Abstract
Individuals with a history of poor interpersonal relationships are more likely to demonstrate negative health outcomes than those who have had high quality relationships. We sought to evaluate how attachment orientations, stress-induced respiratory sinus arrhythmia (RSA), and self-reported stress were associated with length of telomeres measured from peripheral blood mononuclear cells. Participants (N = 213) completed self-report measures of attachment and stress. Measurement of RSA was conducted before and after a stressful task and a blood draw was completed for analysis of telomere length. Attachment orientations were not directly associated with telomere length; however, we found that high attachment anxiety was associated with shorter length of telomeres via high self-reported stress. Attachment avoidance was also associated with telomere length via self-reported stress, but only among those with high stress-induced RSA. Exploratory analyses of T cell subsets indicated that stress was most strongly associated with telomeres from CD8CD28+ cells in comparison to CD8CD28− and CD4 cells. Study findings indicate that attachment orientations are associated with telomere length via stress, providing novel insights into the mechanisms through which close relationships can impact health and aging.
Keywords: close relationships, stress, telomere length, attachment orientations, respiratory sinus arrhythmia
Close relationships are important for physical health (Uchino et al., 2014). Indeed, morbidity and mortality rates are higher among those with unsupportive and hostile relationships in comparison to those with more supportive relationships (Brummett et al., 2001; Repetti et al., 2002). Attachment theory provides a useful framework for understanding the associations between close relationships and health outcomes (Maunder & Hunter, 2008; Pietromonaco, 2013). Low social support has been linked with increased risk of age related health problems (Reblin & Uchino, 2008); however, a better understanding of the biological mechanisms that may drive the link between close relationships, aging, and health is clearly needed.
Telomeres and Age-Related Health Problems
Telomere length is a marker of cellular aging. Each chromosome is capped by telomeres, which are repetitive sequences of nucleotides that prevent chromosomal deterioration (Barrett et al., 2015). Telomere length is relatively stable over time; however, as a consequence of cell division, telomeres become shorter until they are no longer able to divide, which is known as the “end replication problem” or “Hayflick limit” (Shay & Wright, 2000). Human telomeres are comprised of TTAGGG repeats that are bound by the shelterin complex (e.g., Maciejowski & de Lange, 2017). Shelterin protects telomeres and reduces the likelihood of cell arrest (Bilsland, Cairney, & Kieth, 2011). Although telomeres tend to become shorter over time due to cell division, telomerase, an intracellular ribonucleoprotein, can help to maintain and elongate telomeres (Shalev et al., 2013). Genetics plays a central role in determining telomerase activity (e.g., Savage & Bertuch, 2010); however, environmental factors, such as stress, are also associated with telomerase activity (Gilley, Herbert, Huda, Tanaka, & Reed, 2008).
Stress is associated with accelerated shortening of telomeres due to lower telomerase activity (Epel et al., 2004; Shay & Wright, 2005). Shorter telomeres are associated with increased risk of cardiovascular disease (Haycock et al., 2014), type 2 diabetes (Zhao et al., 2013), and some forms of cancer (Wentzensen et al., 2011). Indeed, telomere length is a predictor of the likelihood of onset of disease and mortality (Heidinger et al., 2012; Shalev et al., 2013), indicating that telomeres are a critical component of the aging process. The association between attachment orientations and telomere length remains unexplored.
Attachment Orientations, Stress, and Health
According to attachment theory, those who had supportive and responsive caregivers during childhood develop emotional and relational security that lasts into adulthood (Mikulincer & Shaver, 2009). In contrast, unsupportive and unresponsive parenting can lead to developing emotional and relational insecurity (Mikulincer & Shaver, 2009). Attachment insecurity is represented along two dimensions: attachment anxiety and attachment avoidance (Mikulincer & Shaver, 2007).
Individuals high in attachment anxiety tend to worry about being rejected/abandoned and use ineffective emotional regulation strategies that increase stress and rumination (Brennan et al., 1998). High attachment anxiety is reliably associated with many age-related health problems (McWilliams & Bailey, 2010; Puig et al., 2012). Proposed biobehavioral mechanisms linking attachment anxiety to poor health include poor cellular immunity (Fagundes et al., 2014a), chronic low-grade inflammation (Gouin et al., 2009), maladaptive neuroendocrine responses to stress (Ditzen et al., 2008), and low quality of life (Fagundes et al., 2014b).
Those high in attachment avoidance are described as being uncomfortable with relying on others for support and tend to use “deactivating” emotion regulation strategies that inhibit or suppress distressing relational experiences (Brennan et al., 1998; Fraley & Shaver, 2000). Avoidantly attached individuals tend to keep negative emotions activated internally, while trying not to express them externally (Shaver & Mikulincer, 2005). Accordingly, those high on attachment anxiety and/or attachment avoidance report higher stress than those who are more secure due to the utilization of poor self-regulation strategies (Simpson & Rholes, 2012); however, attachment avoidance is less reliably associated with stress in comparison to attachment anxiety (Dewitte et al., 2010).
