Abstract
Background
Single-walled carbon nanotubes (SWCNTs) have many potential applications in various fields. Especially, the unique physicochemical properties make them as the prime candidates for applications in biomedical fields. However, biocompatibility of SWCNTs has been a major concern for their applications. In the study, biocompatibility of oxidized SWCNTs (O-SWCNTs) was assessed using Saccharomyces cerevisiae (S. cerevisiae) as a model organism.
Results
Cell proliferation and viability were significantly changed after exposure to O-SWCNTs (188.2 and 376.4 mg/L) for 24 h. O-SWCNTs were internalized in cells and distributed in cytoplasm, vesicles, lysosomes and cell nucleus. The average O-SWCNTs contents in S. cerevisiae were ranged from 0.18 to 4.82 mg/g during the exposure from 0 to 24 h, and the maximum content was reached at 18 h after exposure. Both penetration and endocytosis were involved in the internalization of O-SWCNTs in S. cerevisiae, and endocytosis was the main pathway. Cellular structures and morphology were changed after exposure to O-SWCNTs, such as undulating appearance at the membrane, shrinking of the cytosol, increased numbers of lipid droplets and disruption of vacuoles. ROS and antioxidant enzymes activities were observably changed following exposure. For the treatment at 376.4 mg/L, 20.8% of the total cells was undergone apoptosis. Decrease of mitochondrial transmembrane potential and leakage of cytochrome c from mitochondria were observed after exposure. Moreover, expression levels of apoptosis-related genes were significantly increased.
Conclusions
O-SWCNTs can internalize in S. cerevisiae cells via direct penetration and endocytosis, and distribute in cytoplasm, vesicles, lysosomes and cell nucleus. Besides, O-SWCNTs (188.2 and 376.4 mg/L) can induce apoptosis in S. cerevisiae cells, and oxidative stress is involved in activation of the mitochondria-dependent apoptotic pathway.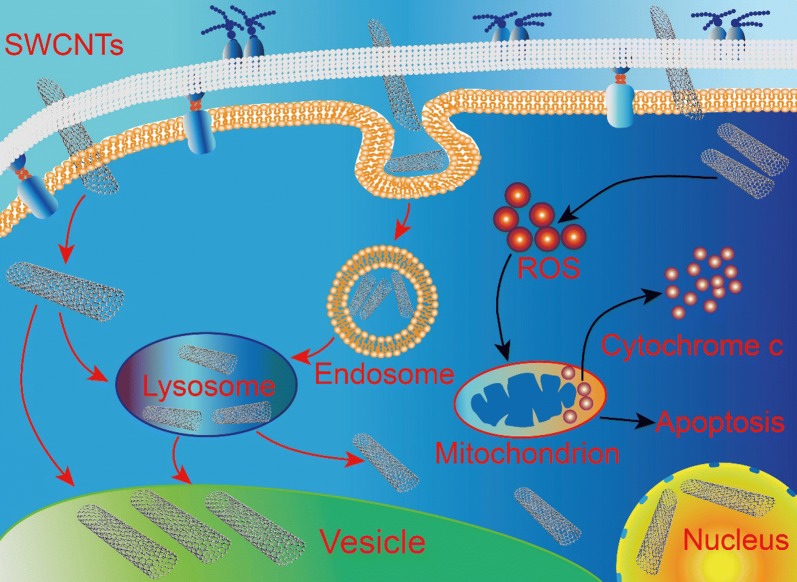
Keywords: Carbon nanotubes, Yeast, Uptake, Oxidative stress, Apoptosis
Background
Single-walled carbon nanotubes (SWCNTs) are important nanomaterials that have potential to be used for a variety of applications in many fields [10]. Especially, they have the potential to revolutionize biomedical research. Merging of SWCNTs with medicine presents an unprecedented opportunity for developing novel materials that can improve treatment and diagnosis of diseases. Several highly promising applications have been reported in some studies, such as drug delivery, gene therapy, growth substrates, tissue scaffolds and biological imaging [2, 3, 17]. When considering their biomedical applications, SWCNTs are expected to have an intrinsically low systemic toxicity [18]. Therefore, the biological, environmental and safe profiles of SWCNTs need to be thoroughly characterized and understood before their broadly used.
A growing number of studies have assessed the potential toxicity of SWCNTs to a number of cell types and organisms [12]. Some studies reported that SWCNTs can internalize in cells and induce cell apoptosis/necrosis. Besides, one of the most common SWCNTs mechanisms that lead to cytotoxicity is related to oxidative stress that can cause mitochondrial impairment and apoptosis [9, 30, 36]. The uptake pathways and intracellular distribution of SWCNTs have also been investigated in previous studies. Data so far supported that endocytosis is the major uptake pathway of SWCNTs in mammalian cells, and SWCNTs are distributed in cytoplasm, lysosomes, mitochondria and cell nucleus [33]. The complexity of SWCNTs toxicity is attributed to various parameters, such as purity, length, agglomeration and surface functionalization of SWCNTs, especially, different types of cells and organisms were used in the toxicity studies [33, 41]. Owing to their lightweight characteristics, SWCNTs are ubiquitously distributed in the environment, including air, water and soil. Various kinds of organisms that active in the environment are at risk of SWCNTs. Therefore, representative organisms are necessary to systematic study the uptake, distribution, accumulation and biocompatibility of SWCNTs.
Saccharomyces cerevisiae (S. cerevisiae) is one of the most intensively used unicellular eukaryotic model organisms in molecular and cell biology studies. Its cellular structure and functional organization share much similarity with cells of plants and animals, furthermore, it also has a short generation time and can be easily cultured like bacteria. Therefore, toxicity studies using S. cerevisiae as a model organism can provide clues to understand nanotoxicity in higher organisms and bacteria [14, 20, 34]. S. cerevisiae is a widely used model organism for the study of oxidative stress and apoptosis [7], and its genome was sequenced [15]. Thus lots of data and genes are available for mechanistic studies. It has been widely used as a model organism in the toxicological studies of nanomaterials, such as TiO2, ZnO, CuO, Al2O3, Mn2O3, CeO2 and SiO2 nanoparticles [14, 20, 34]. In addition, we have investigated the effects of multi-walled carbon nanotubes (MWCNTs), graphene oxide (GO) and α-Fe2O3 nanoparticles on S. cerevisiae [45–47]. Results showed that S. cerevisiae is a suitable model organism for toxicological studies of nanomaterials.
In the study, biocompatibility of oxidized SWCNTs (O-SWCNTs) was assessed using S. cerevisiae as a model organism. Based on previous studies, we hypothesized that (1) O-SWCNTs would be internalized and well distributed in S. cerevisiae; (2) both penetration and endocytosis would involve in the internalization of O-SWCNTs in S. cerevisiae; (3) O-SWCNTs would induce apoptosis in S. cerevisiae cells; and (4) oxidative stress would involve in activation of the mitochondria-dependent apoptotic pathway. The study contributes to a better understanding of the O-SWCNTs biocompatibility, and lays foundation for their applications.
