Abstract
Children aged <9 years may require two doses of influenza vaccine to achieve an adequate immune response to protect against the disease. We analyzed data for >2 million children in each influenza season from 2007 to 2012 from eight Immunization Information System Sentinel Sites to assess trends in two-dose compliance. Compliance was calculated by influenza season, age group, and influenza vaccination history. Two-dose compliance increased from 49% to 60% among 6–23 month olds from 2007 to 2012; no increase was observed for 2–4 or 5–8 year olds. In each season, compliance was 3–12 times higher among 6–23 month olds compared to older children and was two times higher among influenza vaccine naïve children compared to previously vaccinated children. Improved messaging for providers and parents about the importance of the two-dose recommendation, about which children are eligible for two doses, and provider access to complete influenza vaccination histories for all children are needed.
Keywords: Vaccination, Influenza, Information systems
1. Introduction
Influenza vaccination is the most effective way to protect children from influenza and its complications. Circulating influenza virus strains can change from year to year; therefore, in most seasons, one or more seasonal vaccine antigens are changed in order to protect against influenza viruses expected to cause disease during the upcoming season. As a result, unlike other vaccinations for children, seasonal influenza vaccine recommendations need to be updated yearly and providers must re-familiarize themselves with current recommendations.
To achieve high levels of vaccine-induced antibodies and decrease the risk for illness caused by virus strains that are antigenically similar to those strains included in the vaccine, children aged 6 months to 8 years require two influenza vaccine doses during their first vaccination season [1,2]. Children who receive only one vaccine dose in their first year of vaccination are less likely to have protective vaccine-induced antibody response when administered a single dose of an antigenically different vaccine in their second year of vaccination [3,4] consequently, two doses are also recommended for these one-dose vaccine-primed children. However, when vaccine antigens do not change from season to season, a single vaccine dose given to children who received only one dose during their first vaccination season can produce adequate vaccine-induced immune response [4,5]. Once previously vaccinated children enter their third season of influenza vaccination, only one vaccine dose is recommended, regardless of how many vaccine doses were previously administered.
Poor compliance with the two-dose vaccine regimen has been described [6–10], however limited data are available reporting recent trends. We used data from population-based surveillance systems including >2 million children aged 6 months to 8 years to assess trends in seasonal influenza vaccine two-dose compliance from the 2007–2008 through the 2011–2012 seasons.
2. Methods
We used data from the Immunization Information System (IIS) Sentinel Site Project, which is a collaborative project between CDC and selected state/city-based IIS with high data quality to conduct evaluations and estimate vaccination coverage. For the 2008–2012 project period, Arizona, Colorado, Michigan, Minnesota, Oregon, and Wisconsin used subsets of their state IIS as their sentinel sites. North Dakota and New York City used their entire state/city.
Children were selected for inclusion in the study if they were aged 6–23 months, 2–4 years, or 5–8 years during each influenza vaccination season assessed (2007–2008 through 2011–2012). Vaccination seasons were defined from August 1 through March 31, with two exceptions: (1) the 2007–2008 season was defined from September 1 through March 31, as vaccine was not available in August that year; (2) the 2011–2012 influenza vaccination season was defined from August 1 through May 31. Analyses included only those children who were in the specified age groups during the entire influenza season to ensure that all children evaluated had the same opportunity for vaccination; children who aged into or out of an age group during the season were excluded.