A number of studies have evaluated associations between social relationships and telomere length, which may inform the association between attachment orientations and length of telomeres. For example, those with a high number of ambivalent social relationships have shorter telomeres than those with few ambivalent relationships (Uchino et al., 2012). Individuals with low social support have shorter telomeres than those with high social support in late life (Carrol, Diez Roux, Fitzpatrick, & Seeman, 2013). Moreover, early life stress, including non-supportive parenting, is associated with accelerated telomere shortening (Brody, Yu, Beach, & Philibert, 2014; Price, Kao, Burgers, Carpenter, & Tyrka, 2013; Shalev et al., 2012). Heightened stress arousal and reactivity is also associated with accelerated cellular aging (Epel et al., 2006; Kroeneke et al., 2011; Révész, Verhoeven, Milaneschi, de Gues, Wolkowitz, & Penninx, 2014). Given that early life stress promotes attachment insecurity (Murdock & Fagundes, 2017), and high attachment avoidance and anxiety are associated with a greater likelihood of having poor social relationships (e.g., Zimmerman, 2004) and heightened stress arousal (e.g., Brennan et al., 1998), we may expect that high attachment anxiety and avoidance are associated with shorter telomeres.
Although the association between attachment orientations and length of telomeres is unknown, available evidence suggests that high attachment avoidance can be both maladaptive and adaptive for mental and physical health, often depending upon third variable influences. For instance, high attachment avoidance was associated with enhanced production of inflammatory cytokines during a marital disagreement, but not during a social support interaction (Gouin et al., 2009). Further, researchers found that high attachment avoidance was associated with poor quality of life among breast cancer survivors, but only among those with low respiratory sinus arrhythmia (RSA; Fagundes et al., 2014b). Interestingly, high attachment avoidance was associated with better adjustment to the loss of a close social partner in comparison to low attachment avoidance (Fagudnes et al., 2012; Sbarra & Borelli, 2013). Therefore, there may be important factors that explain why attachment avoidance can be adaptive for some individuals, but maladaptive for others.
Respiratory Sinus Arrhythmia
Parasympathetic nervous system functioning is associated with one’s capacity to effectively cope with stress (Thayer & Lane, 2000). RSA reflects the variability in heart rate that is associated with respiration. According to the neurovisceral integration model and polyvagal theory, the ventral vagus complex plays a significant role in parasympathetic regulation of emotion and physiological stress responses (Thayer & Lane, 2000; Porges, 2001). Higher RSA in a stressful context relative to a baseline resting context (also referred to as stress-induced RSA) is thought to reflect active regulatory effort or strength (Beauchaine, 2001; Segerstrom & Nes, 2007) and is relatively stable over time (e.g., El-Sheikh, 2004; Grossman & Svebak, 1987). Indeed, high stress-induced RSA is associated with the use of adaptive emotion regulation strategies (e.g., support seeking, engagement in enjoyable tasks) and lower depression in comparison to low stress-induced RSA (Gentzler et al., 2009). Moreover, when presented with a sad film, high stress-induced RSA was associated with lower depressive symptoms during a recovery period in comparison to low stress-induced RSA (Rottenberg et al., 2005). Therefore, stress-induced RSA is a useful tool for examining one’s ability to regulate emotions. As attachment avoidance is associated with heightened physiological arousal during stressful situations, stress-induced RSA may represent an important factor explaining why attachment avoidance is inconsistently associated with stress and susceptibility to age-related health problems. That is, high ability to regulate emotions during stressful situations may reduce the strength of the associations between attachment avoidance and emotional/physical health outcomes, whereas low ability to regulate emotions may enhance such associations.
Current Study
Given that attachment anxiety has been found to be strongly associated with both self-reported stress (Ditzen et al., 2008) and greater susceptibility to age-related diseases (McWilliams & Bailey, 2010; Puig et al., 2012), we examined if high attachment anxiety would be associated with shorter telomeres via high levels of self-reported stress. Alternatively, as attachment avoidance is associated with poor health and well-being only when stress is high, we examined whether or not attachment avoidance would be indirectly associated with telomere length through stress when stress-induced RSA was high, but not when stress-induced RSA was low.
Primary study hypotheses were based on the extant human subjects literature in which telomere length of peripheral blood mononuclear cells have been targeted; however, the T cell population consists of many functionally and phenotypically different subpopulations. Therefore, there may be clinical utility for evaluating length of telomeres among T cell subsets. For instance, prior work has indicated that shorter telomeres from CD8CD28− cells are associated with an increased risk of an acute upper respiratory infection and illness (Cohen et al., 2013). The strength of the association between telomere length in other T cell subtypes (i.e., CD4 and CD8CD28+) and acute upper respiratory infection and illness was weaker in comparison to CD8CD28− T cells (Cohen et al., 2013). Poor control of viral replication among individuals diagnosed with HIV with shorter telomeres from CD8CD28− cells has also been identified (Effros et al., 1996). Data on the association between telomeres from T cell subtypes and other health outcomes is sparse. Therefore, we explored whether or not the primary hypotheses would be replicated with T cell subtypes.
Methods
Participants and Procedure
The data were collected by the Laboratory for the Study of Stress, Immunity, and Disease at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD; and were accessed via the Common Cold Project website (www.commoncoldproject.com; grant number NCCIH AT006694). Briefly, healthy individuals in Pittsburgh, Pennsylvania were recruited via newspaper advertisements to participate in the present study between the years of 2008 and 2011. All participants (N = 213) provided informed consent and the study protocol was approved by Carnegie Mellon University and the University of Pittsburgh Institutional Review Boards. Participants were compensated with $1,000 for completing the full study.
Participants completed a self-report measure of attachment orientations and stress. RSA was measured before and while engaging in the Trier Social Stress Test (Kirschbaum et al., 1993), a well validated stress paradigm. During the Trier Social Stress Test, participants were told that they would be delivering a five-minute speech in which they were to defend against an alleged transgression (either shoplifting or a traffic violation). They were given five minutes to prepare the speech, which was then videotaped. Additionally, participants provided blood samples to assess telomere length, which was processed by cell separation and stored for later analysis.
