Abstract
Background
Current sleep medicine nosology places increased importance on nocturnal polysomnographic sleep recordings in the diagnosis of central nervous system disorders of hypersomnolence, particularly idiopathic hypersomnia (IH).
Objective
Determine what differences in sleep staging and architecture exist between IH and healthy controls using meta-analysis.
Methods
Systematic review identified relevant studies that included nocturnal polysomnography data for IH and healthy control groups. Meta-analysis compared standardized mean differences (Hedge's g) for total sleep time (TST), sleep onset latency (SOL), sleep efficiency (SE), rapid eye movement (REM) sleep percentage, slow wave sleep (SWS) percentage, and REM latency (REML). Moderator analyses were also conducted for variables with significant heterogeneity among studies.
Results
The meta-analysis included 10 studies. Relative to controls, IH demonstrated increased TST (pooled g = 0.92; 95% CI: 0.46 to 1.38, p < 0.0001) and REM percentage (pooled g = 0.36, 95% CI: 0.09 to 0.64, p = 0.01), decreased SOL (pooled g = −0.46; 95% CI: −0.81 to −0.12, p = 0.009) and SWS percentage (pooled g = −0.28, 95% CI: −0.50 to −0.07, p = 0.01), without significant differences in SE (pooled g = 0.03; 95% CI: −0.32 to 0.38, p = 0.86) or REML (pooled g = 0.14, 95% CI: −0.21 to 0.49, p = 0.42). Moderator analysis demonstrated a significant effect of sex on SE, with a higher proportion of women to men significantly predicting lower SE between in IH and controls (p < 0.0001).
Conclusions
IH is associated with several changes in sleep staging and architecture relative to healthy persons, including alterations in REM and SWS not currently delineated in nosological constructs. Further research is indicated to clarify how these findings are related the pathophysiology of IH and related disorders.
Keywords: Idiopathic hypersomnia, Hypersomnolence, Sleepiness, Polysomnography, Rapid eye movement, Slow wave sleep
1. Introduction
Idiopathic hypersomnia (IH) is a chronic and debilitating disorder of excessive daytime sleepiness, often with prolonged sleep duration [1,2]. Unlike other central nervous system disorders of hypersomnolence such as narcolepsy type 1, the cause of IH remains unknown. IH is a relatively rare disorder [1], and its low prevalence makes large-scale studies logistically difficult. In this context, nosology in sleep medicine has largely relied on a relatively small evidence base and expert opinion to classify and describe IH, which may increase the likelihood of bias influencing diagnostic criteria and descriptive characteristics of the disorder.
The International Classification of Sleep Disorders, currently in its third edition (ICSD-3) [3], has changed from prior iterations to increasingly rely upon nocturnal sleep characteristics in the diagnosis of IH and related disorders. Objectively measured sleep duration on 24-h polysomnography (performed after correction for chronic sleep deprivation) of greater than or equal to 11 h is a criterion for the disorder, which can support the diagnosis of IH in lieu of multiple sleep latency test (MSLT) findings [3]. Also, sleep onset rapid eye movement (SOREM) episodes observed on nocturnal polysomnography, in addition to the MSLT, help distinguish narcolepsy from IH [3]. Since the nocturnal polysomnogram has an ever-growing role in the delineation of disorders of central hypersomnolence, an evidence-based approach in the characterization of nocturnal sleep architecture and sleep stage distribution in IH is crucial for clinical and research purposes.
Beyond the often-prolonged total sleep time that can be observed on polysomnography, the current ICSD-3 describes IH as having non-rapid eye movement (NREM) and REM sleep in expected proportions, normal REM latency, and relatively high sleep efficiency, often above 90%. The primary aim of this systematic review and meta-analysis was to establish the accuracy of these nosologic descriptions of polysomnographic findings in IH, relative to healthy persons. In so doing, the broader goal of this research was to empirically clarify nocturnal polysomnographic findings in IH, which would enhance clinical care and research in the disorder.
2. Methods
2.1. Search strategy
Searches for ‘idiopathic hypersomnia’ (with no limitations on year of publication or language) were conducted in Pubmed and PsychINFO databases, with the final search performed on June 19, 2017. Additional ancestral and waterfall searches of related materials identified in bibliographic citations of articles assessed were performed. Peer reviewed publications and unpublished literature (meeting abstracts, dissertations/theses, etc.) were considered for inclusion/exclusion criteria. The author (DTP) conducted all searches.
2.2. Eligibility
For inclusion, a given study was required to have the following attributes: 1) use of nocturnal polysomnography to assess sleep architecture, as well as 2) a group of adult patients with idiopathic hypersomnia and 3) a healthy comparison group, each of which had polysomnographic data. Comparison groups with a clinical complaint of excessive daytime sleepiness despite normal MSLT findings were not considered to be healthy controls. Exclusion criteria included: 1) absence of polysomnographic data, 2) polysomnography being performed under conditions that were not standard for the patient/subject (e.g. after sleep deprivation), 3) lack of a healthy control group for comparison, and 4) insufficient data for qualitative assessment or meta-analysis. Studies were included that reported polysomnographic variables in patients with IH and a healthy comparison group, even if such measures were not a primary aim of the study. Studies were limited to adult patients given limited data regarding IH in children, as well as sizeable changes in sleep architecture variables that occur during childhood and adolescence [4,5].