For each child, influenza vaccine doses administered prior to the start of each “current” influenza season were reviewed to determine the number of doses the child was recommended to receive. In accordance with Advisory Committee on Immunization Practices (ACIP) recommendations, for the 2007–2008 through 2009–2010 seasons, children were recommended to receive two doses of trivalent inactivated influenza vaccine (TIV) or live attenuated influenza vaccine (LAIV)1 if they: (1) had never received influenza vaccine (“seasonal vaccine naïve”), or (2) received only one dose of seasonal influenza vaccine for the first time in the previous season (“seasonal vaccine primed”) [11–13]. For the 2010–2011 season, children were recommended to receive two doses if they: (1) had no history of influenza A (H1N1)pdm09 vaccination (“pdmH1N1 vaccine naïve”), (2) received ≥1 influenza A (H1N1)pdm09 vaccine dose but had no history of seasonal influenza vaccination (“pdmH1N1 vaccine primed/seasonal vaccine naïve”) at the start of the 2010–2011 season, or (3) received ≥1 influenza A (H1N1)pdm09 vaccine dose and received only one dose of seasonal influenza vaccine for the first time in the 2009–2010 season (“pdmH1N1 vaccine primed/seasonal vaccine primed”) [14]. For the 2011–2012 season, children aged 6 months to 8 years who did not receive ≥1 dose of 2010–2011 seasonal influenza vaccine were recommended to receive two doses [15]. Doses that were invalid according to ACIP recommendations were excluded from the analyses. Doses of TIV were considered invalid if (1) they were administered less than age 6 months minus 4 days or (2) they were administered <24 days from the previous influenza vaccination. Doses of LAIV were considered invalid if (1) they were administered less than age 2 years minus 4 days, (2) were administered <24 days from a previous LAIV dose, or (3) were administered 1–27 days from any other live vaccine.
Full and partial vaccination coverage were calculated for each season. Full vaccination was defined as having received the appropriate number of influenza vaccine doses (one or two), based on ACIP recommendations for that season. Partial vaccination was defined as having received only one influenza vaccine dose when the child was recommended to receive two doses that season. Full and partial vaccination coverage combined reflects the percentage of children in the population that received ≥1 influenza vaccine dose during the evaluated season. Census denominators were used to calculate coverage [16,17]. Two-dose compliance was defined as the percentage of children receiving a second influenza vaccine dose during a season from among those children who were recommended to receive two doses and who had received their first dose that season. Coverage and compliance were calculated by influenza season and age group (6–23 months, 2–4 years, and 5–8 years). Compliance was also calculated by influenza vaccination history (i.e. seasonal vaccine naïve, seasonal vaccine primed, pdmH1N1 naïve, pdmH1N1 vaccine primed/seasonal vaccine naïve, pdmH1N1 vaccine primed/seasonal vaccine primed). Unweighted averages for the eight sites were calculated by summing the percentages at each site and dividing by the total number of sites. This study was exempt from IRB review since it involved examination of secondary, de-identified data.
3. Results
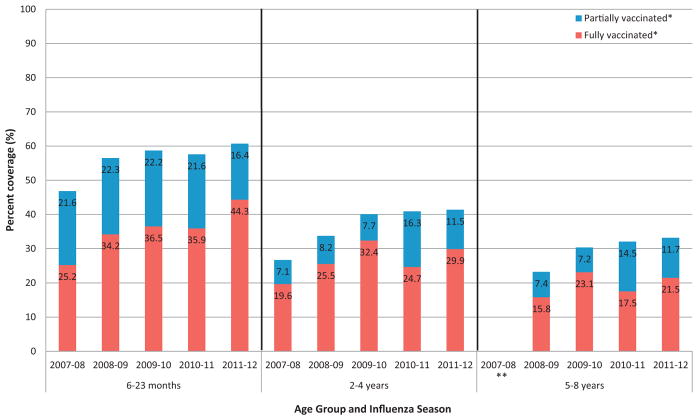
The number of children aged 6 months to 8 years evaluated during each influenza season ranged from 2,069,384 in the 2011–2012 season to 2,186,447 in the 2007–2008 season; sample sizes varied across sentinel sites from a low of 12,901 in Colorado (2011–2012) to a high of 1,027,647 in New York City (2007–2008). From the 2007–2008 through 2011–2012 seasons, vaccination coverage increased for all age groups (Fig. 1). Full vaccination coverage increased from 25.2% to 44.3% among 6–23 month olds, from 19.6% to 29.9% among 2–4 year olds, and 15.8% to 21.5% among 5–8 year olds.
Fig. 1.
Influenza vaccination coverage among children aged 6 months to 8 years by age group and influenza season, 2007–2012.
In each season, the percentage of children recommended to receive two doses was highest among children aged 6–23 months, followed by 5–8 years, and 2–4 years (Fig. 2). In each age group, the percentage of children recommended to receive two doses decreased from the 2007–2008 to the 2009–2010 season, when ACIP recommendations did not change. In the 2010–2011, this increased to the highest percentage in any season, as the majority of these children were pdmH1N1 vaccine naïve.
Fig. 2.
