Abstract
This study aims to investigate and compare the biomarkers in the gingival crevicular fluid between the Han and Uygur subjects with healthy implants and peri-implantitis.
Totally 80 subjects were divided into the H-case (Han patients with peri-implantitis), U-case (Uygur patients with peri-implantitis), H-control (Han subjects with healthy implants), and U-control (Uygur subjects with healthy implants) groups. Cytokine levels in the gingival crevicular fluid were detected, and the dominant bacteria species were analyzed.
The matrix metalloproteinase (MMP)-13 level in the gingival crevicular fluid in the U-control group was significantly higher than the H-control group, whereas the C-reactive protein level in the H-control group was significantly higher than in the U-control group. No significant difference was observed in the dominant subgingival bacteria species between the H- and U-control groups. The levels of interleukin (IL)-1β and MMP-8 were significantly higher in the H-case group than the U-case group, whereas the IL-17A level in the U-case group was significantly higher. The shared dominant subgingival bacteria species of the case groups mainly included Prevotella, clostridium, Porphyromonas, treponema, Streptococcus, neisseria, and hemophilus. Moreover, Acinetobacter, Micrococcus, and Moraxella were found to be the specific dominant subgingival bacteria species for the U-case group. In addition, compared with the H-case group, the IL-1β levels were negatively correlated with Acinetobacter, Micrococcus, and Moraxella in the U-case group.
Han and Uygur populations with healthy implants and peri-implantitis have differentially expressed cytokines in the gingival crevicular fluid. Moreover, dominant subgingival bacteria species differ between the Han and Uygur populations with peri-implantitis.
Keywords: biomarkers, gingival crevicular fluid, Han and Uygur populations, healthy implants, peri-implantitis
1. Introduction
Peri-implantitis is a kind of chronic progressive inflammation, which occurs in the soft and/or hard tissues surrounding the functional osseointegrated implants. Peri-implantitis might induce the loss of alveolar bone and lead to the formation of peri-implant pocket.[1] After the complete absorption of the osseointegration region, the implants would become loose, which would possibly result in the implantation failure. At present, routine periodontal examination and X-ray detection can only served for the disease severity determination, rather than predict or reflect the whole pathogenic process. However, monitoring and detection of the biomarkers in the gingival crevicular fluid of the patients with peri-implantitis would contribute to the prediction of the disease activity.[2,3] Biomarkers in the gingival crevicular fluid, saliva, and serum could also help to evaluate the normal biological processes and disease pathogenesis, as well as the responses to drug treatment.
There are several kinds of biomarkers, which are associated with peri-implantitis. Detection of proteomics biomarkers could intuitively and accurately determine the survival of oral microbes and their responses to the environment changes, including the osteocalcin, alkaline phosphatase, matrix metalloproteinases (MMPs), and C-reactive protein (CRP).[4–8] Moreover, genetic biomarkers have been shown to be associated with the pathogenesis of peri-implantitis, including the interleukin (IL), prostaglandin E2, CD14, lipopolyssacharide receptors, and osteoprotegerin.[9–16] Furthermore, there are numerous microbial biomarkers. More than 600 species of bacteria have been detected and identified in the subgingival plaques, although a relative minority of them has been related to the induction of peri-implantitis. Currently, it has been widely accepted that the pathogenic process for peri-implantitis is similar to that for the periodontal disease, especially in terms of the involved pathogenic bacteria. Based on the clustering characteristics and the relationship with the periodontal status, these subgingival bacteria could be divided into 6 main microbial complexes, designated as red, orange, yellow, green, purple, and blue, respectively. However, as claimed by the report from the European Society of Periodontology, current studies of peri-implantitis-associated microbes have mainly focused on the periodontal pathogens, and the activities and effects of potential pathogenic microbes relatively unimportant for the periodontal diseases might be ignored or underestimated. It has been shown that, compared with the healthy implants, many strains of bacteria are significantly increased in the peri-implantitis cases.[17] Importantly, detection of marker packages, that is, the combination of multiple indicators, is characterized by high accuracy, which can be used as effective detection and prediction tool for peri-implantitis.
Xinjiang, China, is well known as the living region for the minorities, where Han and Uygur populations represent the main residents. There are significant differences in the ethnic origin, living habits, and religious believes between the Han and Uygur populations. Moreover, significant differences have been found in the clinical disease incidence and biomarker expression levels between these 2 populations. In this study, the biomarkers in the gingival crevicular fluid associated with the healthy implants and peri-implantitis for the Han and Uygur subjects were investigated and compared.