Methods
Characterization of O-SWCNTs
SWCNTs (Chengdu Organic Chemicals Co., Ltd., China) were purified and oxidized according to our previous study [44]. O-SWCNTs (600 mg) were weighed on aluminum foil and dispersed in 1 L YPD medium (1% yeast extract, 2% peptone and 2% glucose). The resulting suspension was sonicated for 2 h at 100 W with a 50% on/off cycle. Following the sonication, the suspension was centrifuged at 12,000g for 2 h. The supernatant was collected, and concentration of O-SWCNTs was determined by Raman spectrophotometer. Raman spectrophotometer is a useful tool to quantify CNTs due to the normalised G-band areas show linear concentration behaviour in accordance with Beer’s law [4, 16, 39]. For the quantitative analysis, a standard curve between the concentrations of O-SWCNTs and the normalised G-band areas was established. Then, the supernatant was pipetted into a square quartz groove (side length: 5 mm, height: 1 mm) and dried in vacuum at 80 °C. Contents of O-SWCNTs were measured using the Raman spectrophotometer with an excitation wavelength of 785 nm, and calculated by the standard curve. The supernatant was diluted to create suspensions with a series of concentrations by double dilution method. All suspensions were sterilized and sonicated again before used. For SWCNTs characterization, both SEM (Hitachi S-4800, Japan) and TEM (JEM1200EX, Japan) were used to observe the shape of O-SWCNTs. The purity, chemical states and elemental compositions of pristine SWCNTs (P-SWCNTs) and O-SWCNTs were analyzed using Raman spectrophotometer (Longjumeau Cedex, France) and XPS (PHI-5600, Russia).
Cell proliferation and viability
Saccharomyces cerevisiae was cultivated in O-SWCNTs suspensions with constant shaking (160 rpm) at 30 °C, and the inoculation quantity was approximately 1 × 105 cells/mL. For proliferation assays, cells were counted at 0, 3, 6, 9, 12, 15, 18, 21 and 24 h with a hematocytometer under an optical microscope (Olympus Optical Co., Ltd., Tokyo, Japan). To check the effect of O-SWCNTs on cell viability, cells were collected following exposure for 24 h and stained with 1 mg/mL Trypan Blue (Sigma, USA) for 3–5 min. The stained cells and total cells were counted under the microscope, and mortality rate was calculated as a ratio between stained cells and total cells. All measurements were carried out eight times.
Internalization, distribution and uptake kinetics of O-SWCNTs in S. cerevisiae
Internalization and distribution of O-SWCNTs in S. cerevisiae were observed using the TEM according to Bayat et al. [6]. In order to measure the uptake kinetics, contents of O-SWCNTs in S. cerevisiae were quantitatively assessed after exposure for 0, 3, 6, 9, 12, 15, 18, 21 and 24 h. Dry weight of S. cerevisiae cells was measured before the quantitative assessment. Briefly, cells treated without O-SWCNTs were collected and counted under the microscope. Then, the cells were dried using a freeze dryer (FD5-3, GOLD-SIM) and weighted using a balance (Sartorius Stedim Biotech GmbH, Germany). After that, the average weight of a single cell was calculated. The O-SWCNTs contents were measured based on the dry weight of S. cerevisiae, reflecting the total cell burden across the exposure from 0 to 24 h. For the measurement, cells were collected using density gradient centrifugation [47] at each time point, and then counted under the microscope. The cells were pipetted into a square quartz groove (side length: 5 mm; height: 1 mm), and then dried in vacuum at 80 °C. Contents of O-SWCNTs were measured using the Raman spectrophotometer with an excitation wavelength of 785 nm, and calculated by the standard curve. All measurements were carried out three times.
Uptake mechanism
To elucidate whether O-SWCNTs enter into S. cerevisiae via endocytosis, cells were exposure to O-SWCNTs suspension in the presence of 5% (v/v) ethanol and at 4 °C, respectively. O-SWCNTs in S. cerevisiae were quantitatively measured using Raman spectrophotometer as described above. Moreover, expression levels of endocytosis-related genes (END3, END6, Sla2 and Rsp5) were measured by Real-Time PCR using ribosomal 18S RNA (18S rRNA) as internal standard. Primers for the genes and cycling conditions were designed as previous studies [47]. Relative expression was obtained by using the 2−∆∆Ct [40] method and normalized to the expression of the internal standard gene 18S rRNA in the same sample. All measurements were carried out three times.
Effects of O-SWCNTs on cellular structure
After exposure for 24 h, cells were collected using density gradient centrifugation and washed with PBS (pH = 7.1). The cells were fixed with 2.5% glutaraldehyde in PBS at 4 °C for 24 h and then adhered onto a piece of glass using polylysine. Afterwards, cells were dehydrated through a graded ethanol series (30–90%, 2 × 100%; 15–20 min each) and replaced using isoamyl acetate. The samples were dried overnight and coated with a thin layer of gold, and observed using the SEM. Moreover, effects of O-SWCNTs on cellular structure were also checked using TEM according to Bayat et al. [6].
ROS and antioxidant enzymes activities
After exposure for 24 h, approximately 1 × 108 cells were collected. The cells were mixed with glass beads (0.3–0.4 mm) and thoroughly ruptured by vigorous vortexing for 5–10 min. After vortexing, the homogenates were centrifuged (12,000 rpm, 4 °C) for 10 min. The supernatants were collected for ROS and antioxidant enzymes (SOD, CAT and GPx) activities measurements. Total protein, ROS, SOD, CAT and GPx activities were measured using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to manufacturer’s instructions, and detected by a microplate reader (Multiskan MK3, Thermo Labsystems Co., Beverly, MA). All measurements were carried out three times.
Apoptosis assay
After exposure for 24 h, approximately 2 × 105 cells were collected from each treatment and stained with annexin-V-FITC (5 μL) and PI (5 μL) following the manufacturer’s instruction. Following the staining, flow cytometry (Beckman Coulter Inc., United States) analysis was immediately conducted. FITC fluorescence (FL1) and PI fluorescence (FL2) of each cell were quantitated using the Cell Quest Pro® software (BD, Germany).
Mitochondrial transmembrane potential
Mitochondrial transmembrane potential (MTP, ∆ψm) was detected using JC-1 (Beyotime Biotech, Nantong, China) as described in previous studies [8, 35]. Briefly, cells were collected following exposure for 24 h. The cells were incubated with JC-1 following the manufacturer’s instruction and analyzed using a microplate reader (Multiskan MK3, Thermo Labsystems Co., Beverly, MA). Ratios between red and green fluorescent were calculated, and results were shown as percentage between the ratios of treatments and that of the control. All measurements were carried out three times.
Cytochrome c leakage from mitochondria
After exposure for 24 h, approximately 1 × 106 cells were collected from each treatment. The cells were ruptured as described above, and supernatants were collected. A small amount of Na2S2O3 was added into the supernatants in order to keep cytochrome c in the reduction state. Absorbance of cytochrome c was measured at 540 nm using a UV–visible spectrophotometer (UV-2200, Shimadzu, Japan). All measurements were carried out three times.
