Abstract
In deepwater rice (Oryza sativa), adventitious root primordia initiate at the nodes as part of normal development. Emergence of the roots is dependent on flooding of the plant and is mediated by ethylene action. Root growth was preceded by the induced death of epidermal cells of the node external to the tip of the root primordium. Cell death proceeded until the epidermis split open. Through this crack the root eventually emerged. Induced death was confined to nodal epidermal cells covering the tip of the primordia. Our results suggest that this process facilitates adventitious root emergence and prevents injury to the growing root. Cell death was inducible not only by submergence but also by application of 1-aminocyclopropane-1-carboxylic acid, the natural precursor of ethylene and it was suppressed in the presence of 2,5-norbornadiene (bicyclo[2.2.1]hepta-2,5-diene), an inhibitor of ethylene action. Adventitious root growth and epidermal cell death are therefore linked to the ethylene signaling pathway, which is activated in response to low oxygen stress.
Programmed death of cells is part of plant life. A genetically defined program leads to the death of individual cells in response to developmental signals, and in response to biotic and abiotic environmental signals thereby contributing to the survival of the whole organism (Greenberg, 1996; Havel and Durzan, 1996; Jones and Dangl, 1996). Programmed cell death has been recognized as part of gametophyte development where megaspore cells die (Buckner et al., 1998), during embryo development where the suspensor is aborted and starchy endosperm cells die (Marubashi et al., 1999), during embryo germination, which involves death of aleurone cells (Wang et al., 1996a, 1996b; Fath et al., 1999), during xylogenesis (Groover and Jones, 1999), in root-cap formation, during senescence, as plant defense against pathogens (Dangl et al., 1996), and also in adaptation to low oxygen stress when cortex cells are dissolved to form aerenchyma (He et al., 1996; Kawai et al., 1998; Samarajeewa et al., 1999).
We have studied various aspects of plant adaptation to conditions of partial flooding using deepwater rice (Oryza sativa)as an experimental system (Kende et al., 1998). Rice is a semiaquatic plant and in general is well adapted to submergence. One important adaptation to water logging and flooding is growth of adventitious roots. Adventitious roots functionally replace the basal roots, which upon anaerobiosis are no longer capable of supplying the shoot with minerals and water due to insufficient respiratory energy supply.
In deepwater rice, adventitious root primordia are formed at the nodes as part of the normal developmental program (Bleecker et al., 1986; Lorbiecke and Sauter, 1999). The primordia do not arrest at a defined size, but rather grow at a very slow rate resulting in larger root initials as the nodes get older. However, the adventitious root initials remain within the nodal tissue covered by the epidermis until the proper signal induces them to accelerate their growth rate and to emerge (Lorbiecke and Sauter, 1999). Growth of adventitious root primordia is inducible by submergence, with ethylene being the mediating hormonal signal (Lorbiecke and Sauter, 1999).
Emergence of adventitious roots requires growth through the nodal epidermis and cuticle of the stem. Our study was aimed at answering the question whether penetration of the epidermis is a purely mechanical process driven by the force of the growing root or whether it is facilitated by death of epidermal cells at the site of root emergence. Our results clearly indicate that epidermal cells die prior to root growth. Cell death is induced by submergence and is restricted to the sites of root emergence. It can be viewed as a perforation that may help prevent injury to the extending root.
RESULTS
Growth of Adventitious Roots
Adventitious roots are formed early in nodal development in deepwater rice plants (Lorbiecke and Sauter, 1999). They are induced to grow more rapidly upon submergence (Table I; Bleecker et al., 1986; Lorbiecke and Sauter, 1999) or treatment with an ethylene releasing compound such as 1-aminocyclo-propane-1-carboxylic acid (ACC; Table I; Lorbiecke and Sauter, 1999) or ethephon (Fig. 1; Lorbiecke and Sauter, 1999). Lengths of adventitious roots in submerged plants and stem sections treated with ACC for up to 18 h are given in Table I. The lag phase for submergence-induced growth was between 8 and 10 h, the lag phase of ACC-induced growth was at approximately 12 h (Table I).
Table I.
