Abstract
Rationale:
Meningioma is slow-growing benign neoplasm that derived from the meningothelial cells of the arachnoid mater, and it is the most common type of primary brain tumor. Although cases of extracranial metastatic meningioma have been reported previously, few cases have reported the concurrent occurrence of primary meningioma with intratumoral bleeding and lung metastases. Here we report a case of meningioma with concurrent intratumoral bleeding and lung metastases.
Patient concerns:
The patient was admitted to our hospital with sudden left limb paralysis combined with complaints of headache and dizziness.
Diagnoses:
Histopathological results confirmed the lung metastases derived from intracranial bleeding meningioma.
Interventions:
The magnetic resonance imaging (MRI) of brain and computed tomography (CT) of brain and lung were performed.
Outcomes:
CT and MRI of brain showed an intracranial mass associated with intratumoral hemorrhage. Then, multiple lung metastases were observed in the right lung via CT images. The intracranial mass was excised through craniotomy firstly, and a thoracoscopic biopsy was performed 1 month later. The patient was hospitalized again after 1 year because of suddenly epilepsy and received effective symptomatic treatment until symptoms were relieved. Then the patient is lost to follow-up.
Lessons:
When meningiomas occurred nearby venous sinuses with aggressive behavior, a careful whole-body examination is recommended in order to find distant metastasis as early as possible.
Keywords: bleeding, lung, meningioma, metastasis
1. Introduction
Intracranial meningiomas account for 20% to 30% of all intracranial neoplasms in adult patients.[1] Meningiomas are usually benign neoplasms and rarely metastasize with an incidence rate of 0.1%.[2] Intratumoral hemorrhage of meningioma is also uncommon.[3] However, to the best of our knowledge, concurrent occurrence of intratumoral hemorrhage and distal metastases with meningioma has seldom been reported. This report aimed to describe the clinical features and imaging findings of 1 case with lung metastases from intracranial bleeding meningioma.
2. Case presentation
A 58-year-old male was admitted in our hospital because of sudden left limb paralysis combined with complaints of headache and dizziness for 6 hours. At the time of admission, his blood pressure was 130/80 mm Hg (1 mm Hg = 0.133 kPa). The head computed tomography (CT) scan revealed a 6.0-cm, heterogeneous hyper-attenuating mass in the right-frontal lobe (Fig. 1). Magnetic resonance imaging (MRI) showed an intracranial tumor which demonstrated heterogeneously iso- or hypo-intense on T1-weighted imaging (T1WI) (Fig. 2A) and hyper-intense on T2-weighted imaging (T2WI) (Fig. 2B). There were several stripped low-signal on T2WI, which indicated intratumoral hemorrhage. Peritumoral edema was presented. Post-contrast T1WI showed locally enhancement, and dural tail sign could be seen (Fig. 2C). The tumor is closely attached to the superior sagittal sinus with unclear boundary. A craniotomy with tumor resection was recommended. Noticeably, the routine chest radiograph before surgery revealed abnormal shadows on the right-lung field. Chest CT scan confirmed 3 round well-defined mass in the right lung (Fig. 3A and B). The largest one measured about 3.7 cm. The CT value of the lesions increased from 44 to 68 HU after injection of contrast agent (Fig. 3C).
Figure 1.