Expression of apoptosis-related genes
Expression levels of apoptosis-related genes (SOD, Yca1, Nma111 and Nuc1) were measured by Real-Time PCR as described above. All measurements were carried out three times.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) and analyzed using SPSS Version 11.0 software package (SPSS Inc., Chicago, IL). Differences between the controls and treatments were analyzed using one-way ANOVA followed by Tukey’s test, where p < 0.05 was considered significant.
Results and discussion
Characterization of SWCNTs
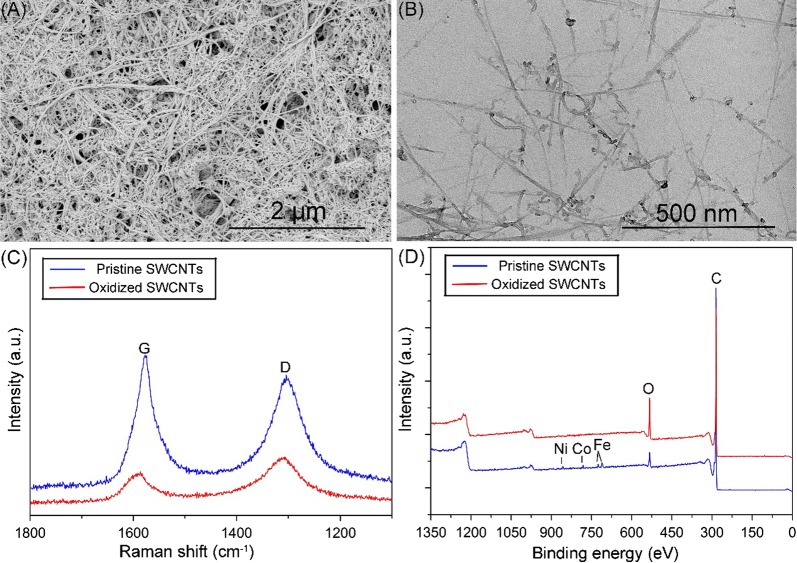
The complexity of SWCNTs toxicity is attributed to various parameters, such as concentration, purity, length and surface functionalization [33]. Therefore, these parameters should be well characterized before the toxicity assessment. In the study, O-SWCNTs suspension was sonicated and centrifuged to acquire well-dispersed supernatant. Concentration of O-SWCNTs in the supernatant was 376.4 mg/L. The supernatant was diluted to create suspensions (23.5, 47.1, 94.1 and 188.2 mg/L) by double dilution method. As shown in SEM (Fig. 1a) and TEM (Fig. 1b) images, O-SWCNTs were fibrous with varying lengths. According to statistical analysis of TEM images, the average length of O-SWCNTs was 263 nm, and shorter than the average length of P-SWCNTs (3.16 μm). As shown in Fig. 1c, typical G (around 1580 cm−1) and D band (around 1303 cm−1) of CNTs [11] were identified from the raman spectra of P- and O-SWCNTs. D/G band intensity ratio (ID/IG) can be used to monitor the defects of CNTs as it is increased with the defects increasing [28, 31]. The ID/IG ratios of P-SWCNTs and O-SWCNTs were 0.82 and 1.32, indicating that defects of SWCNTs were increased following oxidation. Figure 1d shows XPS spectra of P- and O-SWCNTs, indicating SWCNTs surface was consisted of carbon (284 eV) and oxygen (532 eV). Surface oxygen contents for P- and O-SWCNTs were 3.32 and 14.6 atomic %, respectively, suggesting the oxidation was sufficient. There are small amounts of iron [711 (Fe 2p1/2) and 725 (Fe 2p3/2) eV], cobalt (782 eV) and nickel (850 eV) in P-SWCNTs which not found in O-SWCNTs, indicating that O-SWCNTs were free from impurities following oxidation. All the results showed that the oxidation of SWCNTs was sufficient and the impurities were removed.
Fig. 1.
Characterization of SWCNTs. SEM (a) and TEM (b) images of O-SWCNTs. Raman (c) and XPS (d) spectra of P-SWCNTs and O-SWCNTs
Effects of O-SWCNTs on cell proliferation and viability
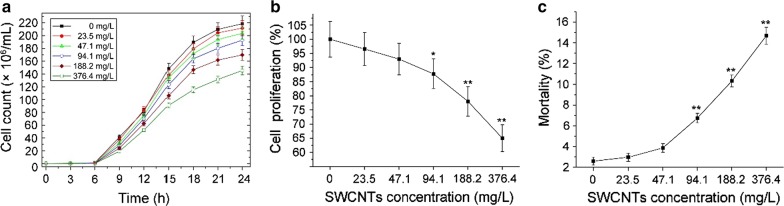
After exposure to O-SWCNTs suspensions, cell proliferation showed a dose-dependent inhibition (Fig. 2a), and was significantly inhibited (p < 0.01) at 188.2 and 376.4 mg/L (Fig. 2b). The cells proliferation at 376.4 mg/L was 64.9% compared with the control after exposure for 24 h. Mortality was notably increased (p < 0.01) at 94.1–376.4 mg/L, and was 14.7% after exposure to 376.4 mg/L for 24 h (Fig. 2c). Effects of MWCNTs on S. cerevisiae have been investigated in our previous study [47]. Proliferation and mortality after exposure to 400 mg/L MWCNTs were 72.2% (compared with the control) and 6.1%, respectively. Therefore, these data indicated that SWCNTs possess a higher toxicity file than MWCNTs.
Fig. 2.
a Growth curves of S. cerevisiae exposed to 0–376.4 mg/L O-SWCNTs suspensions. Effects of O-SWCNTs on cell proliferation (b) and viability (c) after exposure for 24 h. Values are presented as mean ± SD. Values that are significantly different from the control are indicated by asterisks (one-way ANOVA, *p < 0.05; **p < 0.01)
Internalization, distribution and uptake kinetics of O-SWCNTs in S. cerevisiae
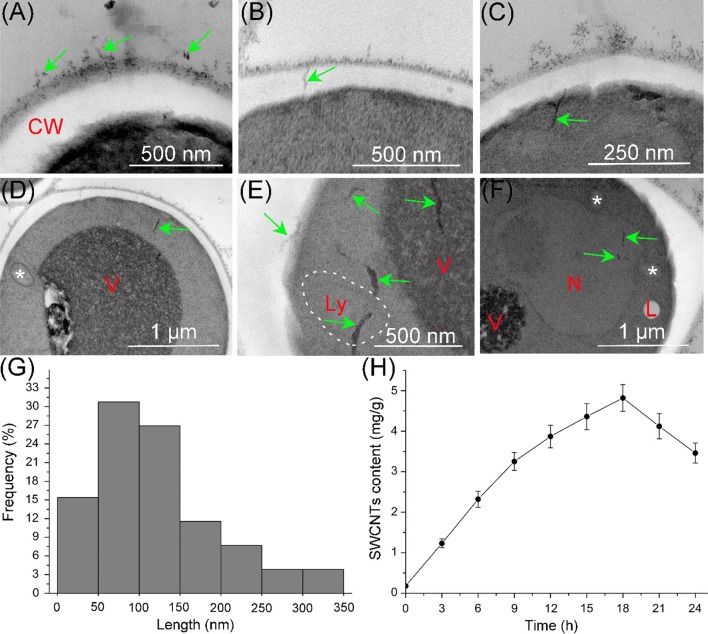
Several studies about the distribution of SWCNTs have been reported and showed that SWCNTs distributed in the cytoplasm, lysosomes, mitochondria, endosome-like vesicles and cell nucleus [33, 37]. The internalization, distribution and uptake kinetics of O-SWCNTs in S. cerevisiae were checked in the study. As shown in Fig. 3a, O-SWCNTs were firstly adsorbed onto the cell wall surface and then penetrated across the cell wall (Fig. 3b) and membrane (Fig. 3c). Subsequently, O-SWCNTs entered into the cells and distributed in cytoplasm (Fig. 3d, e), vesicles (Fig. 3e), lysosomes (Fig. 3e) and cell nucleus (Fig. 3f). These results were similar to that reported by Porter et al. [37], who demonstrated that SWCNTs distributed in cytoplasm, lysosomes, endosome-like vesicles and cell nucleus of human monocyte-derived macrophages [37]. We investigated the distribution of MWCNTs in S. cerevisiae, and showed that MWCNTs were distributed in the perinuclear region without entering into cell nucleus [47]. The difference may be due to that SWCNTs have a smaller diameter and stronger penetrability than MWCNTs.
