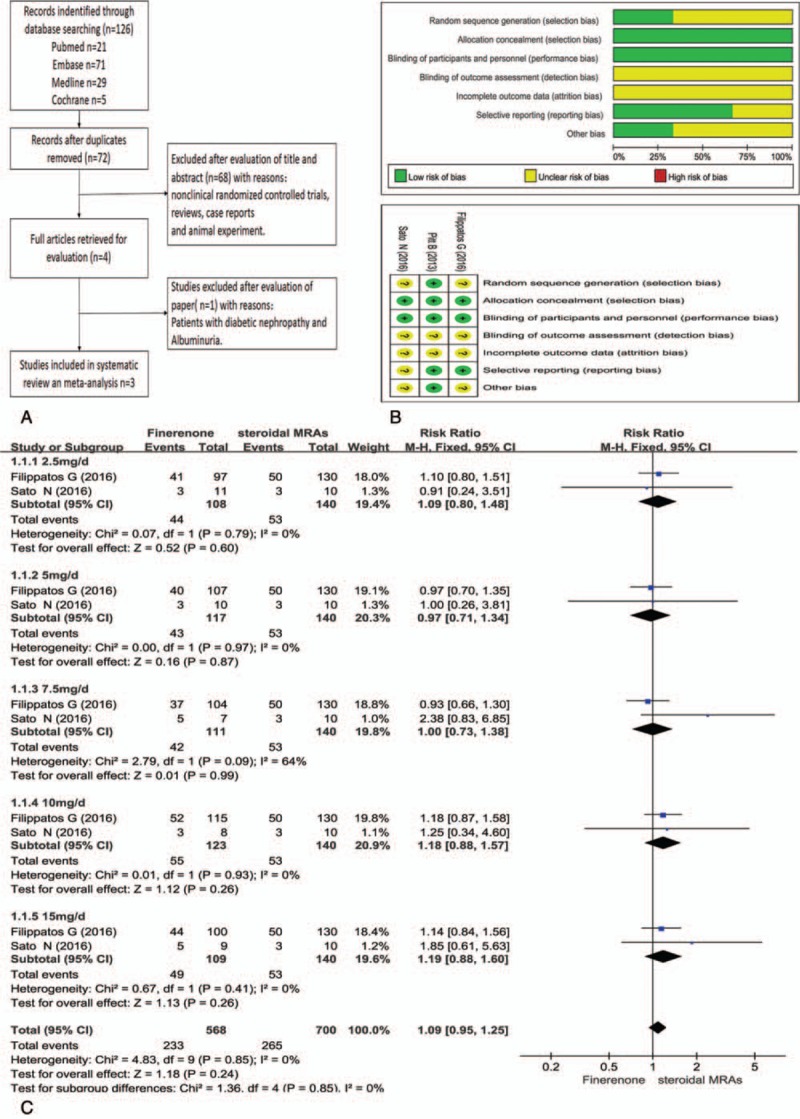
A: Study flow diagram; B: risk of bias graph and risk of bias summary; C: NT-proBNP comparison between ateroidal MRAs and finerenone. CIs = confidence intervals; MRAs = mineralocorticoid receptor antagonists; NT pro-BNP = N-terminal pro-B-type natriuretic peptide.
Table 1.
The basic characteristics of the study.
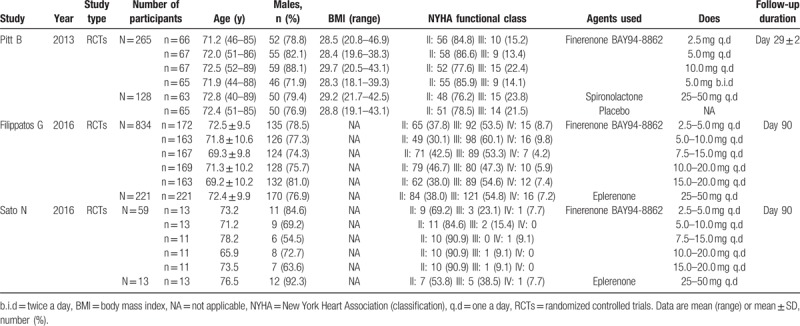
Table 2.
The baseline of patient characteristics before treatment.