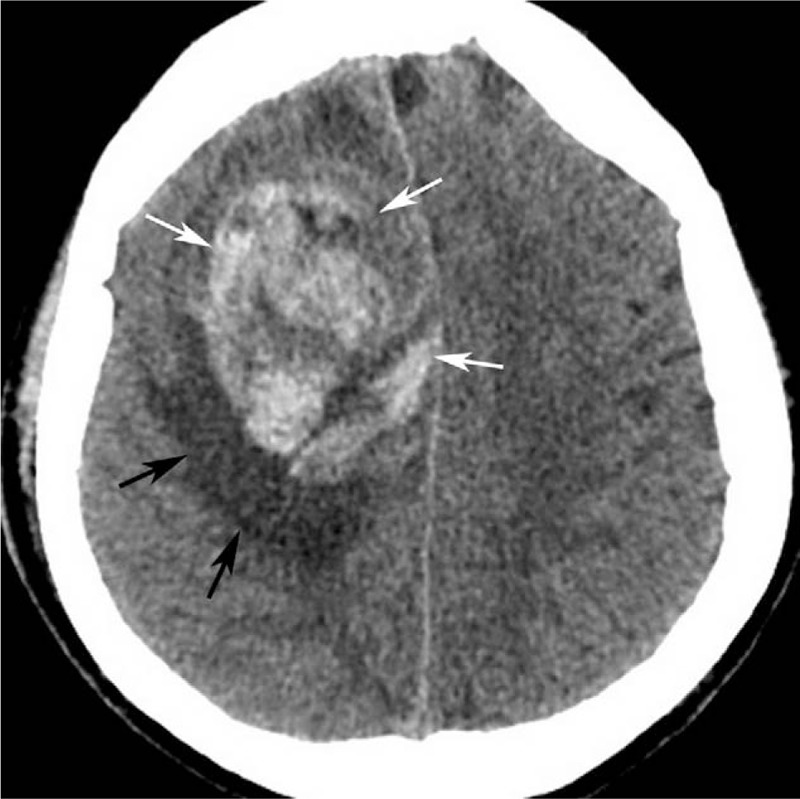
Computed tomography (CT) plain scan showed a round, heterogeneous hyper-attenuating mass (white arrow) with peripheral edema (black arrow) in the right frontal lobe. CT = computed tomography.
Figure 2.
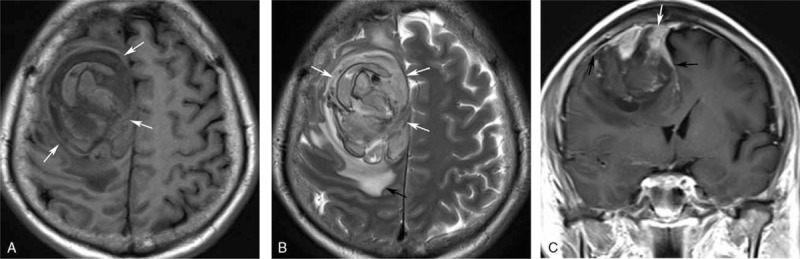
Axial T1-weighted imaging (A) and axial T2-weighted imaging (B) magnetic resonance imaging (MRI) showed a round, nonhomogenous (white arrow), peritumoral edematous (black arrow) mass, which presented dural tail sign (black arrow) and ill-defined boundary with superior sagittal sinus (white arrow) on coronal T1-weighted imaging after gadolinium enhancement (C). MRI = magnetic resonance imaging.
Figure 3.
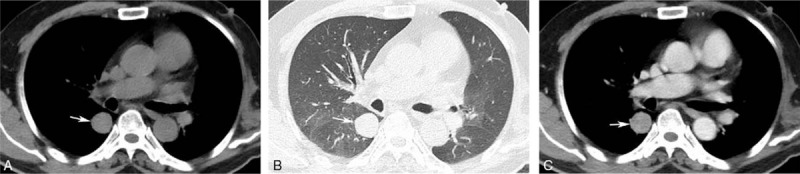
Chest CT scan showed round, homogeneous, sharply circumscribed mass in the right lung (white arrow) (A, B), which revealed homogeneous moderately enhancement (C). CT = computed tomography.
The intracranial mass was excised through craniotomy firstly. The mass was closely adhesion to the skull, and attached to the dura mater, and hematoma was observed surrounding and within the mass. The parenchyma of tumor was demonstrated as grayish white appearance, and the final pathological diagnosis confirmed it was meningioma (Fig. 4). According to the immunohistochemistry results, the tumor was positive in epithelial membrane antigen (EMA), vimentin and Ki-67 (10%), but negative in glial fibrillary acidic protein (GFAP), S100 and CD56. After about 1 month, a thoracoscopic biopsy was performed. The detailed histological exam of the lesions confirmed that there were metastases from meningioma (Fig. 5). Immunophenotype revealed vimentin-positive and S-100-positive.
Figure 4.