Average length of adventitious roots in plants which were submerged for up to 18 h and in stem sections treated with 10 mm 1 aminocyclopropane 1-carboxylic acid (ACC) for up to 18 h
| Time | Length of Adventitious Roots ± se
|
|
|---|---|---|
| Submerged plants | Stem sections 10 mm ACC | |
| h | mm | |
| 0 | 0.65 ± 0.03 | 0.42 ± 0.01 |
| 2 | 0.69 ± 0.03 | 0.56 ± 0.03 |
| 4 | n.d. | 0.49 ± 0.02 |
| 6 | 0.58 ± 0.03 | 0.60 ± 0.02 |
| 8 | 0.65 ± 0.03 | 0.55 ± 0.02 |
| 10 | 0.90 ± 0.03 | n.d. |
| 12 | 1.10 ± 0.06 | 0.63 ± 0.02 |
| 18 | 1.79 ± 0.20 | 0.76 ± 0.02 |
Figure 1.

Adventitious roots at the third node of a deepwater rice shoot. Adventitious root initials are covered by the nodal epidermis during normal growth (left) or have emerged after treatment with 150 μm ethephon for 24 h (right).
Death of Epidermal Cells Is Induced by Submergence
Adventitious root primordia in non-submerged deepwater rice plants grew continuously albeit at a very slow rate resulting in bulging of the epidermis at older nodes (Figs. 1 and 2A). In non-submerged plants, the epidermal cells at the node that covered the primordia were alive and grew along with the roots (Figs. 1, 2A, and 3A, stage I). When plants were submerged, increasing numbers of nodal epidermal cells covering the root primordia died (Fig. 3). A cross-section indicated that only epidermal cells, but not parenchyma cells of the node or cells of the root primordium itself were stained with Evans blue (Fig. 2, B and C). In the epidermis covering a root primordium, cell death started with single cells producing a patchy staining pattern (Figs. 2, B and C, and 3A, stage II). A cross-section of a complete root initial including the connecting vasculature showed that the dye was able to diffuse freely, staining dead cells of the vasculature within the node, but not the living protoxylem cells in the central cylinder of adventitious root primordia (Fig. 2A). This pattern indicated that staining of epidermal cells was specific and not due to limited dye diffusion (Fig. 2). Death of nodal epidermal cells was confined to areas covering protruding root tips (Figs. 2 and 3A). Eventually, most of the nodal epidermal cells covering the root tip were stained blue (Fig. 3A, stage III). When most of the epidermal cells covering a root tip had died, the nodal epidermis cracked open making way for the root beneath (Fig. 3A, stage IV).
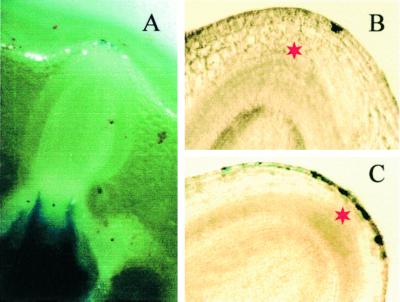
Figure 2.
Cross-sections of the third node stained with Evans blue to indicate dead cells. A, Staining of the nodal vasculature connected to a root primordium, but not of the protoxylem elements of the root primordium itself. B, A single epidermal cell is stained (see Fig. 3A, stage II). All parenchymal cells of the node and all cells of the root primordium itself are not stained, indicating that the primordium does not yet possess a root cap. Asterisk indicates the tip of the root primordium. C, More cells are stained (see Fig. 3A, stage III), but staining is still restricted to the epidermis and is not seen in either the nodal parenchyma cells or in the root primordium. Asterisk indicates the tip of the root primordium.
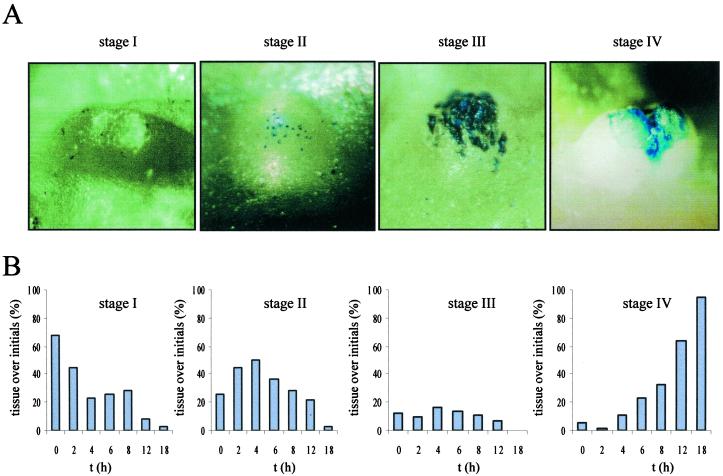
Figure 3.