3.2. Primary outcome: NT-proBNP
NT-proBNP is an important indicator of treatment efficacy, and is closely related to the improvement and prognosis of heart failure. Like most studies, anti-heart failure treatment was considered to be effective when blood NT-proBNP decreased by >30% compared with that prior to the treatment.[18,19] The effective case number with a >30% reduction in NT-proBNP was calculated. Clinical effectiveness of finerenone at different doses were compared with spironolactone or eplerenone at 25 to 50 mg/d. The analysis of effective treatment in patient with CHF showed that adding finerenone treatment group show there is no statistically significant difference in NT-proBNP changes at all doses of finerenone as compared with eplerenone (25–50 mg/d) (P > .05), as shown in Fig. 1C. Finerenone was not superior or inferior to existing steroidal mineralocorticoid antagonist. However, the pooled results of 2 RCTs by Filippatos et al[16] and Sato et al[17] showed that with increasing dose of finerenone, there was an improvement tendency based on NT-proBNP changes in the patients with heart failure. At the dose of 10 and 15 mg/d, the efficacy of finerenone looks more effective or comparable to that of eplerenone (25–50 mg/d) (RR = 1.18, 95% CI 0.88, 1.57), (RR = 1.19, 95% CI 0.88, 1.60), although P-value is >.05. The median data from study of Pitt et al[15] cannot be imported into the above meta-analysis, we can also see this dose tendency. Median changes of serum NT-proBNP from baseline in finerenone group (10 mg/d) is –193.65, and inter-quartile range is –630 to 102. While median changes in spironolactone group (25–50 mg/d) is –170.3, and inter-quartile range is –585 to 70.
3.3. The second outcome: adverse events
A total of 3 articles[15–17] were included the analysis of adverse in patient with CHF showed that the TEAEs of 10 mg/d finerenone is significantly lower than spironolactone or eplerenone (RR = 0.81, 95% CI = 0.66–0.99, P = .04) in Fig. 2A. More importantly, the serious adverse events including hyperkalemia and the discontinuation of treatment due to the adverse events were significantly lower in the finerenone group than those in steroidal MRAs group (RR = 0.60, 95% CI 0.27–1.30, P = .19) and (RR = 0.58, CI 0.25–1.32, P = .19) in Fig. 2B and C.
Figure 2.
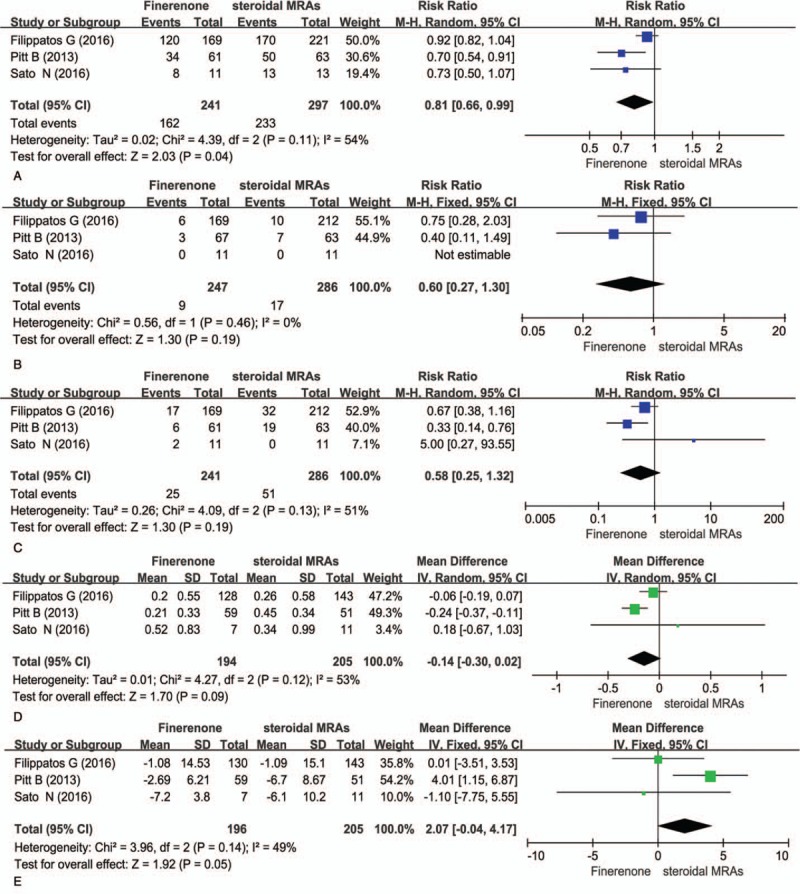
Comparsion of adverse events and renal tolerance between steroidal MRAs and finerenone. A: TEAEs (the treatment-emergent adverse evernts), B: hyperkalemia, C: the discontinuation of treatment due to the adverse events, D: serum potassium, E: eGFR (estimated glomerular filtration rate).