Measures
Attachment insecurity
The Experiences in Close Relationship Scale (ECR)-short form (Wei et al., 2007) was utilized to measure attachment insecurity. The 12-item measure evaluates attachment insecurity within people’s close relationships with two six-item dimensions (i.e., attachment anxiety and attachment avoidance). Participants were asked to indicate the degree to which each statement (e.g., “I am very uncomfortable being close to people.”) is true for them on a scale ranging from 1 (disagree strongly) to 7 (agree strongly). Cronbach’s alpha was .83 for attachment avoidance and .89 for attachment anxiety in the present sample. Those who report low attachment anxiety and avoidance using the ECR have secure attachment orientations (Fraley et al., 2000).
Respiratory sinus arrhythmia
RSA was recorded using three electrocardiogram leads and a respiration band (Vernier Software & Technology, Beaverton, OR). For the baseline period, which lasted 20 minutes, participants were instructed to sit upright in a chair and rest quietly. Interbeat interval (IBI) sequences were recorded using an automated algorithm (Mindware Version 2.51, Mindware Technologies, LTD) and a 250 Hz sampling frequency (Malik, 1996). Measurement of RSA during baseline was separated into five minute epochs. Spectral analysis of IBIs was conducted using a Fast Fourier transform algorithm (Duhamel & Vetterli, 1990). High frequency (HF) band power was utilized in the present study, which was calculated as the sum of the powers associated with any peaks in the range of 0.12 Hz to 0.40 Hz. HF band power across the four epochs for baseline measurement were averaged to form an overall indicator of baseline RSA. The same procedure was utilized to evaluate stress-induced RSA during the Trier Social Stress Test, which consisted of a single five minute epoch.
Self-reported stress
Participants completed the 10-item Perceived Stress Scale (Cohen et al., 1983). On the Perceived Stress Scale, participants are asked to indicate the degree to which they have experienced various symptoms of stress on a scale ranging from 0 (never) to 4 (very often). Cronbach’s alpha was .81 for the Perceived Stress Scale in the present study.
Telomere length
Three 15-ml samples of whole blood were collected into heparinized tubes via standard venipuncture. Peripheral blood mononuclear cells (PBMCs) were separated from serum following the Ficoll-Paque™ PLUS protocol (Cat# 17-1440-03, Amersham Biosciences, Pittsburgh, PA; for an overview, see Cohen et al., 2013). Standard curves and dilution factors of standards associated with the telomere (T) and single-copy gene (S) were calculated using Applied Biosystems SDS software to calculate a ratio (T/S ratio; see O’Callaghan et al., 2008). Samples were run in duplicate, and replicate valuates were averaged to determine a final T/S ratio, which was utilized in analyses described below.
Control variables
During the visit, participants provided self-reported information about their age, gender, and smoking status. Height and weight were measured in order to generate a body mass index (BMI) for each participant. Physical activity was measured via a Yamax SW-401 Digi-Walker pedometer (Yamax Corporation, Tokyo, Japan). Participants were asked to wear the pedometer during all waking hours across a period of four days. The average number of steps across the four days of measurement was utilized as an indicator of physical activity in the present study.
Analytic Strategy
Descriptive statistics, zero-order correlations, and multiple linear regression analyses were performed using SPSS statistical software (IBM, 2012). We utilized full information maximum likelihood estimation to handle missing data, which is superior to listwise deletion (Schafer & Olsen, 1998). Multiple linear regressions examined how attachment dimensions and stress-induced RSA were associated with self-reported stress. Both attachment dimensions were included simultaneously given recommendations for utilizing the ECR (Fraley et al., 2000; Lo et al., 2009). In all of our analyses, we adjusted for baseline RSA (in order to reflect stress-induced RSA relative to baseline), as well as demographic characteristics associated with telomere length (i.e., participant age, gender, ethnicity, BMI, physical activity, and smoking status; Schaefer et al., 2013). EQS structural equation modeling software (version 6.1; Bentler, 2004) was utilized to examine a moderated mediation model. Importantly, 5,000 bias corrected bootstrap samples were utilized to examine indirect effects, consistent with modern approaches to mediation analysis (Hayes, 2009).
Results
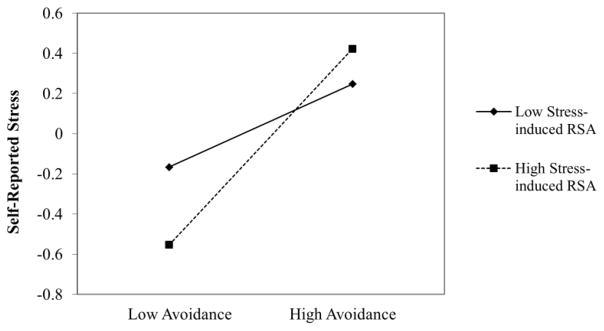
Descriptive statistics are presented in Table 1 and Pearson correlations are presented in Table 2. Higher attachment anxiety and avoidance were associated with greater self-reported stress. Attachment anxiety and avoidance were not directly associated with length of telomeres. Higher self-reported stress was associated with shorter telomeres. Using multiple regression analyses, we examined the interaction between attachment dimensions and stress-induced RSA in predicting self-reported stress. Higher attachment anxiety was associated with greater self-reported stress (β = .18, p = .02); however, neither stress-induced RSA (β = − .03, p = .80) or the interaction between attachment anxiety and stress-induced RSA (β = .03, p = .72) were significantly associated with self-reported stress. Higher attachment avoidance was associated with greater self-reported stress (β = .28, p < .001). The interaction between attachment avoidance and stress-induced RSA was significantly associated with self-reported stress (β = .13, p = .05) such that individuals with low attachment avoidance and high RSA reported less stress than those with high attachment avoidance, regardless of stress-induced RSA (see Figure 1).