Percentage of children recommended to receive two influenza vaccine doses by age group, influenza season, and influenza vaccination history, 2007–2012.
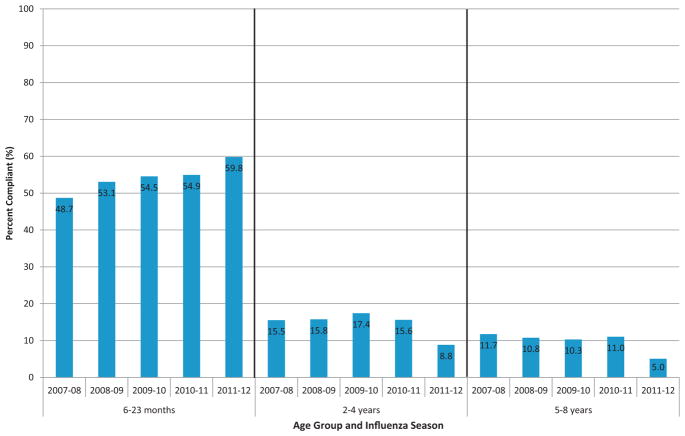
Across seasons, two-dose compliance among 6–23 month olds was 3.1–6.8 times higher than among 2–4 year olds and 4.1–11.9 times higher than among 5–8 year olds (Fig. 3). From the 2007–2008 to 2010–2011 seasons, compliance among 6–23 month olds increased from 48.7% to 59.8%. Little change was observed during this time among children aged 2–4 years (16%) and 5–8 years (11%); in the 2011–2012 season, decreases in two-dose compliance were observed in both age groups to 8.8% and 5.0%, respectively.
Fig. 3.
Two-dose compliance with influenza vaccine among children aged 6 months to 8 years by age group and influenza season, 2007–2012.
From the 2007–2008 through 2009–2010 seasons, seasonal vaccine naïve children were approximately two times more likely to be two-dose compliant than seasonal vaccine primed children; this was true for all age groups (results not shown). In the 2010–2011 season, a similar pattern was observed for pdmH1N1 and seasonal vaccine naïve children compared to children previously vaccinated with pdmH1N1 and seasonal vaccine.
4. Discussion
This study represents the first investigation of two-dose compliance since recommendations for universal influenza vaccination for children ≥6 months of age were issued, utilizing population-based, provider-verified data for several influenza seasons. Findings suggest that while two-dose compliance with seasonal influenza vaccine increased modestly from 2007 to 2012 among 6–23 month olds, it remained unchanged among older children. Overall, two-dose compliance was <50% in all age groups and was lowest among older children and children previously primed with influenza vaccine.
There are several possible reasons for low two-dose compliance. One, the complexity of influenza vaccination recommendations might make it difficult for providers to know which children are recommended to receive two doses in each season. One survey of physicians administering influenza vaccination to children found that 40% did not know which children should receive two doses [18]. Two, providers might have limited access to complete vaccination records to determine the number of doses needed in a season. In 2011, 34% of U.S. children visited more than one vaccination provider and in 2009, 34% of children were vaccinated for influenza at school [unpublished data, CDC]. Thus, children might have fragmented vaccination records in multiple vaccination settings. Three, provider knowledge about the importance of administering two doses to adequately protect children from influenza might be limited. Lastly, parents may not be able or willing to overcome barriers, such as missing work and/or school and difficulty accessing a physician’s office [19], twice for their child’s influenza vaccinations. This might disproportionately affect older children, who have fewer provider visits compared to children aged <2 years for well-child visits and/or administration of other vaccines. Monitoring two-dose compliance, in addition to full vaccination coverage, is important for assessing the success of influenza vaccination programs. Because compliance metrics are based on a subset of the population that is likely less affected by influenza vaccine hesitancy and avoidance, as they have initiated their series, interventions to improve two-dose compliance might focus more on improving access to vaccine, rather than on educating parents on the importance of influenza vaccination and influenza vaccine safety.