Degree Evans blue staining of the nodal epidermis above root primordia at the third node of submerged rice plants. A, Stage I indicates no staining of the nodal epidermis covering an adventitious root primordium; stage II indicates a patchy staining pattern of the nodal epidermis; stage III indicates areal staining of the nodal epidermis; and stage IV staining includes a cracked nodal epidermis with or without the root initial growing through the opening. B, At each time point, the staining patterns as exemplified in A were determined above each root primordium at five to eight nodes with 12 to 15 root primordia per node and attributed to stage I, II, III, or IV. The numbers are expressed as a percentage and add up to 100% for each time point. Analysis was carried out at the third node of intact rice plants that were submerged for up to 18 h.
We analyzed the timecourse of submergence-induced cell death (Fig. 3B) and compared it with the timecourse of induced adventitious root growth (Table I). The epidermis above each root initial was categorized to one of the above described stages at defined times for up to 18 h of submergence. At time 0 h, approximately 70% of all epidermal tissues did not show any staining, approximately 25% displayed single stained cells, and in 5% to 10% of all cases, stage III and IV staining was observed. Within 2 h after submergence the percentage of unstained tissues dropped to around 45% and the number of stage II staining patterns increased accordingly (Fig. 3B). Unstained epidermal tissues covering the root tips continuously declined and were less than 10% after 12 h. The stage II staining pattern with single dead cells transiently increased with maximal numbers after 4 h (Fig. 3B). Stage III staining with large areas of dead cells that started to tear apart existed only briefly. Only few tissues were therefore attributable to this stage at a time (Fig. 3B). Increasing numbers of root initials over which the epidermal tissues were torn open were first observed after 6 h (Fig. 3B, stage IV). The percentage continuously increased until after 18 h more than 90% of all roots were no longer confined by the nodal epidermis. The lag phase of submergence-induced adventitious root growth at the third node was between 8 and 10 h (Table I; Lorbiecke and Sauter, 1999). Induced death of epidermal cells as early as 2 h after submergence clearly preceded induced growth of adventitious roots indicating that cell death was not the result of a mechanical force exerted by the growing root, but rather a growth independent process that was triggered by submergence.
ACC Induces Cell Death
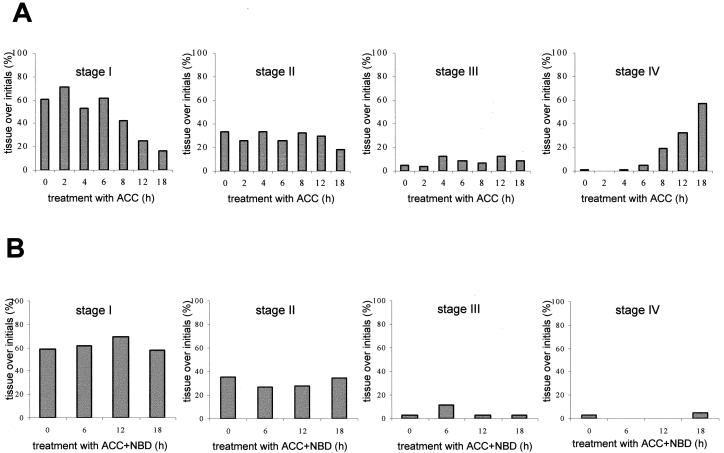
Ethylene has been shown to accumulate in submerged rice. We therefore tested the capacity of the natural ethylene precursor ACC to induce death of epidermal cells. Ten millimolars ACC was shown to be optimal for induction of adventitious root growth (Lorbiecke and Sauter, 1999). We therefore chose this concentration for our experiments. Stem sections containing the third node were treated with ACC for the same times as the submerged plants for up to 18 h. At each time point, epidermal tissue was scored for cell death as indicated by Evans blue staining and classified to stages I to IV as described in Figure 3A. The results of Evans blue staining are summarized in Figure 4A. Eight hours after application of ACC, the percentage of living cells declined from an average of around 60% to approximately 40% and was further reduced to 25% after 12 h (Fig. 4). Stage II staining with scattered dead epidermal cells was observed at a constant level above approximately 30% of all root primordia examined except after 18 h when it was reduced to 18%. Epidermal tissue with a stage III staining pattern was found at an increased frequency after 4 h of treatment with ACC and epidermal tissues began to crack open after 6 h (Fig. 4A). After 18 h, close to 60% of all epidermal tissues above root primordia were dead and cracked open and root primordia began to emerge. Because the lag phase of ACC-induced adventitious root growth was at around 12 h (Table I), ACC-induced cell death occurred prior to or concomitant with root growth.