Table 1.
Descriptive statistics for study variables
| Variable | M or N | SD or % |
|---|---|---|
| Attachment avoidance | 17.26 | 7.97 |
| Attachment anxiety | 20.57 | 8.02 |
| RSA baseline | 6.20 | 1.20 |
| RSA stress | 6.16 | 1.62 |
| Self- reported stress | 12.05 | 5.65 |
| PBML telomere T/S ratio | .80 | .18 |
| CD4 telomere T/S ratio | .53 | .20 |
| CD8CD28+ telomere T/S ratio | .55 | .16 |
| CD8CD28− telomere T/S ratio | .58 | .22 |
| Age | 30.13 | 10.85 |
| Gender | ||
| Male | 123 | 57.75 |
| Female | 90 | 42.25 |
| Ethnicity | ||
| non-White | 71 | 33.33 |
| non-Hispanic White | 142 | 66.67 |
| Body mass index | 27.36 | 6.47 |
| Current smoker | ||
| No | 141 | 66.20 |
| Yes | 72 | 33.80 |
| Physical activity | 7,339.25 | 4,270.91 |
Note. RSA = Respiratory Sinus Arrhythmia. PBML = peripheral blood mononuclear lymphocytes. Physical activity = average number of steps taken per day across four days.
Table 2.
Pearson correlations between primary study variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Attachment avoidance | -- | ||||||||||
| 2. Attachment anxiety | .46** | -- | |||||||||
| 3. Baseline RSA | .08 | − .02 | -- | ||||||||
| 4. Stress-induced RSA | .11 | − .05 | .76** | -- | |||||||
| 5. Self-reported stress | .38** | .32** | .08 | .06 | -- | ||||||
| 6. PBML telomere T/S ratio | − .06 | − .11 | .03 | .09 | − .12 | -- | |||||
| 7. Age | − .07 | − .11 | − .50** | − .51** | − .10 | − .16* | -- | ||||
| 8. Gender | − .09 | .11 | − .04 | − .07 | − .05 | − .12 | .03 | -- | |||
| 9. Ethnicity | − .01 | .10 | .10 | .05 | − .02 | − .06 | − .16* | − .10 | -- | ||
| 10. Body mass index | − .06 | − .17* | − .37** | − .35** | − .02 | − .20** | .32** | .17* | − .23** | -- | |
| 11. Current smoker | .01 | − .09 | − .12 | .01 | − .02 | − .02 | .22** | − .17* | − .13 | − .03 | -- |
| 12. Physical activity | .07 | .06 | .20** | .16 | .09 | .11 | − .13 | − .11 | .13 | − .28** | − .01 |
Note. RSA = respiratory sinus arrhythmia; PBML = peripheral blood mononuclear lymphocytes; Gender coded as 0 = male and 1 = female; Ethnicity coded as 0 = non-White and 1 = non-Hispanic White; Current smoker coded as 0 = no and 1 = yes.
p < .05.
p < .01.
Fig 1.
The interaction between high (+ 1 SD) and low (-1 SD) attachment avoidance and stress-induced respiratory sinus arrhythmia (RSA) in predicting self-reported stress. * p < .05.
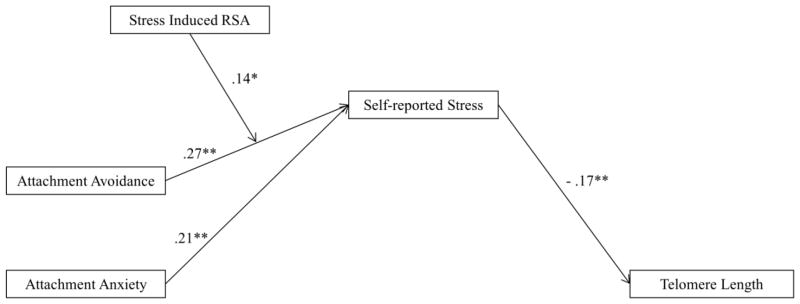
As seen in Figure 2, we examined a moderated mediation model in which self-reported stress mediated associations between attachment dimensions and length of telomeres, with stress- induced RSA moderating the association between attachment avoidance and self-reported stress. The interaction between stress-induced RSA and attachment anxiety was not included given that it was not significant in the analyses described above. Direct paths between attachment orientations and telomere length were not evaluated in the model given the non-significant associations identified in Table 2. Consistent with prior analyses, higher attachment anxiety and avoidance were associated with greater self-reported stress. Attachment anxiety was indirectly associated with telomere length through self-reported stress. Stress-induced RSA changed the association between attachment avoidance and self-reported stress such that those with low avoidance and high stress-induced RSA self-reported lower stress than those with high avoidance or low stress-induced RSA. Moreover, a conditional indirect effect was identified as attachment avoidance was negatively associated with length of telomeres via stress when stress-induced RSA was one standard deviation above the mean (− .07; 95% CI = −.14, −.03), but not when stress-induced RSA was one standard deviation below the mean (− .02; 95% CI = −.07, .01). Accordingly, full support for the moderated mediation model was identified.
Fig. 2.