Fig. 3.
Internalization, distribution and uptake kinetics of O-SWCNTs (green arrows) in S. cerevisiae. O-SWCNTs were firstly adsorbed onto the cell wall surface (a) and then penetrated across the cell wall (b) and membrane (c). O-SWCNTs entered into the cells and distributed in cytoplasm (d), vesicles and lysosomes (e), and cell nucleus (f). g Length distribution of O-SWCNTs in S. cerevisiae cells. h Uptake kinetics of O-SWCNTs in S. cerevisiae cells. Values are presented as mean ± SD. CW cell wall, V vesicle, Ly lysosome, N nucleus, L lipid droplet, *mitochondrion
As shown in Fig. 3g, the average length of SWCNTs in cells was around 110 nm, which was shorter than the SWCNTs (263 nm). The result indicated that shorter SWCNTs are more easily enter into cells. The uptake kinetics of O-SWCNTs in S. cerevisiae was shown in Fig. 3h. The average O-SWCNTs contents were ranged from 0.18 to 4.82 mg/g, and the maximum content was reached at 18 h after exposure. A decrease was observed from 18 to 24 h probably due to the discharge of O-SWCNTs. Uptake kinetics of MWCNTs in S. cerevisiae was investigated in previous study. Result showed that the maximum MWCNTs content was reached at 3 h after exposure [47], indicating that SWCNTs presented a slower uptake compared with MWCNTs.
Uptake mechanism
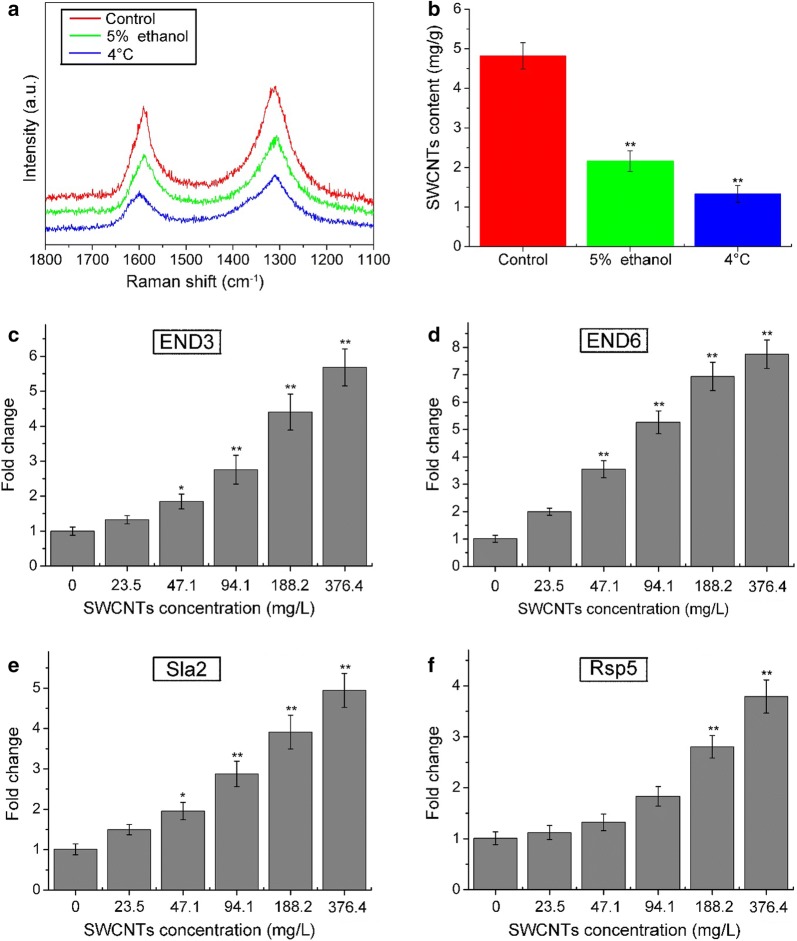
Several findings suggested that endocytosis is the major uptake pathway of SWCNTs in mammalian cells [19, 33]. To investigate whether endocytosis occurs for the internalized O-SWCNTs in S. cerevisiae cells, we studied the effect of endocytosis inhibiting conditions (5% ethanol [23] and 4 °C [29]) on cellular uptake. As shown in Fig. 4a, b, the contents of O-SWCNTs in cells were significantly decreased after incubation with 5% ethanol (2.16 mg/g) and at 4 °C (1.33 mg/g) compared with the control (4.82 mg/g). Contents of O-SWCNTs in cells were reduced by 55.2 and 72.4% after incubation with 5% ethanol and at 4 °C compared with the control, respectively. Besides, expression levels of endocytosis-related genes [END3 (Fig. 4c), END6 (Fig. 4d), Sla2 (Fig. 4e) and Rsp5 (Fig. 4f)] were increased after exposure to 0–376.4 mg/L O-SWCNTs suspensions. These data indicated that cellular uptake is predominantly an endocytosis process. Similar result was reported in other studies [27, 33].
Fig. 4.