2. Materials and methods
2.1. Study subjects and grouping
Totally 105 samples of gingival crevicular fluid of patients with healthy implants and peri-implantitis were included in this study, who were admitted to the Fifth Affiliated Hospital of Xinjiang Medical University, the Second Affiliated Hospital of Xinjiang Medical University, the Urumqi Stomatological Hospital, and three other minority clinics (including the Aizezi Oral Clinic). All the study subjects receive dental implantation, from 2013 to 2016, with at least 1 implant for over half a year. According to the Guidelines for Periodontal Disease in the United States, peri-implantitis was defined as bleeding of probing (BOP) and/or periodontal pocket depth (PPD), accompanied by bone tissue loss below the first thread of the implant.[18] Exclusion criteria were as follows: patients with systematic diseases; patients who accepted periodontal therapy, or received immunosuppressive agents and antibiotics, within 6 m; patients who received long-term contraceptives; and pregnant women, or smokers. Previous written and informed consents were obtained from every patient and the study was approved by the local ethics review board.
These subjects were divided into the following 4 groups: the H-case group contained 20 cases of Han subjects with peri-implantitis; the U-case group contained 20 cases of Uygur subjects with peri-implantitis; the H-control group contained 20 cases of Han subjects with healthy implants; and the U-control group contained 20 cases of Uygur subjects with healthy implants.
2.2. Sample collection
After gargling, the sampling site within the patient's mouth was gently wiped with the sterile cotton. Sterile absorbent paper tip (the sharp tip was cut off) was softly inserted into the gingival sulcus around the implant. There were totally 6 sampling points for each case, including the proximal, middle, and distal points on the buccal and tongue (palate) sides, respectively. After 30 seconds, the paper tip with the gingival crevicular fluid was taken out (with no blood or pus), and immediately placed in the frozen tube and stored at −80°C.
2.3. Cytokine detection
Cytokines in the gingival crevicular fluid were detected with the ProcartaPlex TM Multiplex Immunoassay. Briefly, 50 μL of microsphere mix solution was put into each well in the 96-well detection kit. After 30 minutes, the solution was discarded. Following washing, 30-μL Universal Assay Buffer + 20-μL standard or sample were added. The kit was sealed and incubated with vibration at room temperature for 2 hours. After unsealing, the 96-well kit was placed on the magnetic separation plate for 3 minutes, and the solution within each well was discarded. Following washing, 25-μL biotin-labeled detection antibody was added into each well for 30 minutes. The 96-well kit was again placed on the magnetic separator for 3 minutes, and the solution was discarded. After washing, 50-μl Streptavidin-PE was added into each well, followed by incubation with vibration at room temperature for 30 minutes. The 96-well kit was again placed on the magnetic separator for 3 minutes, and the solution was discarded. After washing, 100-μL Reading Buffer was added into each well, followed by vibration at room temperature for 5 minutes and then detection.
2.4. Bacterial diversity monitoring
Genomic DNA was extracted with the DNA extraction kit. Bacterial diversity identification was performed based on the 16S V3-V4 region (primers 343F and 798R). For the purification, PCR products were analyzed by electrophoresis, and mixed according to the sample products, which were then sequenced. The 16S rRNA gene clone library was constructed.
2.5. Statistical analysis
Cytokine detection results were analyzed using the ProcartaPlex Analyst 1.0 analysis software. The spearman statistical method was used for statistical analysis. Subgingival bacterial diversity were determined with the UPARSE software, and classified into multiple operational taxonomic units (OTUs) based on the sequence similarity (no <97%). Representative OUT sequences were picked out with the QIIME software package, which were compared against the databases and annotated. The 16S rRNA gene clone library was compared against the Greengenes or Silva (Version 123) databases. Species comparison and annotation were performed using the RDP classifier software, and the annotation results with the confidence interval >0.7 were preserved.