Similarly, a study[14] of patients with diabetic nephropathy found that there was no difference in the overall incidence of adverse events and serious adverse events between the finerenone groups and the placebo group. There was no relevant increase in adverse events with finerenone dosages increased. Drug-related serious adverse events occurred in 1.46% of patients receiving finerenone >10 mg/d.
3.4. The other results: tolerability of the kidney
Patients with heart failure usually have comorbidities such as diabetes and chronic kidney diseases. The steroidal mineralocorticoid antagonist including spironolactone and eplerenone could cause kidney damage. Therefore, it is important to determine and compare the safety of finerenone versus spironolactone or eplerenone on renal function.[15] We analysis the changes of serum potassium and eGFR before and after treatments in 3 studies.[15–17] The results showed that as compared with 25 to 50 mg/d of steroidal MRAs, 10 mg/d of finerenone treatment had less side effects on renal functions. Finerenone-induced serum potassium change was less than that induced by steroidal MRAs treatments (MD = –0.14, 95% CI –0.30–0.02, P = .09) in Fig. 2D. Similarly, finerenone caused less changes in the eGFR in comparison to steroidal MRAs (MD = 2.07, 95% CI –0.04–4.17, P = .05) in Fig. 2E.
Similar to the conclusion of above research, study of Pitt et al[15] shows that the geometric mean ratio of serum UACR in finerenone group (10 mg/d) is 0.72 ± 2.34, while geometric mean ratio of serum UACR in spironolactone group (25–50 mg/d) is 0.61 ± 2.63. Compared with steroidal MRAs, finerenone has the same effect of reducing proteinuria in patients with CHF and mild or moderate chronic kidney disease. While, the research of Bakris et al,[14] was more clear, found that finerenone demonstrated a dose-dependent reduction in UACR. The primary outcome, the placebo-corrected mean ratio of the UACR at day 90 relative to baseline, was reduced in the finerenone for 10 mg/d, 0.76 (90% CI, 0.65–0.88; P = .001); for 15 mg/d, 0.67 (90% CI, 0.58–0.77; P < .001); for 20 mg/d, 0.62 (90% CI, 0.54–0.72; P < .001).
4. Discussion
The steroidal mineralocorticoid antagonist (spironolactone, eplerenone) can significantly improve the prognosis and the quality of life of patients with heart failure, reduce hospitalization, and mortality.[20–22] However, due to the low selectivity, the classic mineralocorticoid antagonist may lead to elevated serum potassium,[23,24] development of male breast,[4] female menstrual disorder,[25] and other adverse effects,[26] which limit their clinical use. A new generation of non-steroidal mineralocorticoid antagonist, BAY 94–8862 (finerenone), developed by Bayer Company of Germany, has been on its Phase III clinical trial. It has a better selectivity than spironolactone and a better affinity than eplerenone. Studies showed that finerenone had excellent selectivity to steroida mineralocorticoid receptor, and its half maximal inhibitory concentration (IC50) was only 17.8 nmol/L (spironolactone IC50 = 24.2 nmol/L, eplerenone IC50 = 990 nmol/L). Its selectivity to mineralocorticoid receptor is significantly higher than (>500 folds) glucocorticoid receptor, androgen receptor, and progesterone receptor.[27]
In clinical practice, we found that with the worsening of heart failure in patients, the patient's kidney function will be subject to varying degrees of damage. For CHF patients receiving MRAs treatment who have renal dysfunction, the use potassium sparing diuretics increases the risk of hyperkalemia. Thus, it is important to monitor the serum potassium levels. As compared with spironolactone or eplerenone, finerenone has less unfavorable effects on blood potassium and eGFR. This might be primarily due to different pharmacological properties in metabolism and tissue distribution of these drugs. Finerenone is evenly distributed in the heart and kidneys, whereas eplerenone is at least 3 times higher in the kidneys than in the heart. This might be the reason why finerenone has a cardiac effect at a relatively low dose and the incidence of hyperkalemia induced by finerenone is lower than that of spironolactone. Further clinical trials found that in heart failure patients with coexisting mild to moderate chronic kidney disease, finerenone reduced BNP, NT-proBNP level, UACR, and other biochemical indicators, in a dose-dependent manner. The primary endpoint of the ARTS-DN[14] study was the improvement in UACR. Compared with placebo, 4 high-dose of finerenone treatment reduced UACR in a dose-dependent manner, while the incidence of adverse events was relatively low.