Internalization of O-SWCNTs in S. cerevisiae cells via endocytosis. Raman spectra (a) and contents (b) of O-SWCNTs uptake by S. cerevisiae cells under different treatment (control, 5% ethanol and 4 °C). Expression levels of endocytosis-related genes [END3 (c), END6 (d), Sla2 (e) and Rsp5 (f)] after exposure to 0–376.4 mg/L O-SWCNTs suspensions. Values are presented as mean ± SD. Values that are significantly different from the control are indicated by asterisks (one-way ANOVA, *p < 0.05; **p < 0.01)
Figure 3 clearly illustrated the penetration and internalization of O-SWCNTs into the cells. Direct penetration through the cell wall and membrane could be designated as one of the routes to deliver the O-SWCNTs into the cytoplasm. It was demonstrated that CNTs could readily permeate through the cell wall, driven by van der Waals forces or hydraulic forces [42]. Based on the combined results of Figs. 3 and 4, it can be concluded that internalization of O-SWCNTs in S. cerevisiae cells via: (1) direct penetration. O-SWCNTs were firstly adsorbed onto the cell wall surface, and then penetrated across the cell wall and membrane. After the penetration, O-SWCNTs distributed in the cytoplasm, vesicles, lysosomes and cell nucleus; (2) endocytosis. O-SWCNTs adsorbed and penetrated across the cell wall. Subsequently, the cell membrane was invaginated and wrapped up the O-SWCNTs. O-SWCNTs entered into the cells within the endosomes. The endosomes merged with lysosomes, and then lysosomes merged with vacuoles. The findings are somewhat similar to the results of previous studies [1, 47].
Effects of O-SWCNTs on cellular structure
One of the CNTs-cytotoxicity mechanisms was proposed to be CNTs-induced cellular structure damages [22, 33]. As shown in Fig. 5a, surface of cells treated without O-SWCNTs was plump and smooth. After exposure to O-SWCNTs, cells were deformed and shrank (Fig. 5b, c). Cell wall of crinkled cell was incrassate, and its thickness was not uniform (Fig. 5d). Other damages, such as increased numbers of lipid droplets (Fig. 5e) and disruption of vacuoles (Fig. 5f) were also observed. Our findings are similar to the results reported by Bayat et al. [6], who investigated the effects of engineered nanoparticles on the cellular structure of S. cerevisiae. They demonstrated that increase in the number of lipid droplets, disruption or lack of vacuoles, shrinking of the cytosol and undulating appearance at the membrane were visible after exposure to CuO and Ag nanoparticle [6].
Fig. 5.
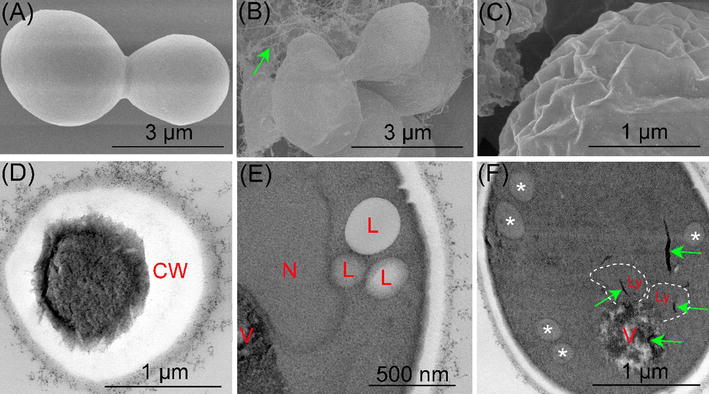
Effects of O-SWCNTs (green arrows) on cellular structure. a Surface of cells treated without O-SWCNTs was plump and smooth. b, c After exposure to O-SWCNTs, cells were deformed and shrank. d Cell wall of crinkled cell was incrassate, and its thickness was not uniform. Increased numbers of lipid droplets (e) and disruption of vacuoles (f) were also observed. CW cell wall, V vesicle, N nucleus, L lipid droplet, Ly lysosome, *mitochondrion
ROS and antioxidant enzymes activities
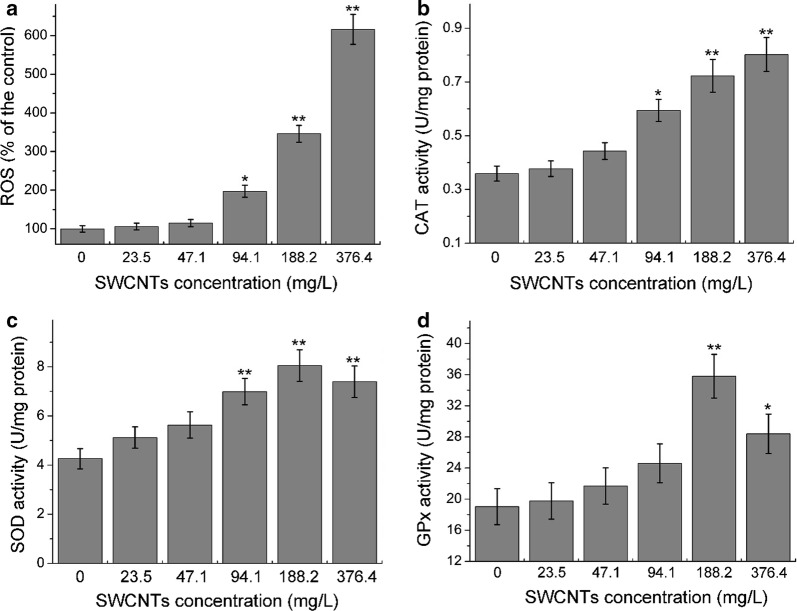
SWCNTs internalized into the cells would induce oxidative stress by producing ROS, which could undoubtedly be one of the factors responsible for the acceleration of cell apoptosis or death. Exposure to SWCNTs has been associated with altered levels of components in the antioxidant defense system, such as CAT, SOD and GPx [9, 25, 30]. Production of ROS and antioxidant enzymes activities were detected in the study. As shown in Fig. 6a, ROS was observably (p < 0.01) increased following exposure to 188.2 and 376.4 mg/L O-SWCNTs compared with the control. CAT activity showed a dose-dependent increase and dramatically increased (p < 0.01) at 188.2 and 376.4 mg/L (Fig. 6b). Interestingly, SOD (Fig. 6c) and GPx (Fig. 6d) activities were firstly increased and follow by decreases. Increases of CAT, SOD and GPx activities may be due to responses to the superoxide, high antioxidant enzymes activities can efficiently degrade superoxide. Decreases of SOD and GPx activities may be due to the depletion of SOD and GPx, or decrease in cell viability. Similar phenomenon was reported in other studies [32, 38]. In general, the data indicated that oxidative stress was induced following exposure to O-SWCNTs.
Fig. 6.
Effects of O-SWCNTs on ROS generation (a), CAT (b), SOD (c) and GPx (d) activities. Values are presented as mean ± SD. Values that are significantly different from the controls are indicated by asterisks (one-way ANOVA, *p < 0.05; **p < 0.01)
Cell apoptosis
Several studies reported that SWCNTs can induce cell apoptosis/necrosis. Besides, one of the most common SWCNTs mechanisms that lead to cytotoxicity is related to oxidative stress that can cause mitochondrial impairment and apoptosis [9, 36]. For example, Cheng et al. [9] investigated the effect of SWCNTs on rat aorta endothelial cells and demonstrated that SWCNTs induced apoptosis in the cells. Moreover, they also showed that ROS generation was involved in activation of the mitochondria-dependent apoptotic pathway [9]. To study whether O-SWCNTs can induce cellular apoptosis/necrosis, Annexin V/PI staining was performed and analyzed by flow cytometry. As shown in Fig. 7, there was no obvious apoptosis following exposure to 0–94.1 mg/L. However, it showed a significant increase (p < 0.01) after exposure to 188.2 (11.5%) and 376.4 (20.8%) mg/L for 24 h compared with the control. The data indicated that the increase of mortality was related to apoptosis.