3. Results
3.1. Baseline characteristics of study subjects
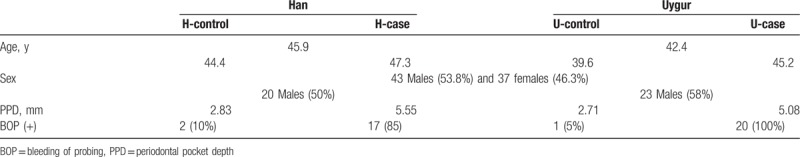
Basic information of these subjects was shown in Table 1. Totally 80 subjects were included in this study, 43 males (53.8%) and 37 females (46.3%), with the average age of 44.1 years. In details, the average ages for the Han and Uygur subjects were 45.9 and 42.4 years, respectively, with no significant difference between these 2 populations. Moreover, there was no significant difference in the male/female rate between these 2 populations. Male subjects accounted for 50% (20) and 58% (23) in the Han and Uygur populations, respectively. Significant differences were observed in the PPD between the H-control (2.83 mm) and H-case (5.55 mm) groups (P < .01), as well as between the U-control (2.71 mm) and U-case (5.08 mm) groups (P < .01), rather than between the H-control and U-control groups, or between the H-case and U-case groups. Moreover, significant differences were observed in the BOP incidence between the H-control (10%) and H-case (85%) groups, as well as between the U-control (5%) and U-case (100%) groups, rather than between the H-control and U-control groups, or between the H-case and U-case groups (Table 1). These results suggest that no significant differences are observed in the age and sex ratio between the Han and Uygur populations, and no significant differences are observed in the clinical indicators for the healthy implants and peri-implantitis between these 2 populations.
Table 1.
Basic information of study subjects.
3.2. Evaluation and comparison of cytokine levels in gingival crevicular fluid between Han and Uygur populations
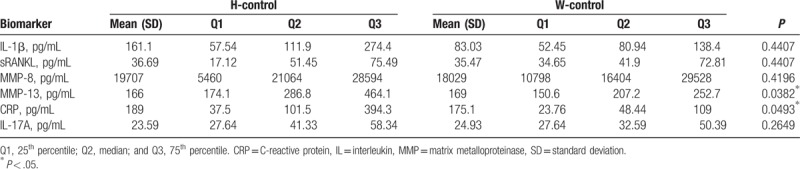
The cytokine contents in the gingival crevicular fluid were investigated and compared between the Han and Uygur populations, with healthy implants and peri-implantitis, respectively. For the subjects with healthy implants, our results showed that the MMP-13 level in the U-control group (169 pg/ml) was significantly higher than the H-control group (166 pg/ml) group, whereas the CRP level in the H-control group (189 pg/mL) was significantly higher than the U-control group (175.1 pg/mL). However, no significant differences were observed in the IL-1β (161.1 vs. 83.03 pg/mL), soluble receptor activator of nuclear factor-kappa B ligand (36.69 vs. 35.47 pg/mL), MMP-8 (19707 vs. 18029 pg/mL), or IL-17A (23.59 vs. 24.93 pg/mL), between the H-control and U-control groups, respectively (Table 2). These results suggest that higher levels of MMP-13 are observed in the gingival crevicular fluid for the Uygur subjects with healthy implants, whereas higher levels of CRP are observed for the Han subjects with healthy implants.
Table 2.
Analysis of Han and Uygur subjects with healthy implants.
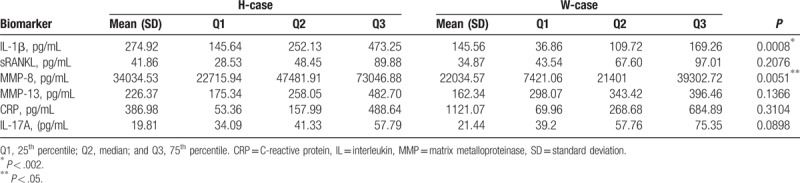
On the contrary, for the subjects with peri-implantitis, our results showed that higher levels of IL-1β (274.9 pg/ml) and MMP-8 (34035 pg/mL) were observed in the H-case group compared with the U-case group (145.6 pg/mL IL-1β, and 22035 pg/mL MMP-8) (both P < .01), whereas the IL-17A level in the U-case group (21.44 pg/mL) was significantly higher than the H-case group (19.81 pg/mL) (Table 3). These results suggest that higher levels of IL-1β and MMP-8 are observed in the gingival crevicular fluid for the Han subjects with peri-implantitis, whereas higher levels of IL-17A are observed for the Uygur subjects with peri-implantitis.
Table 3.
Analysis of Han and Uygur subjects with peri-implantitis.