Pitt et al[5] confirmed this finding that 10 mg/d of finerenone decreased BNP and NT-proBNP at a greater magnitude than 25 to 50 mg/d of spironolactone or eplerenone. Interestingly, we also found a relative paradox place in Bertram Pitt's research. The 25 to 50 mg/d spironolactone group had higher serum aldosterone levels and lower systolic blood pressure than the 10 mg/d finerenone group. Leaving aside the high affinity and selectivity of finerenone, the only explanation is that the effect of antagonist mineralocorticoid receptor in finerenone 10 mg group is inferior to that of 25 to 50 mg MRAs groups. Therefore, future research on finerenone should increase the dose of the drug, at least ≥10 mg. Increasing the drug dose of finerenone from 10 mg/d can also achieve other cardiovascular benefits. As the dose increases, especially 15 to 20 mg, the risk of cardiovascular death and the composite endpoint (death from any cause, cardiovascular hospitalization, or emergency presentation for worsening chronic heart failure) was reduced.
5. Conclusions
Finerenone reduced NT-proBNP level, UACR, and other biochemical indicators, in a dose-dependent manner. In terms of anti-ventricular remodeling in patient with chronic heart failure, finerenone at 10 mg/d is as effective as 20 to 50 mg/d of steroidal MRAs. However, finerenone is much safer to patients with chronic kidney disease.
5.1. Limitation
This meta-analysis was limited by the small number of studies and the scarcity of data for some of the results. This article only provides preliminary results on the efficacy and safety of finerenone. Furthermore, the relatively small sample sizes in each component trials make the results of meta-analysis prone to small-study effect. Some meta-epidemiological studies found that small study effect might overestimate the effectiveness of an intervention.[28] Thus, the results of the study had been interpreted with caution. Fortunately, multi-center phase III clinical trial of FINESSE-HF planned to recruit >3600 cases of chronic heart failure patients with HFrEF, with coexisting type 2 diabetes and/or chronic kidney disease is coming. We are expecting more evidence and conclusive results regarding the efficacy and safety of finerenone in comparison to spironolactone or eplerenone.
Author contributions
Conceptualization: Zhuo Zhao.
Data curation: Hui Pei.
Formal analysis: Wei Wang, Zhuo Zhao.
Funding acquisition: Lei Wang.
Investigation: Wei Wang.
Methodology: Guo-Hai Su, Zhuo Zhao.
Project administration: Di Zhao, Guo-Hai Su, Zhuo Zhao.
Resources: Di Zhao, Zhuo Zhao.
Software: Di Zhao.
Supervision: Lei Wang, Guo-Hai Su.
Visualization: Lei Wang, Zhuo Zhao.
Writing – original draft: Hui Pei.
Writing – review & editing: Zhuo Zhao.
Footnotes
Abbreviations: BNP = brain natriuretic peptide, CHF = chronic heart failure, CIs = confidence intervals, eGFR = estimated glomerular filtration rate, HFrEF = heart failure of reduced ejection fraction, IC50 = half maximal inhibitory concentration, MD = mean differences, MRAs = mineralocorticoid receptor antagonists, NT pro-BNP = N-terminal pro-B-type natriuretic peptide, RCT = randomized controlled trial, RR = Risk ratio, TEAEs = treatment-related adverse events, UACR = urinary albumin/creatinine ratio.
HP and WW have contributed equally to this work.
Supported by the National Natural Science Foundation of China of Zhuo Zhao [81270175/81670245], the National Natural Science Foundation of China of Guohai Su[81170087].
The authors report no conflicts of interest.