Figure 4.
Degree of epidermal Evans blue staining above root primordia at the third node of isolated stem sections. A, Stem sections were treated with 10 mm ACC for the times indicated. Results are averages of at least 15 stem sections analyzed in three independent experiments. Each stem section contained 12 to 15 adventitious root primordia. B, Stem sections were treated with 10 mm ACC and 50 μL/L NBD for the times indicated. The staining patterns are given as percentages of stage I to stage IV as described in Figure 3A. Results are averages of at least five stem sections analyzed.
2,5-Norbornadiene (bicyclo[2.2.1]hepta-2,5-diene) (NBD) Inhibits Ethylene-Induced Cell Death
NBD binds to the ethylene receptor thereby inhibiting ethylene activity (Bleecker et al., 1987). In deepwater rice, ethephon, a chemical precursor of ethylene, induced adventitious root growth similar to ethylene (Lorbiecke and Sauter, 1999). When ethephon was supplied together with NBD, the growth response was completely abolished (Lorbiecke and Sauter, 1999). In this study incubation of stem sections with 10 mm ACC, the natural precursor of ethylene and 50 μL/L NBD, blocked root emergence and drastically reduced induction of cell death (Fig. 4B). Measurements at 0, 6, 12, and 18 h yielded essentially the same distribution of staining patterns indicating that cell death was dependent on proper perception of the ethylene signal.
DISCUSSION
We have shown that submergence-induced growth of adventitious roots is preceded by death of epidermal cells at the node external to the root primordia. We view this cell death as a way to weaken and then perforate the epidermal cell layer and to facilitate emergence of the root. One might postulate that cell wall degrading enzymes such as cellulase are active as was shown for cortical cell lysis in aerenchyma formation (He et al., 1994). The details of cell disintegration have however not been looked at yet.
The epidermis and cuticle protect the plant against desiccation, mechanical injury, and pathogen attack. It provides resistance to penetration from outside and is therefore, by default, resistant to penetration from within. If the adventitious root primordia had to force its way through the nodal epidermis, the root apex might get damaged, which in turn would compromise or completely prevent further root growth. Weakening of the epidermis at the site of root penetration may therefore be seen as a means to prevent self-damage.
Would a plant be able to distinguish between an attack from outside and an attack from within? Hardly. If the growing roots were to penetrate the epidermis forcefully, would the plant respond with concerted defense actions? If so, it would waste energy and resources on an uncalled for response at a time when the plants' energy supply and metabolism are restricted by reduced oxygen tension and resultant limitations in respiration and carbohydrate resources (Sauter, 2000). Programmed degradation of the boundary cell layer prior to root growth prevents a defense response.