3.3. Analysis of dominant subgingival bacteria of Han and Uygur populations
The dominant subgingival bacteria were next investigated and compared between the Han and Uygur populations, with healthy implants and peri-implantitis, respectively. For the subjects with healthy implants, as shown in Figure 1, the dominant subgingival bacteria (>1%) in the H-control group mainly included neisseria (11.807%), Prevotella (8.015%), Streptococcus (6.596%), Clostridium (5.609%), Hemophilus (4.155%), Treponema (3.514%), Porphyromonas (3.187%), Vibrio (2.831%), Leptothrix (2.250%), and Actinobacillus actinomycetem comitans (1.837%). The dominant subgingival bacteria species (>1%) in the U-control group mainly included Neisseria (8.208%), Hemophilus (7.943%), Streptococcus (7.475%), Vibrio (6.732%), Prevotella (4.399%), Clostridium (3.600%), Porphyromonas (3.453%), Actinobacillus actinomycetem comitans (2.576%), Leptothrix (2.070%), and Treponema (1.981%). These results suggest that no significant difference is observed in the dominant subgingival bacteria species between the Han and Uygur subjects with healthy implants.
Figure 1.
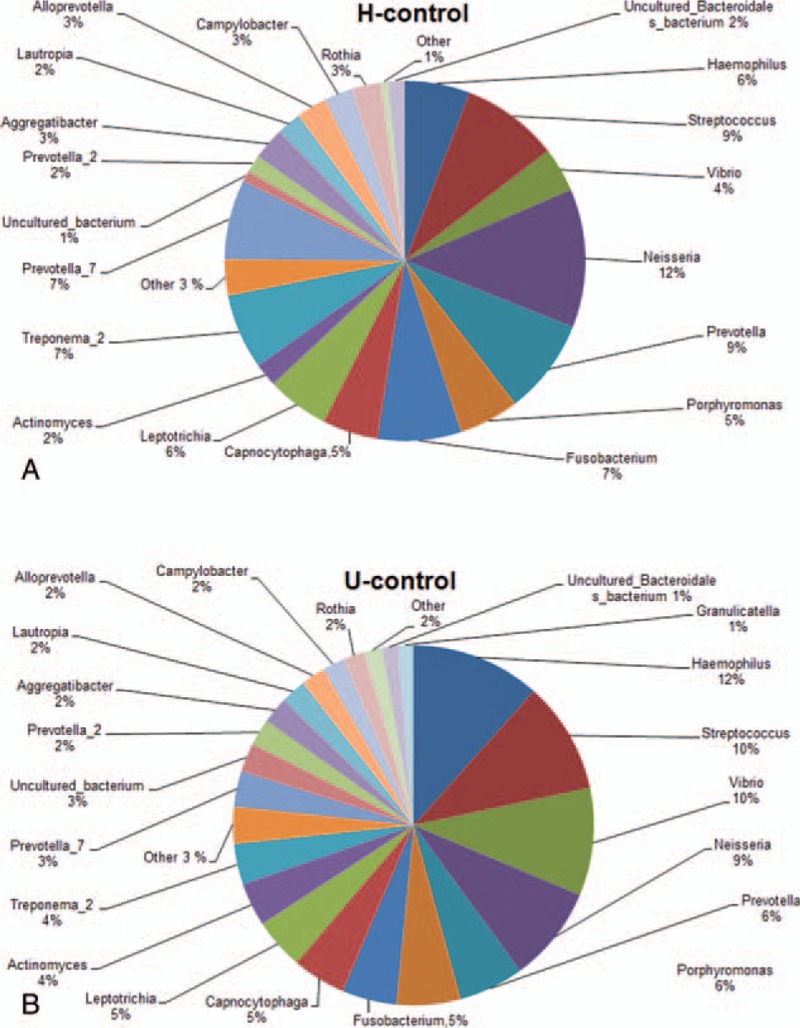
Relative bacterial abundance in the gingival crevicular fluid of the Han and Uygur subjects with healthy implants. (A) Han subjects with healthy implants (H-control group). (B) Uygur subjects with healthy implants (U-control group).
On the contrary, for the subjects with peri-implantitis, as shown in Figure 2, the dominant subgingival bacteria (> 1%) in the H-case group mainly included Prevotella (10.625%), Clostridium (8.467%), Porphyromonas (6.763%), treponema (5.345%), Streptococcus (4.824%), Neisseria (3.594%), Hemophilus (3.284%), Rothia (3.007%), Leptothrix (2.033%), and Campylobacter (1.790%). The dominant subgingival bacteria (>1%) in the U-case group mainly included Streptococcus (8.007%), Clostridium (6.207%), Acinetobacter (5.911%), Neisseria (5.458%), Porphyromonas (5.345%), Prevotella (4.210%), Treponema (3.534%), Micrococcus (3.023%), Moraxella (2.100%), and Hemophilus (1.841%). These results suggest that significant differences are observed in the following dominant subgingival bacteria species between the Han and Uygur subjects with peri-implantitis: Rothia, Leptothrix, Campylobacter, Acinetobacter, Micrococcus, and Moraxella.