A moderated-mediation model of associations between attachment anxiety and avoidance, stress induced respiratory sinus arrhythmia, self-reported stress, and telomere length. Values represent standardized regression coefficients. Control variables (not pictured) include participant age, sex, ethnicity, body mass index, smoking history, and physical activity. Indirect effect of attachment anxiety on telomere through self-reported stress using 5,000 bootstrap samples length (− .04; 95% CI = − .08, − .01). Indirect effect of the interaction between attachment avoidance and stress-induced RSA on telomere length through self-reported stress using 5,000 bootstrap samples length (− .02; 95% CI = − .06, − .01). Tests of model fit: χ2 (4, N = 213) = 3.06, p = .55; comparative fit index = .99; root mean-square error of approximation = .00, 90% confidence interval = .00, .09. * p < .050. ** p < .001.
In exploratory analyses, telomeres from CD8CD28− cells were longer than CD8CD28+ cells (t = 2.45, p = .02). Similarly, telomeres from CD8CD28− cells were longer than CD4 cells (t = 3.45, p = .001). There was no difference between telomeres from CD8CD28+ cells and CD4 cells (t = 1.40, p = .16). When examining the overall model using telomeres from CD8CD28− cells as the dependent variable, an association with perceived stress was identified (β = − .14, p = .03). The indirect effect (−.02; 95% CI = −.06, − .01) was also significant indicating that attachment avoidance was indirectly associated with telomere length from CD8CD28− cells via stress when stress-induced RSA was one standard deviation above the mean (− .06; 95% CI = − .13, − .02), but not when stress-induced RSA was one standard deviation below the mean (− .02; 95% CI = − .06, .01). Attachment anxiety was also associated with telomere length of CD8CD28− cells via stress (−.03; 95% CI = − .07, − .01). The association between perceived stress and telomeres from CD8CD28− cells was no longer significant when other T cell subsets were included in the analyses (β = − .04, p = .51), resulting in non-significant indirect effects for attachment avoidance (− .01; 95% CI = − .03, .01) and attachment anxiety (− .01; 95% CI = − .04, .01).
When evaluating telomeres from CD8CD28+ cells, there was an association with perceived stress (β = − .25, p < .001) and evidence for moderated-mediation (− .04; 95% CI = − .09, − .01). Specifically, attachment avoidance was indirectly associated with length of telomeres from CD8CD28+ cells when stress-induced RSA was one standard deviation above the mean (− .10; 95% CI = − .19, − .04), but not when stress-induced RSA was one standard deviation below the mean (− .03; 95% CI = − .09, .01). Attachment avoidance was also indirectly associated with telomere length of CD8CD28+ cells via stress (− .05; 95% CI = − .11, − .02). Findings remained when other T cell subsets were entered as covariates for both attachment avoidance (− .03; 95% CI = − .07, − .01) and attachment anxiety (− .04; 95% CI = − .08, − .01).
Perceived stress was not associated with telomere length from CD4 cells (β = − .05, p = .46). As a result, attachment avoidance was not associated with telomere length from CD4 cells (− .01; 95% CI = − .03, .01) regardless of stress-induced RSA. Moreover, attachment anxiety was not associated with length of telomeres from CD4 cells via perceived stress (− .01; 95% CI = − .04, .01).
Discussion
Telomere length is a reliable predictor of susceptibility to age-related health concerns (Barrett et al., 2015). Given the growing multidisciplinary interest between attachment theory and health (Pietromonaco et al., 2013), this study is significant and timely. Anxious and avoidant attachment dimensions were indirectly associated with shorter telomeres via self-reported stress. Moreover, we found that attachment avoidance was associated with shorter telomeres via self-reported stress among those with high stress-induced RSA, but not among those with low stress-induced RSA. Specifically, self-reported stress was lower among those with low attachment avoidance and high-stress-induced RSA than among those with high attachment avoidance and high stress-induced RSA. Study results were independent of age, gender, body mass index, smoking status, and physical activity. Thus, our findings provide insight into the connection between psychological (i.e., perceived stress) and biological (i.e., telomere length) mechanisms through which attachment insecurity might influence health (Cohen et al., 2013; Fagundes et al., 2014a; Wolkowitz et al., 2011). Furthermore, findings provide initial evidence for why attachment avoidance has been found to be less reliably associated with age-related health outcomes in comparison to attachment anxiety given that those with low attachment avoidance and high RSA reported less stress than those with high attachment avoidance, regardless of stress-induced RSA.
The quality of one’s close relationships is strongly associated with health outcomes, including medical morbidity and mortality (Brummett et al., 2001; Repetti et al., 2002; Uchino et al., 1996), and attachment theory provides a powerful framework to understand this relationship. One hypothesis is that insecure attachment styles have physiological costs that foster poor health. Our findings suggest that shortening of telomere length is one of these physiological costs and that psychological stress might be a pathway, among others, through which it becomes activated. Shorter telomere length is associated with increased risk of infection (Cohen et al., 2013), cardiovascular disease (Haycock et al., 2014), type 2 diabetes (Zhao et al., 2013), and some forms of cancer (Wentzensen et al., 2011). Further, chronic stress, a recurrent experience among people reporting high levels of attachment insecurity, has been reliably associated with accelerated shortening of telomeres among older adults (Epel et al., 2004; Tyrka et al., 2010). We replicated such findings among younger adults. Future work may benefit from examining associations between attachment orientations and specific health outcomes through stress and autonomic nervous system pathways given present study findings.