IIS can assist providers with some of the challenges mentioned above as they consolidate vaccination records for each child, regardless of where vaccine was administered, to create a complete history. All U.S. IIS have the ability to remind providers about the number and timing of recommended vaccine doses, based on a child’s vaccination history, and generate reminder notifications for children who are due for vaccination. State and local health departments, vaccination providers, and other vaccine stakeholders should work to ensure that all influenza vaccinations are reported to IIS by traditional and complementary vaccination providers. These efforts can be supported through training, education, policy making and legislation. Increased provider awareness about the importance of administering two doses to adequately protect children from influenza, and about which children are recommended to receive two doses, is also needed. Effective interventions for improving influenza vaccination, which might also improve two-dose compliance, include expanded hours of operation, walk-in influenza vaccination clinics within provider practices, reminder/recall notifications, year-round scheduling, standing orders, and parent education [20,21].
This investigation has several limitations. Our results are based on eight localities and might not be generalizable to the entire U.S. population. Incomplete vaccination histories might exist in this study, which could underestimate coverage and compliance and overestimate the percentage of children recommended to receive two doses. Incomplete vaccination histories might have a greater impact on estimates for older children, who are more likely to be vaccinated in complementary settings, have fewer co-administered vaccinations, and fewer vaccinations co-reported to IIS, compared to younger children. However, IIS Sentinel Sites have >85% provider site participation, which reduces the potential impact of incomplete histories. The National Immunization Survey (NIS) last published provider-verified vaccination coverage estimates for children aged 6–23 months in the 2007–2008 season [22]. NIS-based vaccination coverage estimates for this age group were comparable to IIS-based estimates for this season. Vaccination coverage estimates from the NIS in more recent seasons are parent-reported, not provider-verified, making comparisons difficult [23].
In conclusion, the majority of children aged <9 years eligible for two-dose influenza vaccination have not received two doses, leaving them susceptible to influenza disease and its complications. While a modest increase in two-dose compliance was observed among 6–23 month olds, no progress has been made among older children who are at highest risk of infection and might transmit the virus to their family and community. Steps should be taken to emphasize the importance of the two-dose recommendation to providers and parents, to make complete vaccination histories available at the time of vaccination, and to maximize all opportunities to vaccinate children against influenza.
Acknowledgments
The authors gratefully acknowledge the contributions of the Sentinel Site Project staff: Patty Gast, MS, Lisa Rasmussen, Rob Bailey: Arizona Department of Health Services; Diana Herrero, MS, Paul Gillenwater, MPH: Colorado Department of Public Health and Environment, Immunization Program; Rachel Potter, DVM, MS, Beatrice Salada: Michigan Department of Community Health; Emily Emerson, Karen E. White, MPH: Minnesota Department of Health; Molly Sander, MPH, Mary Woinarowicz: North Dakota Department of Health; Vikki Papadouka, PhD, MPH, Alexandra Ternier, MPH: New York City Department of Health and Mental Hygiene; Andrew Osborn, MBA, Mary Beth Kurilo, MPH: Oregon Immunization Program, Public Health Division, Department of Human Services; Thomas Maerz, Stephanie Schauer, PhD: Wisconsin Immunization Program.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Live attenuated influenza vaccine is recommended for healthy people 2 through 49 years of age, who are not pregnant and do not have certain health conditions such as asthma, heart/lung/kidney/liver diseases or diabetes.