Figure 2.
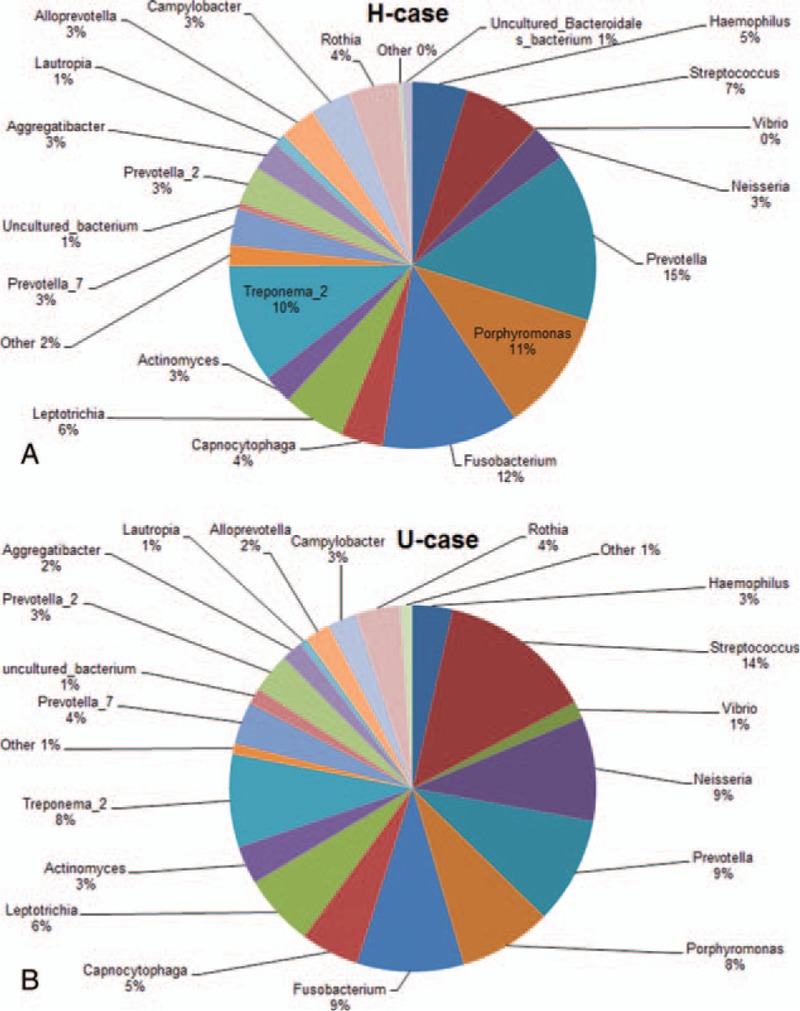
Relative bacterial abundance in the gingival crevicular fluid of the Han and Uygur subjects with peri-implantitis. (A) Han subjects with healthy implants (H-case group). (B) Uygur subjects with healthy implants (U-case group).
3.4. Correlation analysis between cytokines and dominant subgingival bacteria of Han and Uygur populations
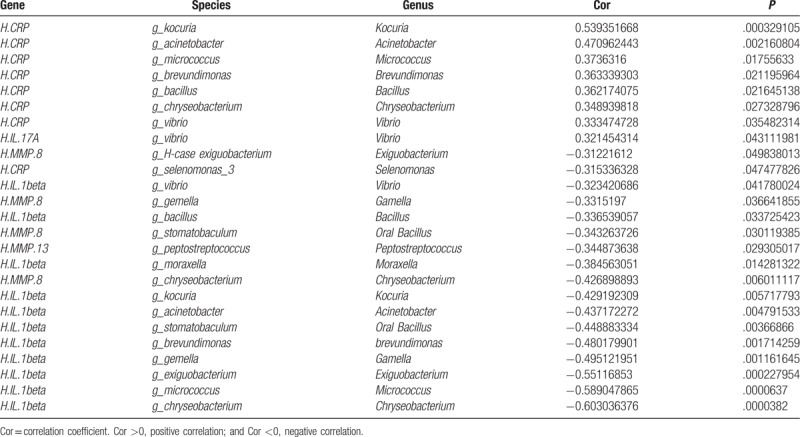
According to the cytokine measurement and dominant subgingival bacteria detection results, correlation analysis was performed (correlation coefficient greater than 0.3 was considered to be correlated). As shown in Table 4, for the subjects with healthy implants, the MMP-13 level was negatively correlated with the Peptostreptococcus, while the CRP level was positively correlated with the Kocuria, Acinetobacter, Micrococcus, Brevundimonas, Bacillus, Chryseobacterium, and Vibrio, respectively. However, for the subjects with peri-implantitis, the MMP-8 level was negatively correlated with the Exiguobacterium, Gamella, Oral Bacillus, and Chryseobacterium, respectively, and the IL-1β level was negatively correlated with the Vibrio, Bacillus, Moraxella, Kocuria, Acinetobacter, Oral Bacillus, brevundimonas, Gamella, Exiguobacterium, Micrococcus, and Chryseobacterium. However, the IL-17A level was positively correlated with the Vibrio. Taken together, these results suggest that, compared with the Han subjects with peri-implantitis, the IL-1β level is negatively correlated with the Moraxella, Acinetobacter, and Micrococcus, respectively, for the Uygur subjects with peri-implantitis.