Fig. 7.
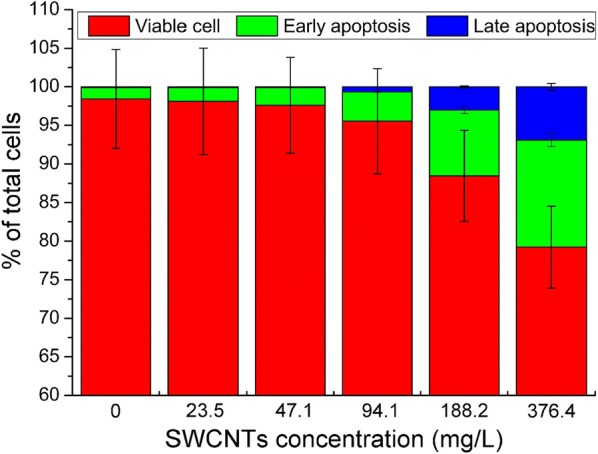
Flow cytometric analysis of apoptosis using Annexin V/PI staining. The annexin V-FITC−/PI−, annexin V-FITC+/PI− and annexin V-FITC+/PI+ populations were regarded as viable cells, early apoptosis and late apoptosis, respectively. Values are presented as mean ± SD
Mitochondrial transmembrane potential
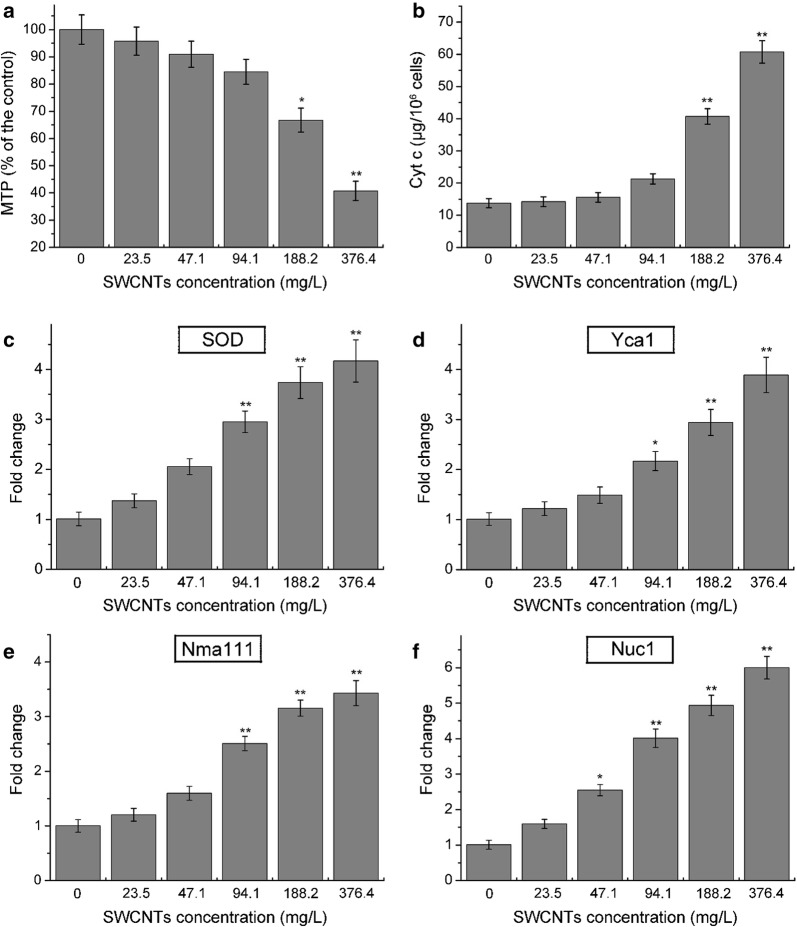
Mitochondria have been suggested to play a key role in the apoptotic process. Their control of apoptosis has been described as: (1) maintenance of ATP production; (2) MTP and mitochondrial membrane permeability for the release of certain apoptogenic factors from the intermembrane space into the cytosol [24]. Reduction in MTP has been postulated to be an early and obligate event in the apoptotic signaling pathway [43]. In the study, changes of MTP after exposure to O-SWCNTs suspensions for 24 h were measured using JC-1 staining. As shown in Fig. 8a, MTP was significantly decreased (p < 0.01) at 376.4 mg/L compared with the control, indicating that the apoptosis induced by O-SWCNTs was related to mitochondrial impairment. The result is in accordance with previous studies [9, 36].
Fig. 8.
a Mitochondrial transmembrane potential (MTP) of S. cerevisiae cells was evaluated using JC-1, and measured by microplate reader. b Contents of cytochrome c in cytoplasm after exposure to 0–376.4 mg/L O-SWCNTs. Expressions of SOD (c), Yca1 (d), Nma111 (e) and Nuc1 (f) following exposure to O-SWCNTs (0–376.4 mg/L). Values are presented as mean ± SD. Values that are significantly different from the control are indicated by asterisks (one-way ANOVA, **p < 0.01)
Leakage of cytochrome c from mitochondria
Decrease of MTP is associated with the opening of the mitochondria permeability transition pore. Mitochondria become so permeable that they are unable to maintain a sufficient force to retain cytochrome c (Cyt c), thus, Cyt c will release from the mitochondria into the cytoplasm. Contents of Cyt c in cytoplasm were detected in the study. As shown in Fig. 8b, significant increases in Cyt c were observed compared with the control after exposure to 188.2 and 376.4 mg/L. The result indicated that mitochondrial membrane permeability was increased, and Cyt c was released from mitochondria to cytoplasm following exposure to O-SWCNTs.
Expression of apoptosis-related genes
Superoxide dismutase gene encodes superoxide dismutase, which is a crucial enzyme in removing superoxide anion. It is sensitive to a variety of stress agents due to escalated oxidative stress. Moreover, it also plays a key role in the apoptotic process [21]. Yca1 mediates the apoptotic process, and encodes a yeast protein with structural homology and similar function to mammalian caspases [26]. Nma111 encodes a protein which belongs to the HtrA family of serine proteases, and its pro-apoptotic activity depends on its serine-protease activity [13]. Nuc1 has roles in mitochondrial recombination and apoptosis [5]. Expressions of SOD (Fig. 8c), Yca1 (Fig. 8d), Nma111 (Fig. 8e) and Nuc1 (Fig. 8f) were significantly increased following exposure to O-SWCNTs, indicating that S. cerevisiae cells were undergone apoptosis related to oxidative stress and mitochondrial impairment. The findings were consistent with previous studies [9, 30].