Ethylene plays a central role in submergence-tolerant species (Armstrong et al., 1994; Voesenek and Blom, 1999). In deepwater rice, adventitious root primordia are formed as part of the normal developmental program. Emergence of the primordia is dependent on an appropriate signal, i.e. submergence (Bleecker et al., 1986), which in turn is mediated by ethylene (Lorbiecke and Sauter, 1999). Formation of adventitious roots in response to ethylene has been described for other plants as well and is connected with the flooding resistance of plant species in general (Drew et al., 1979; Voesenek and van der Veen, 1994; Visser et al., 1996). Our results indicate that ethylene is also the signal that induces death of epidermal cells at the node since cell death was induced by the natural ethylene precursor ACC and was inhibited by simultaneous application of norbornadien, an inhibitor of ethylene action (Bleecker et al., 1987; Lorbiecke and Sauter, 1999). The cell death program of cortical cells during aerenchyma formation was also shown to depend on the transduction of an ethylene signal (Drew et al., 1979; He et al., 1996). Formation of air spaces, growth of adventitious roots, and death of epidermal cells at the node adjacent to the root primordia are adaptive processes that help plants to cope with water logging or submergence. It can be seen as an advantage that these processes are induced by the same signal. With the different lag phases of the ethylene-regulated responses the plant ensures appropriate timing of cell death and cell proliferation events.
Lateral and adventitious roots are both secondary roots. Just like adventitious roots, lateral roots are formed in response to environmental conditions and, in fact, provide the major portion of most mature root systems (Malamy and Benfey, 1997). Formation of the secondary root system strongly determines a plants' survival ability. Mutants exist in Arabidopsis (Celenza et al., 1995) and in maize (Hetz et al., 1996), which have normal primary roots, but which lack secondary, i.e. lateral and adventitious roots, suggesting a common pathway for lateral and adventitious root formation. Thus understanding growth and development of adventitious roots may be helpful in elucidating growth and development of secondary roots in general, including the long-standing question how secondary roots traverse cortical and epidermal cells (Bell and McCully, 1970; Kosslak et al., 1997).
MATERIALS AND METHODS
Plant Material and Incubation Conditions
Seeds of deepwater rice (Oryza sativa L., Pin Gaew 56) were obtained from the International Rice Research Institute (Los Baños, Philippines). Plants were grown essentially as described (Sauter, 1997) for 12 to 14 weeks. Whole plants were submerged in a 600-L plastic tank filled with tap water at 25°C with approximately 30 cm of the leaf tips remaining above water. Incubation was in an environmental growth chamber under continuous light (200 μE m−2 s−1). Control plants were kept in the same growth chamber.
To analyze the effects of the ethylene precursor ACC and of NBD, an inhibitor of ethylene action on cell death at the third node, isolated stem sections were used. These were cut from 2 cm below the third highest node extending upward with a total length of 20 cm. The sections contained the third and the second node and the second youngest internode between them. The sections were incubated in 150-mL beakers containing 25 mL of aqueous solution of ACC at a final concentration of 10 mm or 10 mm ACC and NBD at a concentration of 50 μL/L in the gas phase. The beakers with the stem sections were placed in plastic cylinders to ensure high humidity. Incubation was at 25°C under continuous light.
To determine the length of the adventitious roots in ACC-treated stem sections, roots were isolated from the node and photographed through binoculars. The enlarged photographed roots were then measured with a ruler and root length was calculated to scale.
Evans Blue Staining
After submergence treatment of plants or treatment of stem sections with ACC or ACC and NBD, the third node was excised along a total length of 10 mm. The nodes were stained in 2% (w/v) Evans blue in water for 3 min and subsequently washed in water. Evans blue enters cells with a freely permeable plasma membrane, that is dead cells only (Gaff and Okong'O-gola, 1971; Kanai and Edwards, 1973). Microscopic analysis of staining patterns was performed immediately afterward using binoculars. Cross-sections were cut following Evans blue staining and washing to avoid staining artifacts from wounded cells.
Footnotes
This work was supported by a grant from the Arthur and Aenne Feindt Foundation.