Table 4.
Correlation analysis.
4. Discussion
In the process of human evolution, owing to genetic drift in populations of different races and regions, the genetic structure has been gradually changing, leading to differential gene frequency distribution and different susceptibility genes.[19] Currently, majority of the studies concerning the peri-implantitis have been focusing on the Caucasian race. Xinjiang, China, is one of the most typical regions of multi-ethnic settlement, among which the Han and Uygur populations account for a considerable proportion. Zhao et al[20] have shown that the Uygur people is the descendants of Turks, with both the descent from white people and the genetic characteristics from the Oriental Mongolian, whereas the Han population belongs to the Mongolian race. In the present study, our results showed that there were some differences in the gene expression associated with peri-implantitis between the 2 populations with different race origins.
MMP-13 has been shown to be present in the tissues with periapical disease,[21] which is generally less expressed in normal adult tissues.[18] Recker et al[4] have shown that there is no significant difference in the CRP contents in the gingival crevicular fluid between from the implants and natural teeth. It has been shown that MMP-13 and CRP are present in the normal oral tissues. In this study, our results showed that the contents of MMP-13 and CRP were among the normal ranges in the healthy implants, whereas they were differentially expressed between the Han and Uygur populations. Significant higher level of MMP-13 was observed in the gingival crevicular fluid for the Uygur subjects with healthy implants, whereas CRP was highly expressed in the Han subjects with healthy implants. However, Siamak et al[22] have found that the incidence of peri-implantitis is increased along with the increasing IL-β content in the gingival crevicular fluid. Moreover, Christoph et al[23] have shown that the elevated contents of IL-β and MMP-8 are closely related to the pathogenesis of periodontitis and peri-implantitis. Emilia et al[24] have demonstrated that the increased level of MMP-8 in the gingival crevicular fluid is closely associated with the development of peri-implantitis. Furthermore, Arakawa et al[25] have shown that MMP-8 is the most important collagenase in the gingival crevicular fluid in the active phase of bone destruction. Mahdi et al[26] have shown that, compared with the subjects with healthy implants, the serum IL-17 levels were significantly higher for the patients with peri-implantitis or chronic periodontal diseases. Severino et al[27] have suggested that IL-17 is closely associated with the pathogenesis of peripheral mucositis and peri-implantitis. These findings are in line with our results. Compare with the Han and Uygur subjects with healthy implants, the contents of IL-β, MMP-8, and L-17 in the gingival crevicular fluid were significantly higher than the patients with peri-implantitis. Moreover, our results showed that, compared with the U-case group, the IL-1β and MMP8 level were significantly higher in the H-case group, whereas the IL-17A level in the U-case group was significantly higher than the H-case group. These results suggest that these three cytokines are differentially expressed between these 2 populations. Furthermore, our results showed no significant difference in the dominant subgingival bacteria between the H-control and U-control groups. However, the H-case and U-case groups shared the following dominant subgingival bacteria: Prevotella, clostridium, Porphyromonas, treponema, Streptococcus, neisseria, and hemophilus. These findings were in line with the common pathogenic bacteria for peri-implantitis.[28] In addition to this, the following specific dominant subgingival bacteria were observed for the U-case group: Acinetobacter, Micrococcus, and Moraxella. In these specific dominant subgingival bacteria species, Acinetobacter is a kind of Gram-negative bacteria which is usually found in the human tissues such as skid, respiratory tract, digestive tract, and genitourinary tract. It can be a conditional pathogen for those with low immunity and has certain resistance to immunosuppression. Acinetobacter has been commonly reported in the respiratory tract infection, but rarely seen in the pathogenesis of peri-implantitis. Micrococcus is a kind of Gram-positive cocci commonly parasitic in the human skin and throat, which is conditional pathogen usually observed in the open fracture infection in clinic.[29] The Gram-negative Moraxella induces infantile ear infection, which is also the main cause of heart disease in children. In recent years, Moraxella has been reported as pathogen for child caries. These bacteria species have been rarely seen in the reports of peri-implantitis. Further in-depth studies are still needed to investigate their roles in the pathogenesis of peri-implantitis in the Uygur population.