Conclusion
Taken together, results so far indicated that: (1) O-SWCNTs (< 47.1 mg/L) show no obvious effects on S. cerevisiae cells; (2) O-SWCNTs can internalize in S. cerevisiae cells and distribute in cytoplasm, vesicles, lysosomes and cell nucleus; (3) internalization of O-SWCNTs in S. cerevisiae cells via direct penetration and endocytosis; (4) the maximum content of O-SWCNTs in S. cerevisiae cells is reached at 18 h after exposure; (5) O-SWCNTs (188.2 and 376.4 mg/L) induce apoptosis in S. cerevisiae cells, and oxidative stress is involved in activation of the mitochondria-dependent apoptotic pathway.
Authors’ contributions
SZ and FL performed the experimental work. JL contributed to the analysis and representation of data. SZ wrote the manuscript. All authors revised the manuscript. BZ and GXW designed the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Consent for publication
All authors agree to be published.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. U1701233), the Special Funds for Talents in Northwest A&F University to B. Zhu (Program No. Z111021510), the special grade of the financial support from the China Postdoctoral Science Foundation (Program No. 2016T90956) and the Fundamental Research Funds for the Central Universities (Program No. 2452017078).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- SEM
scanning electron microscope
- TEM
transmission electron microscope
- XPS
X-ray photoelectron spectroscopy
- PBS
phosphate buffer solution
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- CAT
catalase
- GPx
glutathione peroxidase
Contributor Information
Song Zhu, Email: 18792830934@163.com.
Fei Luo, Email: luofei1121@126.com.
Jian Li, Email: lj18829352358@163.com.
Bin Zhu, Phone: +86 29 87092102, Email: zhubin1227@126.com.
Gao-Xue Wang, Phone: +86 29 87092102, Email: wanggaoxue@126.com.
References
- 1.Al-Jamal KT, Nerl H, Müller KH, Ali-Boucetta H, Li S, Haynes PD, Jinschek JR, Prato M, Bianco A, Kostarelos K. Cellular uptake mechanisms of functionalised multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale. 2011;3:2627–2635. doi: 10.1039/c1nr10080g. [DOI] [PubMed] [Google Scholar]
- 2.Alshehri R, Ilyas AM, Hasan A, Arnaout A, Ahmed F, Memic A. Carbon nanotubes in biomedical applications: factors, mechanisms and remedies of toxicity. J Med Chem. 2016;59:8149–8167. doi: 10.1021/acs.jmedchem.5b01770. [DOI] [PubMed] [Google Scholar]
- 3.Amenta V, Aschberger K. Carbon nanotubes: potential medical applications and safety concerns. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:371. doi: 10.1002/wnan.1317. [DOI] [PubMed] [Google Scholar]
- 4.Anoshkin IV, Nefedova II, Lioubtchenko DV, Nefedov IS, Räisänen AV. Single walled carbon nanotube quantification method employing the Raman signal intensity. Carbon. 2017;116:547–552. doi: 10.1016/j.carbon.2017.02.019. [DOI] [Google Scholar]
- 5.Büttner S, Eisenberg T, Carmonagutierrez D, Ruli D, Knauer H, Ruckenstuhl C, Sigrist C, Wissing S, Kollroser M, Fröhlich KU. Endonuclease G regulates budding yeast life and death. Mol Cell. 2007;25:233. doi: 10.1016/j.molcel.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Bayat N, Rajapakse K, Marinseklogar R, Drobne D, Cristobal S. The effects of engineered nanoparticles on the cellular structure and growth of Saccharomyces cerevisiae. Nanotoxicology. 2014;8:363–373. doi: 10.3109/17435390.2013.788748. [DOI] [PubMed] [Google Scholar]
- 7.Carmona-Gutierrez D, Eisenberg T, Büttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 2010;17:763–773. doi: 10.1038/cdd.2009.219. [DOI] [PubMed] [Google Scholar]
- 8.Cavalieri E, Rigo A, Bonifacio M, Prati ACD, Guardalben E, Bergamini C, Fato R, Pizzolo G, Suzuki H, Vinante F. Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells. J Transl Med. 2011;9:45. doi: 10.1186/1479-5876-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng WW, Lin ZQ, Wei BF, Zeng Q, Han B, Wei CX, Fan XJ, Hu CL, Liu LH, Huang JH. Single-walled carbon nanotube induction of rat aortic endothelial cell apoptosis: reactive oxygen species are involved in the mitochondrial pathway. Int J Biochem Cell Biol. 2011;43:564. doi: 10.1016/j.biocel.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 10.De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 11.Dresselhaus MS, Dresselhaus G, Saito R, Jorio A. Raman spectroscopy of carbon nanotubes. Phys Rep. 2005;409:47–99. doi: 10.1016/j.physrep.2004.10.006. [DOI] [Google Scholar]
- 12.Ema M, Gamo M, Honda K. A review of toxicity studies of single-walled carbon nanotubes in laboratory animals. Regul Toxicol Pharmacol Rtp. 2016;74:42. doi: 10.1016/j.yrtph.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Fahrenkrog B, Sauder U, Aebi U. The S. cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J Cell Sci. 2004;117:115–126. doi: 10.1242/jcs.00848. [DOI] [PubMed] [Google Scholar]
- 14.Garcíasaucedo C, Field JA, Oterogonzalez L, Sierraálvarez R. Low toxicity of HfO2, SiO2, Al2O3 and CeO2 nanoparticles to the yeast, Saccharomyces cerevisiae. J Hazard Mater. 2011;192:1572–1579. doi: 10.1016/j.jhazmat.2011.06.081. [DOI] [PubMed] [Google Scholar]
- 15.Goffeau A. Four years of post-genomic life with 6000 yeast genes. FEBS Lett. 2000;480:37–41. doi: 10.1016/S0014-5793(00)01775-0. [DOI] [PubMed] [Google Scholar]
- 16.Han A, Lefrant S. A correlated method for quantifying mixed and dispersed carbon nanotubes: analysis of the Raman band intensities and evidence of wavenumber shift. J Raman Spectrosc. 2005;36:400–408. doi: 10.1002/jrs.1305. [DOI] [Google Scholar]
- 17.Heister E, Brunner EW, Dieckmann GR, Jurewicz I, Dalton AB. Are carbon nanotubes a natural solution? Applications in biology and medicine. ACS Appl Mater Interfaces. 2013;5:1870–1891. doi: 10.1021/am302902d. [DOI] [PubMed] [Google Scholar]
- 18.Jafar A, Alshatti Y, Ahmad A. Carbon nanotube toxicity: the smallest biggest debate in medical care. Cogent Med. 2016;3:1–12. doi: 10.1080/2331205X.2016.1217970. [DOI] [Google Scholar]
- 19.Jin H, Heller DA, Strano MS. Single-particle tracking of endocytosis and exocytosis of single-walled carbon nanotubes in NIH-3T3 cells. Nano Lett. 2008;8:1577–1585. doi: 10.1021/nl072969s. [DOI] [PubMed] [Google Scholar]