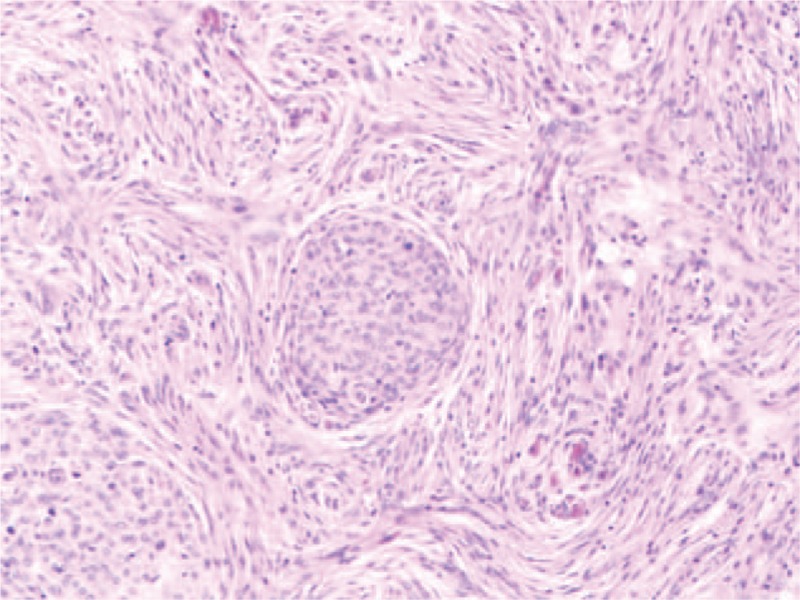
Histological examination on the intracranial tumor diagnosed as meningioma (hematoxylin–eosin, ×100).
Figure 5.
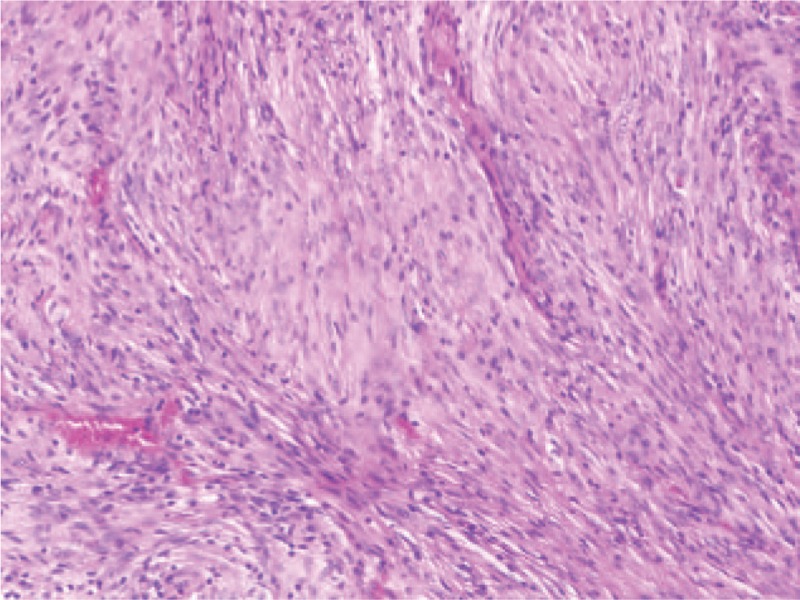
Pathological examination of the lung specimen showing spindle-shaped cells similar to meningioma (hematoxylin–eosin, ×100).
The patient discharged from our hospital after 9 days, and received no further treatment for both the intracranial and lung lesions. One year later, he was hospitalized again because of a suddenly epilepsy, which was considered to be a postoperative complication. Effective symptomatic treatment was provided until symptoms were relieved. Then the patient is lost to follow-up.
The institutional review board of our hospital approved this study was a case report for retrospective analysis, and the requirement of informed consent was waived. We anonymized all of the data before analysis.
3. Discussion
Intracranial neoplasms accompanying spontaneous hemorrhage are accounting for 1.4% to 10% of all intracranial neoplasms, among them, the intratumoral hemorrhage of meningioma is extremely rare (only 0.5%–2.4%).[3] Meanwhile, the extracranial metastasis of benign meningioma is particularly rare[2], and clinical information including radiological and pathological features of its extracranial metastasis are not well characterized. Therefore, our case was unusual since it was a primary meningioma with intratumoral bleeding and presented lung metastases simultaneously.