References
- [1].Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008;10:933–89. [DOI] [PubMed] [Google Scholar]
- [2].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- [3].Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- [4].Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- [5].Pitt B, Pedro Ferreira J, Zannad F. Mineralocorticoid receptor antagonists in patients with heart failure: current experience and future perspectives. Eur Heart J Cardiovasc Pharmacother 2017;3:48–57. [DOI] [PubMed] [Google Scholar]
- [6].Parthasarathy HK, Menard J, White WB, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens 2011;29:980–90. [DOI] [PubMed] [Google Scholar]
- [7].McMurray J, Swedberg K. Treatment of chronic heart failure: a comparison between the major guidelines. Eur Heart J 2006;27:1773–7. [DOI] [PubMed] [Google Scholar]
- [8].Bramlage P, Swift SL, Thoenes M, et al. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail 2016;18:28–37. [DOI] [PubMed] [Google Scholar]
- [9].Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 2014;64:69–78. [DOI] [PubMed] [Google Scholar]
- [10].Orena S, Maurer TS, She L, et al. PF-03882845, a non-steroidal mineralocorticoid receptor antagonist, prevents renal injury with reduced risk of hyperkalemia in an animal model of nephropathy. Front Pharmacol 2013;4:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arai K, Homma T, Morikawa Y, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol 2015;761:226–34. [DOI] [PubMed] [Google Scholar]
- [12].Yang C, Balsells J, Chu HD, et al. Discovery of benzimidazole oxazolidinediones as novel and selective nonsteroidal mineralocorticoid receptor antagonists. ACS Med Chem Lett 2015;6:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [14].Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 2015;314:884–94. [DOI] [PubMed] [Google Scholar]
- [15].Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013;34:2453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Filippatos G, Anker SD, Bohm M, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016;37:2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sato N, Ajioka M, Yamada T, et al. A randomized controlled study of finerenone vs. eplerenone in Japanese patients with worsening chronic heart failure and diabetes and/or chronic kidney disease. Circ J 2016;80:1113–22. [DOI] [PubMed] [Google Scholar]
- [18].McQuade CN, Mizus M, Wald JW, et al. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017;166:180–90. [DOI] [PubMed] [Google Scholar]
- [19].Leto L, Testa M, Feola M. The predictive value of plasma biomarkers in discharged heart failure patients: role of plasma NT-proBNP. Minerva Cardioangiol 2016;64:157–64. [PubMed] [Google Scholar]
- [20].Dhillon S. Eplerenone: a review of its use in patients with chronic systolic heart failure and mild symptoms. Drugs 2013;73:1451–62. [DOI] [PubMed] [Google Scholar]
- [21].Vizzardi E, Sciatti E, Bonadei I, et al. Effects of spironolactone on ventricular-arterial coupling in patients with chronic systolic heart failure and mild symptoms. Clin Res Cardiol 2015;104:1078–87. [DOI] [PubMed] [Google Scholar]
- [22].Eschalier R, McMurray JJ, Swedberg K, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (eplerenone in mild patients hospitalization and survival study in heart failure). J Am Coll Cardiol 2013;62:1585–93. [DOI] [PubMed] [Google Scholar]
- [23].Lopes RJ, Lourenco AP, Mascarenhas J, et al. Safety of spironolactone use in ambulatory heart failure patients. Clin Cardiol 2008;31:509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cruz CS, Cruz AA, Marcilio de Souza CA. Hyperkalaemia in congestive heart failure patients using ACE inhibitors and spironolactone. Nephrol Dial Transplant 2003;18:1814–9. [DOI] [PubMed] [Google Scholar]
- [25].Hughes BR, Cunliffe WJ. Tolerance of spironolactone. Br J Dermatol 1988;118:687–91. [DOI] [PubMed] [Google Scholar]
- [26].Anton C, Cox AR, Watson RD, et al. The safety of spironolactone treatment in patients with heart failure. J Clin Pharm Ther 2003;28:285–7. [DOI] [PubMed] [Google Scholar]
- [27].Fagart J, Hillisch A, Huyet J, et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem 2010;285:29932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].van Enst WA, Naaktgeboren CA, Ochodo EA, et al. Small-study effects and time trends in diagnostic test accuracy meta-analyses: a meta-epidemiological study. Syst Rev 2015;4:66. [DOI] [PMC free article] [PubMed] [Google Scholar]