- 20.Kasemets K, Ivask A, Dubourguier HC, Kahru A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol Vitro. 2009;23:1116. doi: 10.1016/j.tiv.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Kwolek-Mirek M, Zadrąg-Tęcza R, Bednarska S, Bartosz G. Acrolein-induced oxidative stress and cell death exhibiting features of apoptosis in the yeast Saccharomyces cerevisiae deficient in SOD1. Cell Biochem Biophys. 2014;71:1525–1536. doi: 10.1007/s12013-014-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long Z, Ji J, Yang K, Lin D, Wu F. Systematic and quantitative investigation of the mechanism of carbon nanotubes’ toxicity toward algae. Environ Sci Technol. 2012;46:8458–8466. doi: 10.1021/es301802g. [DOI] [PubMed] [Google Scholar]
- 23.Lucero P, Penalver E, Moreno E, Lagunas R. Moderate concentrations of ethanol inhibit endocytosis of the yeast maltose transporter. Appl Environ Microbiol. 1997;63:3831–3836. doi: 10.1128/aem.63.10.3831-3836.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis Int J Progr Cell Death. 2003;8:115. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- 25.Møller P, Christophersen DV, Jensen DM, Kermanizadeh A, Roursgaard M, Jacobsen NR, Hemmingsen JG, Danielsen PH, Cao Y, Jantzen K. Role of oxidative stress in carbon nanotube-generated health effects. Arch Toxicol. 2014;88:1939–1964. doi: 10.1007/s00204-014-1356-x. [DOI] [PubMed] [Google Scholar]
- 26.Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S. A caspase-related protease regulates apoptosis in yeast. Mol Cell. 2002;9:911–917. doi: 10.1016/S1097-2765(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 27.Meng L, Zhang X, Lu Q, Fei Z, Dyson PJ. Single walled carbon nanotubes as drug delivery vehicles: targeting doxorubicin to tumors. Biomaterials. 2012;33:1689–1698. doi: 10.1016/j.biomaterials.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Miyata Y, Mizuno K, Kataura H. Purity and defect characterization of single-wall carbon nanotubes using raman spectroscopy. J Nanomater, 2011,(2010-12-05) 2010;2011:84–95. [Google Scholar]
- 29.Mu Q, Broughton DL, Yan B. Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: developing a model for cell uptake. Nano Lett. 2009;9:4370–4375. doi: 10.1021/nl902647x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naserzadeh P, Ghanbary F, Seydi E, Ghasemi A, Joghataei MT, Ashtari K, Akbari M. Mitochondrial oxidative stress and dysfunction induced by single- and multi-wall carbon nanotubes: a comparative study. J Biomed Mater Res Part A. 2017;105:2047–2055. doi: 10.1002/jbm.a.36063. [DOI] [PubMed] [Google Scholar]
- 31.Neves V, Heister E, Costa S, Tîlmaciu C, Borowiak-Palen E, Giusca CE, Flahaut E, Soula B, Coley HM, McFadden J. Uptake and release of double-walled carbon nanotubes by mammalian cells. Adv Func Mater. 2010;20:3272–3279. doi: 10.1002/adfm.201000994. [DOI] [Google Scholar]
- 32.Oncel I, Yurdakulol E, Keleş Y, Kurt L, Yildiz A. Role of antioxidant defense system and biochemical adaptation on stress tolerance of high mountain and steppe plants. Acta Oecol. 2004;26:211–218. doi: 10.1016/j.actao.2004.04.004. [DOI] [Google Scholar]
- 33.Ong L-C, Chung FF-L, Tan Y-F, Leong C-O. Toxicity of single-walled carbon nanotubes. Arch Toxicol. 2016;90:103–118. doi: 10.1007/s00204-014-1376-6. [DOI] [PubMed] [Google Scholar]
- 34.Otero-González L, García-Saucedo C, Field JA, Sierra-Álvarez R. Toxicity of TiO2, ZrO2, Fe0, Fe2O3, and Mn2O3 nanoparticles to the yeast, Saccharomyces cerevisiae. Chemosphere. 2013;93:1201–1206. doi: 10.1016/j.chemosphere.2013.06.075. [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Brandau W, Simon U, Jahnen-Dechent W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5:2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 36.Park EJ, Zahari NEM, Kang MS, Sang JL, Lee K, Lee BS, Yoon C, Cho MH, Kim Y, Kim JH. Toxic response of HIPCO single-walled carbon nanotubes in mice and RAW264.7 macrophage cells. Toxicol Lett. 2014;229:167–177. doi: 10.1016/j.toxlet.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Porter AE, Gass M, Muller K, Skepper JN, Midgley PA, Welland M. Direct imaging of single-walled carbon nanotubes in cells. Nat Nanotechnol. 2007;2:713. doi: 10.1038/nnano.2007.347. [DOI] [PubMed] [Google Scholar]
- 38.Radu M, Munteanu MC, Petrache S, Serban AI, Dinu D, Hermenean A, Sima C, Dinischiotu A. Depletion of intracellular glutathione and increased lipid peroxidation mediate cytotoxicity of hematite nanoparticles in MRC-5 cells. Acta Biochim Pol. 2010;57:355–360. [PubMed] [Google Scholar]
- 39.Salzmann CG, Chu BT, Tobias G, Llewellyn SA, Green ML. Quantitative assessment of carbon nanotube dispersions by Raman spectroscopy. Carbon. 2007;45:907–912. doi: 10.1016/j.carbon.2007.01.009. [DOI] [Google Scholar]
- 40.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 41.Sohn EK, Chung YS, Johari SA, Kim TG, Jin KK, Ji HL, Yong HL, Kang SW, Yu IJ. Acute toxicity comparison of single-walled carbon nanotubes in various freshwater organisms. Biomed Res Int. 2015;2015:1–7. doi: 10.1155/2015/323090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone MB. Differential uptake of carbon nanoparticles by plant and Mammalian cells. Small. 2010;6:612. doi: 10.1002/smll.200901911. [DOI] [PubMed] [Google Scholar]
- 43.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssière JL, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu B, Liu GL, Ling F, Song LS, Wang GX. Development toxicity of functionalized single-walled carbon nanotubes on rare minnow embryos and larvae. Nanotoxicology. 2014;9:1–12. doi: 10.3109/17435390.2014.957253. [DOI] [PubMed] [Google Scholar]
- 45.Zhu S, Luo F, Zhu B, Wang GX. Toxicological effects of graphene oxide on Saccharomyces cerevisiae. Toxicol Res. 2017a. [DOI] [PMC free article] [PubMed]
- 46.Zhu S, Luo F, Zhu B, Wang GX. Mitochondrial impairment and oxidative stress mediated apoptosis induced by α-Fe2O3 nanoparticles in Saccharomyces cerevisiae. Toxicology Research. 2017b; 6. [DOI] [PMC free article] [PubMed]
- 47.Zhu S, Zhu B, Huang A, Hu Y, Wang G, Ling F. Toxicological effects of multi-walled carbon nanotubes on Saccharomyces cerevisiae: the uptake kinetics and mechanisms and the toxic responses. J Hazard Mater. 2016;318:650–662. doi: 10.1016/j.jhazmat.2016.07.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.