Correlation analysis between cytokines and dominant subgingival bacteria of Han and Uygur populations showed that, compared with the H-case group, the IL-1β level is negatively correlated with the Moraxella, Acinetobacter, and Micrococcus in the U-case group. These results suggest that along with the increasing IL-1β in the gingival crevicular fluid, the growth of these correlated bacteria species would be inhibited. Further in-depth studies are still needed to clarify the related mechanisms.
In conclusion, our results showed that the Han and Uygur populations with healthy implants and peri-implantitis had differentially expressed cytokines in the gingival crevicular fluid. Moreover, dominant subgingival bacteria differed between the Han and Uygur populations with peri-implantitis. Compared with the Han subjects with peri-implantitis, the IL-1β levels were negatively correlated with Acinetobacter, Micrococcus, and Moraxella in the Uygur subjects with peri-implantitis. These findings might contribute to the understanding of the pathogenesis of peri-implantitis and the disease treatment in the Uygur population in clinic.
Author contributions
Data curation: Lin Wang.
Formal analysis: Jing Zhou.
Investigation: Yue Sun.
Methodology: Xiaowei Gao.
Software: Xiaowei Gao.
Supervision: Yanmin Zhou.
Writing – original draft: Xiaowei Gao.
Writing – review & editing: Yanmin Zhou.
Footnotes
Abbreviations: BOP = bleeding of probing, CRP = C-reactive protein, IL = interleukin, MMPs = matrix metalloproteinases, PPD = periodontal pocket depth.
This work was supported by the National Natural Science Foundation of China (grant number 81570983).
Author contributions: L.W. contributed in data curation; J.Z. contributed in formal analysis; Y.S. contributed in investigation; X.G. contributed in Methodology and software, and in writing of the original draft; Y.Z. contributed in supervision and writing-review and editing.
The authors report no conflicts of interest.
References
- [1].Mir-Mari J, Mir-Orfila P, Figueiredo R, et al. Prevalence of peri-implant diseases. A cross-sectional study based on a private practice environment. J Clin Periodontol 2012;39:490–4. [DOI] [PubMed] [Google Scholar]
- [2].Syndergaard B, Al-Sabbagh M, Kryscio RJ, et al. Salivary biomarkers associated with gingivitis and response to therapy. J Periodontol 2014;85:e295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pavankumar A, Jagdishreddy G, Raja Babu P. Biomarkers in periodontal disease. J Mol Biomark Diagn 2015;6:232. [Google Scholar]
- [4].Recker EN, Avila-Ortiz G, Fischer CL, et al. A cross-sectional assessment of biomarker levels around implants versus natural teeth in periodontal maintenance patients. J Periodontol 2015;86:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Malik N, Naik D, Uppoor A. Levels of myeloperoxidase and alkaline phosphatase in periimplant sulcus fluid in health and disease and after nonsurgical therapy. Implant Dent 2015;24:434–40. [DOI] [PubMed] [Google Scholar]
- [6].Wei M, Yu N. Significance of detection of MMP-9 and MMP-2 in the implant gingival crevicular fluid. Chinese Medical Journal of Metallurgical Industry 2015;32:298–9. [Google Scholar]
- [7].Farhan D, Royana S. Myeloperoxidase level around dental implants as anindicator of an inflammatory process. Indian J Dent 2015;6:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xie Q, Liu Z. Relationship between C-reactive protein concentration and surrounding inflammation in gingival crevicular fluid around implant. Guangdong Med J 2015;36:2172–3. [Google Scholar]
- [9].Yaghobee S, Khorsand A, Rasouli Ghohroudi AA, et al. Assessment of interleukin-1beta and interleukin-6 in the crevicular fluid around healthy implants, implants with peri-implantitis, and healthy teeth: a cross-sectional study. J Korean Assoc Oral Maxillofac Surg 2014;40:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fonseca FJ, Moraes Junior M, Lourenco EJ, et al. Cytokines expression in saliva and peri-implant crevicular fluid of patients with peri-implant disease. Clin Oral Implants Res 2014;25:e68–72. [DOI] [PubMed] [Google Scholar]