LITERATURE CITED
- Armstrong W, Brändle R, Jackson MB. Mechanisms of flood tolerance in plants. Acta Bot Neerl. 1994;43:307–358. [Google Scholar]
- Bell JK, McCully ME. A histological study of lateral root initiation and development in Zea mays. Protoplasma. 1970;70:179–205. [Google Scholar]
- Bleecker AB, Rose-John S, Kende H. Evaluation of 2,5-norbornadien as a reversible inhibitor of ethylene action in deepwater rice. Plant Physiol. 1987;84:395–398. doi: 10.1104/pp.84.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Schuette JL, Kende H. Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta. 1986;169:490–497. doi: 10.1007/BF00392097. [DOI] [PubMed] [Google Scholar]
- Buckner B, Janick-Buckner D, Gray J, Johal GS. Cell-death mechanisms in maize. Trends Plant Sci. 1998;3:218–223. [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard S. Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta. 1979;147:83–88. doi: 10.1007/BF00384595. [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke PC, Jones RL. Barley aleurone cell death is not apoptotic: characterization of nuclease activities and DNA degradation. Plant J. 1999;20:305–315. [PubMed] [Google Scholar]
- Gaff DF, Okong'O-Ogola O. The use of non-permeating pigments for testing the survival of cells. J Exp Bot. 1971;22:757–758. [Google Scholar]
- Greenberg JT. Programmed cell death: a way of life for plants. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel L, Durzan DJ. Apoptosis in plants. Bot Acta. 1996;109:268–277. [Google Scholar]
- He CJ, Drew MC, Morgan PW. Induction of enzymes associated with lysigenous earenchyma formation in roots of Zea mays during hypoxia or nitrogen starvation. Plant Physiol. 1994;105:861–865. doi: 10.1104/pp.105.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 1996;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz W, Hochholdinger F, Schwall M, Feix G. Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J. 1996;10:845–857. [Google Scholar]
- Jones AM, Dangl JL. Logjam at the styx: programmed cell death in plants. Trends Plant Sci. 1996;1:114–119. [Google Scholar]
- Kanai R, Edwards GE. Purification of enzymatically isolated mesophyll protoplasts from C3, C4 and crassulacean acid metabolism plants using aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973;52:484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Samarajeewa PK, Barrero RA, Nishiguchi M, Uchimiya H. Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation in rice roots. Planta. 1998;204:277–287. [Google Scholar]
- Kende H, van der Knaap E, Cho HT. Deepwater rice: a model plant to study stem elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak RM, Chamberlin MA, Palmer RG, Bowen BA. Programmed cell death in the root cortex of soybean root necrosis mutants. Plant J. 1997;11:729–745. doi: 10.1046/j.1365-313x.1997.11040729.x. [DOI] [PubMed] [Google Scholar]
- Lorbiecke R, Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999;119:21–29. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Down and out in Arabidopsis: the formation of lateral roots. Trends Plant Sci. 1997;2:390–396. [Google Scholar]
- Marubashi W, Yamada T, Niwa M. Apoptosis detected in hybrids between Nicotiana glutinosa and N. repanda expressing lethality. Planta. 1999;210:168–171. doi: 10.1007/s004250050667. [DOI] [PubMed] [Google Scholar]
- Samarajeewa PK, Barrero RA, Umeda-Hara C, Kawai M, Uchimiya H. Cortical cell death, cell proliferation, macromolecular movements and rTip1 expression pattern in roots of rice (Oryza sativa L.) under NaCl stress. Planta. 1999;207:354–361. [Google Scholar]
- Sauter M. Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes during the cell cycle and in response to gibberellin. Plant J. 1997;11:181–190. doi: 10.1046/j.1365-313x.1997.11020181.x. [DOI] [PubMed] [Google Scholar]
- Sauter M. Rice in deep water: how to take heed against a sea of troubles. Naturwissenschaften. 2000;87:289–303. doi: 10.1007/s001140050725. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Bögemann GM, Blom CWPM, Voesenek LACJ. Ethylene accumulation in waterlogged Ryme plants promotes formation of adventitious roots. J Exp Bot. 1996;47:403–410. [Google Scholar]
- Voesenek LACJ, Blom CWPM. Stimulated shoot elongation: a mechanism of semiaquatic plants to avoid submergence stress. In: Lerner HR, editor. Plant Responses to Environmental Stress: From Phytohormones to Genome Reorganization. New York: Marcel Dekker; 1999. pp. 431–448. [Google Scholar]
- Voesenek LACJ, Van Der Veen R. The role of phytohormones in plant stress: too much or to little water. Acta Bot Neerl. 1994;43:91–127. [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG. Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell. 1996a;8:375–391. doi: 10.1105/tpc.8.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Oppedijk BJ, Lu X, Dujin BV, Schilperoort RA. Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol Biol. 1996b;32:1125–1134. doi: 10.1007/BF00041396. [DOI] [PubMed] [Google Scholar]