Our results also suggest that individual differences in autonomic nervous system activity are important for describing the association between attachment security dimensions, stress, and telomere length. Attachment avoidance is less reliably associated with chronic stress than attachment anxiety (Dewitte et al., 2010) and present study results indicate that individual differences in stress-induced RSA may explain previous findings. Present study findings may reflect that individuals who are exposed to relatively low interpersonal stress, such as those with low attachment avoidance, are able to effectively regulate stressful emotions they encounter if they have the regulatory strength (i.e., high stress-induced RSA) to do so. Alternatively, those with high attachment avoidance may be ineffective at regulating emotions over time given that frequent stress leads to self-regulatory failure even if self-regulatory strength is high (Baumeister & Heatherton, 1996; Wagner & Heatherton, 2014). Accordingly, interventions designed to improve RSA such as exercise (e.g., Routledge, Campbell, McFetridge-Durdle, & Bacon, 2010) and biofeedback (e.g., Lehrer & Gervirtz, 2014) therapies may be more effective at reducing stress among those with low attachment avoidance in comparison to those with high attachment avoidance; however, much of the work on improving RSA has been focused solely on baseline RSA indicating that future research addressing this possibility is clearly needed.
Results from exploratory analyses indicate that attachment orientations may be most strongly associated with length of telomeres from CD8CD28+ cells via perceived stress in comparison to other T cell subsets (i.e., CD8CD28− and CD4). CD8CD28+ cells are mostly considered to belong to the CD8+ suppressor T cells. Antigenic stimulation of naïve and memory CD8+ cells needs the presence of a co-stimulatory molecule CD28 for effective clonal expansion. CD8 cells are considered to lose their CD28 expression due to repetitive antigen-specific stimulation, a phenomenon which is often associated with a replicative senescence and shortened telomeres (Strioga, Pasukoniene, & Characiejus, 2011). Functionally they can exert cytotoxic or suppressive functions. It is unclear why stress in the present study was most strongly associated with length of telomeres from CD8CD28+ cells. Any explanations for these findings would be purely speculative. Further research is clearly needed to elucidate present study results.
Attachment orientations and telomeres were assessed within a short timeframe, which could be interpreted as a limitation. However, given that both attachment orientations and accompanying stress levels are somewhat fluid based on people’s current relationship status and telomeres are slow to change (Chen et al., 2011), a longitudinal investigation where attachment orientation was assessed many years before telomere length would not address the study question. In order to more closely examine causality, one possibility is for researchers to examine changes in telomerase, rather than telomeres, before and after an event that was particularly threatening to the attachment system as telomerase supports tight regulation of the telomere structure and is more malleable to acute changes (Xie et al., 2015). Moreover, the measure of short term stress utilized in the present study may underestimate the association between stress and telomere length in comparison to measures of long-term chronic stress (Mathur et al., 2016). The sample in the present study was predominantly non-Hispanic White. It is important to investigate if primary findings exist among more racially diverse groups in future work. Ethnic differences in inflammation and reactivation of herpesviruses, two important correlates of telomere length, have been identified in prior work (Dowd et al., 2010; Ford & Stowe, 2013).
Conclusions
Attachment theory provides a useful tool for understanding why experiences in close relationships are associated with stress and health outcomes. Indeed, attachment anxiety was indirectly associated with length of telomeres via stress in the present study, whereas attachment avoidance was indirectly associated with telomere length via stress among those with high, but not low, stress-induced RSA. Such findings provide valuable information about the biobehavioral mechanisms that may explain why unsupportive relationships can lead to poor health outcomes given that telomere length provides a useful index of cellular aging and susceptibility to age-related diseases. Moreover, findings provide insight into why attachment avoidance is inconsistently associated with stress and health in prior work by demonstrating the importance of the autonomic nervous system.
Acknowledgments
The data used for this article were collected by the Laboratory for the Study of Stress, Immunity, and Disease at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD; and were accessed via the Common Cold Project (CCP) website (www.commoncoldproject.com). CCP data are made publically available through a grant from the National Center for Complementary and Integrative Health (AT006694); the conduct of the studies was supported by grants from the National Heart, Lung, and Blood Institute (HL65111; HL65112) and National Institute for Allergy and Infectious Diseases (R01 AI066367); secondary support was provided by a grant from the National Institutes of Health to the University of Pittsburgh Clinical and Translational Science Institute (UL1 RR024153 and UL1 RT000005); and supplemental support was provided by John D. and Catherine T. MacArthur Foundation Research Network on Socioeconomic Status & Health. Preparation of the manuscript was supported by the National Heart, Lung, and Blood Institute (R01HL127260; F32HL131353).
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the appropriate institutional committees.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Conflict of Interest: Kyle W. Murdock, Samuele Zilioli, Khadija Ziauddin, Cobi J. Heijnen, & Christopher P. Fagundes declare that they have no conflict of interest.