- [11].Faot F, Nascimento GG, Bielemann AM, et al. Can peri-implant crevicular fluid assist in the diagnosis of peri-implantitis? A systematic review and meta-analysis. J Periodontol 2015;86:631–45. [DOI] [PubMed] [Google Scholar]
- [12].Basegmez C, Yalcin S, Yalcin F, et al. Evaluation of periimplant crevicular fluid prostaglandin E2 and matrix metalloproteinase-8 levels from health to periimplant disease status: a prospective study. Implant Dent 2012;21:306–10. [DOI] [PubMed] [Google Scholar]
- [13].Rakic M, Petkovic-Curcin A, Struillou X, et al. CD14 and TNFalpha single nucleotide polymorphisms are candidates for genetic biomarkers of peri-implantitis. Clin Oral Invest 2015;19:791–801. [DOI] [PubMed] [Google Scholar]
- [14].Rakic M, Lekovic V, Nikolic-Jakoba N, et al. Bone loss biomarkers associated with peri-implantitis. A cross-sectional study. Clin Oral Implants Res 2013;24:1110–6. [DOI] [PubMed] [Google Scholar]
- [15].Kadkhodazadeh M, Ebadian AR, Gholami GA, et al. Analysis of RANKL gene polymorphism (rs9533156 and rs2277438) in Iranian patients with chronic periodontitis and periimplantitis. Arch Oral Biol 2013;58:530–6. [DOI] [PubMed] [Google Scholar]
- [16].Duarte PM, Serrao CR, Miranda TS, et al. Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J Periodontal Res 2016;51:689–98. [DOI] [PubMed] [Google Scholar]
- [17].Persson GR, Renvert S. Cluster of bacteria associated with peri-implantitis. Clin Implant Dent Relat Res 2014;16:783–93. [DOI] [PubMed] [Google Scholar]
- [18].Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol 2002;37:149–66. [DOI] [PubMed] [Google Scholar]
- [19].Yuan Y, Zhao R. A preliminary study on the genetic distance between seventeen ethnic groups in China. J Genet Genom 1983;10:398–405. [Google Scholar]
- [20].Zhao T. The mystery of Rh gene structure. Chin J Blood Transfus 1998;11:216–7. [Google Scholar]
- [21].Andrade ALDLD, Santos EDM, Freitas RDA, et al. PP-immunoexpression of MMP-13 and analysis of tryptase-positive cells in chronic periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2017;123:e69–70. [Google Scholar]
- [22].Yaghobee S, Khorsand A, Paknejad M. Comparison of interleukin-1beta levels in gingival crevicular fluid and peri-implant crevicular fluid and its relationship with clinical indexes. J Dent (Tehran) 2013;10:1–9. [PMC free article] [PubMed] [Google Scholar]
- [23].Ramseier CA, Eick S, Bronnimann C, et al. Host-derived biomarkers at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin Oral Implants Res 2016;27:211–7. [DOI] [PubMed] [Google Scholar]
- [24].Janska E, Mohr B, Wahl G. Correlation between peri-implant sulcular fluid rate and expression of collagenase2 (MMP8). Clin Oral Investig 2016;20:261–6. [DOI] [PubMed] [Google Scholar]
- [25].Arakawa H, Uehara J, Hara ES, et al. Matrix metalloproteinase-8 is the major potential collagenase in active peri-implantitis. J prosthodont Res 2012;56:249–55. [DOI] [PubMed] [Google Scholar]
- [26].Kadkhodazadeh M, Baghani Z, Ebadian AR, et al. IL-17 gene polymorphism is associated with chronic periodontitis and peri-implantitis in Iranian patients: a cross-sectional study. Immunol Invest 2013;42:156–63. [DOI] [PubMed] [Google Scholar]
- [27].Severino VO, Beghini M, de Araujo MF, et al. Expression of IL-6, IL-10, IL-17 and IL-33 in the peri-implant crevicular fluid of patients with peri-implant mucositis and peri-implantitis. Arch Oral Biol 2016;72:194–9. [DOI] [PubMed] [Google Scholar]
- [28].Kumar PS, Mason MR, Brooker MR, et al. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol 2012;39:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li J. Advances in Micrococcus classification. Clinical Biochemistry and Laboratory Medicine, Foreign Medical Sciences 2004;1:39–41. [Google Scholar]