References
- 1.Hirota Y, Kaji M, Ide S, Kajiwara J, Kataoka K, Goto S, et al. Antibody efficacy as a keen index to evaluate influenza vaccine effectiveness. Vaccine. 1997;15:962–7. doi: 10.1016/s0264-410x(96)00302-7. [DOI] [PubMed] [Google Scholar]
- 2.Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naïve 5–8-year-old children. J Infect Dis. 2006;194:1032–9. doi: 10.1086/507309. [DOI] [PubMed] [Google Scholar]
- 3.Englund JA, Walter EB, Fairchok MP, Monto AS, Neuzil KM. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics. 2005;115:1039–47. doi: 10.1542/peds.2004-2373. [DOI] [PubMed] [Google Scholar]
- 4.Walter EB, Neuzil KM, Zhu Y, Fairchok MP, Gagliano ME, Monto AS, et al. Influenza vaccine immunogenicity in 6- to 23-month old children: are identical antigens necessary for priming. Pediatrics. 2006;118:e570–8. doi: 10.1542/peds.2006-0198. [DOI] [PubMed] [Google Scholar]
- 5.Allison MA, Daley MF, Crane LA, Barrow J, Beaty BL, Allred N, et al. Influenza vaccine effectiveness in healthy 6- to 21-month-old children during the 2003–2004 season. J Pediatr. 2006;148:755–62. doi: 10.1016/j.jpeds.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Influenza vaccination coverage among children aged 6–23 months—United States, 2005–2006 influenza season. MMWR: Morb Mortal Wkly Rep. 2007;56:59–63. [PubMed] [Google Scholar]
- 7.Jackson LA, Neuzil KM, Baggs J, Davis RL, Black S, Yamasaki KM, et al. Compliance with the recommendations for 2 doses of trivalent inactivated influenza vaccine in children less than 9 years of age receiving influenza vaccine for the first time: a Vaccine Safety Datalink study. Pediatrics. 2006;118:2032–7. doi: 10.1542/peds.2006-1422. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt P, Block SL, Toback SL, Ambrose CS. A prospective observational study of US in-office pediatric influenza vaccination during the 2007 to 2009 influenza seasons: use and factors associated with increased vaccination rates. Clin Pediatr. 2010;49:954–63. doi: 10.1177/0009922810370868. [DOI] [PubMed] [Google Scholar]
- 9.Pabst LJ, Fiore AE, Cullen KA. Completion of the 2-dose influenza vaccine series among children aged 6 to 59 months: immunization information system sentinel sites, 2007–2008 influenza season. Clin Pediatr. 2011;50:1068–70. doi: 10.1177/0009922810385928. [DOI] [PubMed] [Google Scholar]
- 10.Hofstetter AM, Natarajan K, Martinez RA, Rabinowitz D, Vawdrey DK, Stockwell MS. Influenza vaccination coverage and timeliness among children requiring two doses, 2004–2009. Prev Med. 2013;56:165–70. doi: 10.1016/j.ypmed.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR: Morb Mortal Wkly Rep. 2007;56(RR-6):1–54. [PubMed] [Google Scholar]
- 12.CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR: Morb Mortal Wkly Rep. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 13.CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR: Morb Mortal Wkly Rep. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 14.CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR: Morb Mortal Wkly Rep. 2010;59(RR-8):1–62. [Google Scholar]
- 15.CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR: Morb Mortal Wkly Rep. 2011;60:1128–32. [PubMed] [Google Scholar]
- 16.United States Census Bureau. County intercensal estimates (2000–2010) [accessed 02.01.13];Intercensal estimates of the resident population by five-year age groups, sex, race, and Hispanic origin for counties: April 1, 2000 to July 1, 2010; 2010. Available at: http://www.census.gov/popest/data/intercensal/county/county2010.html.
- 17.United States Census Bureau. County characteristics: vintage 2011. [accessed 02.01.13];Age, sex, race, and Hispanic origin. 2013 Available at: http://www.census.gov/popest/data/counties/asrh/2011/index.html.
- 18.Dominguez SR, Daum RS. Physician knowledge and perspectives regarding influenza and influenza vaccination. Hum Vaccine. 2005;1:74–9. doi: 10.4161/hv.1.2.1604. [DOI] [PubMed] [Google Scholar]
- 19.Bhat-Schelbert K, Lin CJ, Matambanadzo A, Hannibal K, Nowalk MP, Zimmerman RK. Barriers to and facilitators of child influenza vaccine—perspectives from parents, teens, marketing and healthcare professionals. Vaccine. 2012;30:2448–52. doi: 10.1016/j.vaccine.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 20.Paul IM, Eleoff SB, Shaffer ML, Bucher RM, Moyer KM, Gusic ME. Improving influenza vaccination rates for children through year-round scheduling. Ambul Pediatr. 2006;6:230–4. doi: 10.1016/j.ambp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Pickering LK, Baker CJ, Freed GL, Gall SA, Grogg SE, Poland GA, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2009;49:817–40. doi: 10.1086/605430. [DOI] [PubMed] [Google Scholar]
- 22.CDC. Influenza vaccination coverage among children aged 6–23 months—United States, 2007–2008 influenza season. MMWR: Morb Mortal Wkly Rep. 2009;58:1063–6. [PubMed] [Google Scholar]
- 23. [accessed 23.04.13];Influenza vaccination coverage: FluVaxView. 2013 Available at: http://www.cdc.gov/flu/fluvaxview/