References
- Barrett JH, Iles MM, Dunning AM, Pooley KA. Telomere length and common disease: Study design and analytical challenges. Human Genetics. 2015;134:679–689. doi: 10.1007/s00439-015-1563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: An overview. Psychological Inquiry. 1996;7(1):1–15. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Bentler PM. EQS (Version 6.1) Encino, CA: Multivariate Software; 2004. [Google Scholar]
- Bilsland AE, Cairney CJ, Keith WN. Targeting the telomere and shelterin complex for cancer therapy: Current views and future perspectives. Journal of Cellular and Molecular Medicine. 2011;15(2):179–186. doi: 10.1111/j.1582-4934.2010.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KA, Clark CL, Shaver PR. Self-reported measurement of adult attachment: An integrative overview. In: Simpson JA, Rholes WS, editors. Attachment Theory and Close Relationships. Guilford Press; New York: 1998. pp. 46–76. [Google Scholar]
- Brody GH, Yu T, Beach SRH, Philibert RA. Prevention effects ameliorate the prospective association between nonsupportive parenting and diminished telomere length. Prevention Science. 2015;16(2):171–180. doi: 10.1007/s11121-014-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Diez Roux AV, Fitzpatrick AL, Seeman T. Low social support is associated with shorter leukocyte telomere length in late life: Multi-Ethnic Study of Atherosclerosis (MESA) Psychosomatic Medicine. 2013;75(2):171–177. doi: 10.1097/PSY.0b013e31828233bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Kark JD, Aviv A. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: Age-dependent telomere shortening is the rule. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66a(3):312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA. 2013;309(7):699–705. doi: 10.1001/jama.2013.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. doi: http://dx.doi.org/10.2307/2136404. [PubMed] [Google Scholar]
- Dewitte M, De Houwer J, Goubert L, Buysse A. A multi-modal approach to the study of attachment related distress. Biological Psychiatry. 2010;85(1):149–162. doi: 10.1016/j.biopsycho.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schmidt S, Strauss B, Nater UM, Ehlert U, Heinrichs M. Adult attachment and social support interact to reduce psychological but not cortisol responses to stress. Journal of Psychosomatic Research. 2008;64:479–486. doi: 10.1016/j.jpsychores.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3–16 years. American Journal of Preventative Medicine. 2010;39(4):314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel P, Vetterli M. Fast Fourier transforms: A tutorial review and state of the art. Signal Process. 1990;19:259–299. doi: 10.1016/0165-1684(90)90158-U. [DOI] [Google Scholar]
- Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, … Giorgi JV. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10(8):17–22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal investigation. Developmental Psychobiology. 2004;46(1):66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. PNAS. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Willhelm FH, Wolkowitz OM, Cawthon R, Adler NE, … Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Diamond LM, Allen KP. Adolescent attachment insecurity and parasympathetic functioning predict future loss adjustment. Personality and Social Psychology Bulletin. 2012;38(6):821–832. doi: 10.1177/0146167212437429. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Jaremka LM, Glaser R, Alfano CM, Povoski SP, Lipari AM, Agnese DM, … Kiecolt-Glaser JK. Attachment anxiety is related to Epstein-Barr virus latency. Brain, Behavior, and Immunity. 2014a;41:232–238. doi: 10.1016/j.bbi.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Jaremka LM, Malarkey WB, Kiecolt-Glaser JK. Attachment style and respiratory sinus arrhythmia predict post-treatment quality of life in breast cancer survivors. Psycho-Oncology. 2014b;23:820–826. doi: 10.1002/pon.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JL, Stowe RP. Racial-ethnic differences in Epstein-Barr virus antibody titers among U.S. children and adolescents. Annals of Epidemiology. 2013;23(5):275–280. doi: 10.1016/j.annepidem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Shaver PR. Adult romantic attachment: Theoretical developments, emerging controversies, and unanswered questions. Review of General Psychology. 2000;4(2):132–154. doi: 10.1037//1089-2680.4.2.132. [DOI] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley D, Herbert BS, Huda N, Tanaka H, Reed T. Factors impacting human telomere homeostasis and age-related disease. Mechanisms of Ageing and Development. 2008;129:27–37. doi: 10.1016/j.mad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Loving TJ, Malarkey WB, Stowell J, Houts C, Kiecolt-Glaser JK. Attachment avoidance predicts inflammatory responses to marital conflict. Brain, Behavior, and Immunity. 2009;23:898–904. doi: 10.1016/j.bbi.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Svebak S. Respiratory sinus arrhythmia as an index of parasympathetic cardiac control during active coping. Psychophysiology. 1987;24(2):228–235. doi: 10.1111/j.1469-8986.1987.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leukocyte telomere length and risk of cardiovascular disease: Systematic Review and meta-analysis. British Medical Journal. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication monographs. 2009;76(4):408–420. doi: 10.1080/03637750903310360. [DOI] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe N, Monaghan P. Telomere length in early life predicts lifespan. Proceedings of the National Academy of Sciences in the United States of America. 2012;109(5):1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The 'Trier Social Stress Test': A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradovic J, Lin J, … Boyce WT. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosomatic Medicine. 2011;73(7):533–540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laboratory for the Study of Stress, Immunity, and Disease. Common Cold Project. 2016 Retrieved from http://www.commoncoldproject.com.