Ki-67 index has been suggested to be a potential predictor for aggressive meningioma and increased risk of metastasis. The mean Ki-67 proliferative index (PI) of metastatic meningiomas (14.7%) was much higher than that of no-metastatic ones (1%).[4] Ki-67 PI of this patient was about 10% in the primary lesion, which can be served as a predictor for metastatic potential. However, it is not a requirement of high cellular proliferative for the occurrence of extracranial metastasis. Any histological subtype of meningiomas may be distant metastasis.[2]
The most common lesions of extracranial metastasis are the lungs (37.2%), followed by the bones (16.5%), intraspinal (15.2%), and liver (9.2%).[5] But other sites of metastasis such as skin are also reported in the literature.[6] Pulmonary metastases rarely present corresponding symptoms. The metastatic lesions are usually observed as single or multiple round, various sizes, well-defined and solid nodules.[2]
The pathophisiology mechanisma of the intratumoral bleeding in meningioma remain unclear. However, several hypotheses have been proposed. Someone suspects that blood vessels may rupture due to the direct invasion of tumor cells. Besides, a few kinds of meningiomas can release vaso-active substances, resulting in vasodilatation and tumoral hemorrhage.[3] Similarly, it is still not completely understood about the mechanisms of extracranial metastasis of meningiomas. The proposed ways of extracranial spread includes metastasis via the venous system, lymphatics or cerebrospinal fluid (CSF).[6] Hematogenous metastasis is the most likely way for lung metastases. Seeded tumor cells access to the dural venous sinuses and cranial veins, then diffuse into the pulmonary circulation and cause lung metastasis. Surgery is thought to be the major reason for this mechanism.[7] In our present case, the boundary between the tumor and superior sagittal sinus is indistinct. It was hypothesized that the sinus was invaded by the primary tumor, which caused intratumoral bleeding and lung metastases.
4. Conclusion
This report is the extremely rare case of primary meningioma with intratumoral bleeding and lung metastases simultaneously. When meningiomas originated nearby venous sinuses and showed invasion signs, a careful whole-body examination should be performed in order to detect distant metastasis as early as possible.
Author contributions
Data curation: Yue Sun, Yang Yu, Jia Wang.
Formal analysis: Feng Sun.
Writing – original draft: Su Hu.
Writing – review & editing: Yaqi Zhang, Hui Dai, Chunhong Hu.
Footnotes
Abbreviations: CSF = cerebrospinal fluid, CT = computed tomography, EMA = epithelial membrane antigen, GFAP = glial fibrillary acidic protein, MRI = magnetic resonance imaging, PI = proliferative index, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging.
This study was supported by the National Natural Science Foundation of China (No. 31271066), the Medical Research Project of Jiangsu Provincial Commission of Health and Family Planning (No. H2017063), and the Research Innovation Program for College Graduates of Jiangsu Province (No. KYZZ15_0335).
The authors have no conflicts of interest to disclose.
References
- [1].Motebejane MS, Kaminsky I, Choi IS. Intracranial meningioma in patients age <35 years: evolution of the disease in the era of human immunodeficiency virus infection. World Neurosurg 2018;109:e292–7. [DOI] [PubMed] [Google Scholar]
- [2].Wang KD, Su YB, Zhang Y. Recurrent intracranial meningioma with multiple pulmonary metastases: a case report. Oncol Lett 2015;10:2765–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sinurat R, Banjarnahor JD. Incidental bleeding meningioma. Asian J Neurosurg 2017;12:247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakayama Y, Horio H, Horiguchi S, et al. Pulmonary and pleural metastases from benign meningeal meningioma: a case report. Ann Thorac Cardiovasc Surg 2014;20:410–3. [DOI] [PubMed] [Google Scholar]
- [5].Ito K, Imagama S, Ando K, et al. Intraspinal meningioma with malignant transformation and distant metastasis. Nagoya J Med Sci 2017;79:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moir JA, Haugk B, French JJ. Hepatic metastasis via a ventriculo-peritoneal shunt from an intracranial meningioma: case report and review of the literature. Case Rep Gastroenterol 2010;4:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee GC, Choi SW, Kim SH, et al. Multiple extracranial metastases of atypical meningiomas. J Korean Neurosurg Soc 2009;45:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