- Lehrer PM, Gevirtz R. Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology. 2014;5:756. doi: 10.3389/fpsyg.2014.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, … Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. Journal of Immunology Methods. 2010;352(1–2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C, Walsh A, Mikulincer M, Gagliese L, Zimmermann C, Rodin G. Measuring attachment security in patients with advanced cancer: Psychometric properties of a modified and brief Experiences in close Relationships scale. Psycho-Oncology. 2009;18:490–499. doi: 10.1002/pon.1417. [DOI] [PubMed] [Google Scholar]
- Maciejowski J, de Lange T. Telomeres in cancer: Tumour suppression and genome instability. Nature Reviews Molecular Biology. 2017;18:175–186. doi: 10.1038/nrm.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, Khazeni N. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain, Behavior, and Immunity. 2016;54:158–169. doi: 10.1016/j.bbi.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunder RG, Hunter JJ. Attachment relationships as determinants of physical health. Journal of the American Academy of Psychoanalysis and Dynamic Psychiatry. 2008;36(1):11–32. doi: 10.1521/jaap.2008.36.1.11. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Bailey SJ. Associations between adult attachment ratings and health conditions: Evidence from the National Comorbidity Survey Replication. Health Psychology. 2010;29(4):446–4453. doi: 10.1037/a0020061. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in adulthood: Structure, dynamics, and change. Guilford Press; New York, NY, US: 2007. [Google Scholar]
- Mikulincer M, Shaver PR. An attachment and behavioral systems perspective on social support. Journal of Social and Personal Relationships. 2009;26(1):7–19. doi: 10.1177/0265407509105518. [DOI] [Google Scholar]
- Murdock KW, Fagundes CP. Attachment orientations, respiratory sinus arrhythmia, and stress are important for understanding the link between childhood socioeconomic status and adult self-reported health. Annals of Behavioral Medicine. 2017;51(2):189–198. doi: 10.1007/s12160-016-9842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan NJ, Dhillon VS, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. BioTechniques. 2008;44(6):807–809. doi: 10.2144/000112761. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, van Tilburg T, Kriegsman DMW, Deeg DJH, Boeke AJP, van Ejik JTM. Effects of social support and personal coping resources on mortality in older age: The Longitudinal Aging Study Amsterdam. American Journal of Epidemiology. 1997;146(6):510–519. doi: 10.1093/oxfordjournals.aje.a009305. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, Uchino B, Dunkel-Schetter CD. Close relationship processes and health: Implications of attachment theory for health and disease. Health Psychology. 2013;32(5):499–513. doi: 10.1037/a0029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42(2):123–146. doi: 10.1016/S0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: An overview. Biological Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Englund MM, Simpson JA, Collins WA. Predicting adult physical illness from infant attachment: A prospective longitudinal study. Health Psychology. 2012;32(4):409–417. doi: 10.1037/a00288889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reblin M, Uchino BN. Social and emotional support and its implication for health. Current Opinion in Psychiatry. 2008;21(2):201–205. doi: 10.1097/YCO.0b013e3282f3ad89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. doi: 10.1037//0033-2909.128.2.330. [DOI] [PubMed] [Google Scholar]
- Révész D, Verhoeven JE, Milaneschi Y, de Geus EJCN, Wolkowitz OM, Penninx BWJH. Dysregulated physiological stress systems and accelerated cellular aging. Neurobiology of Aging. 2014;35(6):1422–1430. doi: 10.1016/j.neurobiolaging.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Robles TF, Carroll JE, Bai S, Reynolds BM, Esquivel S, Repetti RL. Emotions and family interactions in childhood: Associations with leukocyte telomere length. Psychoneuroendocrinology. 2016;63:343–350. doi: 10.1016/j.psyneuen.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42:277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Routlege FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvement in heart rate variability with exercise therapy. The Canadian Journal of Cardiology. 2010;26(6):303–312. doi: 10.1016/S0828-282X(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genetics in Medicine. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra DA, Borelli JL. Heart rate variability moderates the association between attachment avoidance and self-concept reorganization following marital separation. International Journal of Psychophysiology. 2013;88(3):253–260. doi: 10.1016/j.ijpsycho.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Schaefer C, Sciortino S, Kvale M, Lapham K, Ranatunga D, Rowell S, … Whitmer R. Demographic and behavioral influence on telomere length and relationship with all-cause mortality: Early results from the Kaiser Permanente Research Program on Genes, Environment, and Health (RPGEH) Clinical Medicine & Research. 2013;11(3):145. doi: 10.3121/cmr.2013.1176.b4-3. [DOI] [Google Scholar]
- Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analyst’s perspective. Multivariate Behavioral Research. 1998;33(4):545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science. 2007;18(3):275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver PR, Mikulincer M. Attachment theory and research: Resurrection of the psychodynamic approach to personality. Journal of Research in Personality. 2005;39:22–45. doi: 10.1016/j.jrp.2004.09.002. [DOI] [Google Scholar]
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nature Reviews Molecular Cell Biology. 2000;1(1):72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Senescence and immortalization: The role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- Strioga M, Pasukoniene V, Characiejus D. CD8+CD28− and CD8+CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61(3):201–216. doi: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488. doi: http://dx.doi.org/10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cawthon RM, Smith TW, Light CK, Mckenzie J, Carlisle M, … Bowen K. Social relationships and health: Is feeling positive, negative, or both (ambivalent) about your social ties related to telomeres. Health Psychology. 2012;31(6):789–796. doi: 10.1037/a0026836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Smith TW, Berg CA. Spousal relationship quality and cardiovascular risk: Dyadic perceptions of relationship ambivalence are associated with coronary-artery calcification. Psychological Science. 2014;25(4):1037–1042. doi: 10.1177/0956797613520015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF. Emotion and self-regulation failure. In: Gross JJ, editor. Handbook of Emotion Regulation. 2. New York, New York: The Guilford Press; 2014. pp. 613–628. [Google Scholar]
- Wei M, Russell DW, Mallinckrodt B, Vogel DL. The Experiences in Close Relationship Scale (ECR)-short form: Reliability, validity, and factor structure. Journal of Personality Assessment. 2007;88(2):187–204. doi: 10.1080/00223890701268041. [DOI] [PubMed] [Google Scholar]
- Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association between telomere length and cancer: A meta-analysis. Cancer Epidemiology, Biomarkers and Prevention. 2011;20(6):1238–150. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Jay KA, Smith DL, Zhang Y, Liu Z, Zheng J, Tian R, Li H, Blackburn EH. Early telomerase inactivation accelerates aging independently of telomere length. Cell. 2015;160(5):928–939. doi: 10.1016/j.cell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Miao K, Wang H, Ding H, Wang DW. Association between telomere length and type 2 diabetes mellitus: A meta-analysis. PLoS One. 2013;8:e79993. doi: 10.1371/journal.pone.0079993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman P. Attachment representations and characteristics of friendship relations during adolescence. Journal of Experimental Child Psychology. 2004;88(1):83–101. doi: 10.1016/j.jecp.2004.02.002. [DOI] [PubMed] [Google Scholar]