2.3. Data extraction
The author extracted all data (unblinded). Extracted data included: author/journal, year of publication, study design, number and demographics (ages and sex) of IH and healthy control groups, criteria used to define IH, pertinent medical and psychiatric comorbidities, medication use, nature of polysomnographic protocol, as well as polysomnographic variables of interest. The author assessed study quality (unblinded) using the Methodological Index for Non-Randomized Studies (MINORS) rating scale [6].
2.4. Analysis
All studies that met inclusion/exclusion criteria were analyzed in the qualitative assessment of the literature on this topic. The author attempted to obtain data from studies published within the last twenty years that did not report sufficient data for meta-analysis, but might otherwise qualify for inclusion. No unpublished data meeting inclusion/exclusion criteria was identified.
Meta-analysis was performed via restricted maximum likelihood random-effects models conducted in R using the Metafor package [7]. The primary variables of interest were: total sleep time (TST), sleep onset latency (SOL), sleep efficiency (SE), slow wave sleep (SWS; percentage of N3 or S3+S4 of TST), REM sleep (REM; percentage of REM sleep of TST), and REM latency (REML, latency to first epoch of REM sleep). Hedge's g was utilized as the effect size for meta-analysis. I2 was used to assess heterogeneity among studies, with cutoffs 0%, 25%, 50%, and 75% used to define no, small, medium, and large heterogeneity [8,9]. It was anticipated a priori that likely moderators that would affect meta-analysis could include age, sex, and whether polysomnography was conducted with fixed time in bed or using ad libitum/extended duration recordings. Moderator analyses were conducted for all variables demonstrating significant heterogeneity (p < 0.05) among studies, as well as trend-level heterogeneity (p < 0.1) on an exploratory basis.
3. Results
3.1. Study inclusion and assessment
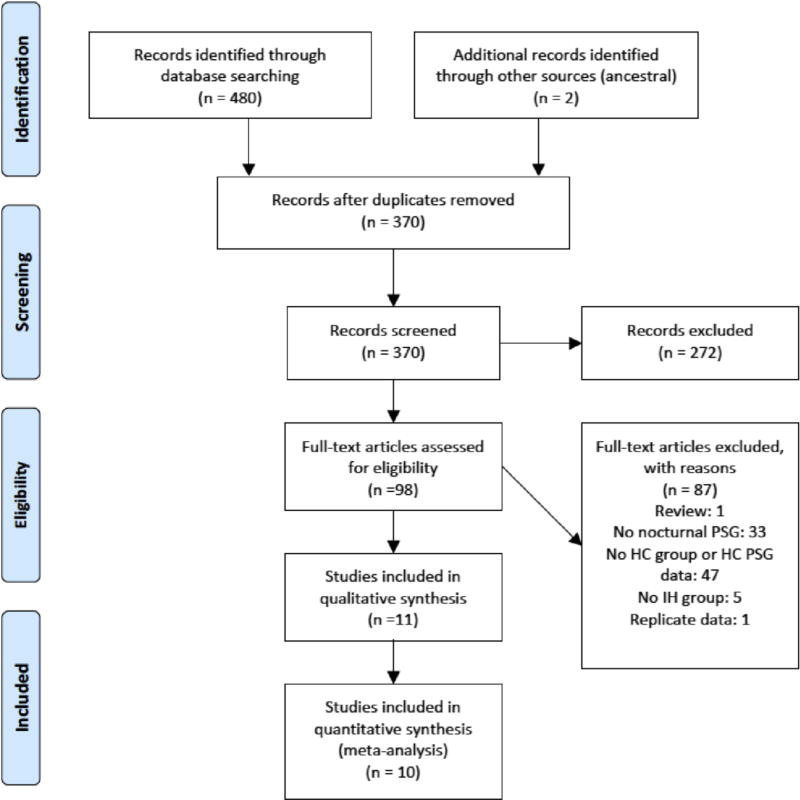
Fig. 1 displays the Preferred Reporting Items for Systematic Reviews (PRISMA) flow diagram [10]. 370 possible unique records were identified for subsequent screening. Rationale for exclusion of full-text articles is enumerated in Fig. 1. One study met inclusion/ exclusion criteria, but was considered to represent replicate data as it was drawn from the same research group within overlapping timeframe [11], and was thus excluded to minimize potential bias. In all, 11 studies met inclusion/exclusion criteria for qualitative review [12–22], 10 of which met inclusion criteria for meta-analysis of at least one primary polysomnographic variable of interest [12–21]. The article excluded from meta-analysis but included in the qualitative discussion [22] did not report sufficient data for individual polysomnographic variables or their variance required to be included in the quantitative analysis. Of the studies included in meta-analysis, one individual study did not report sufficient data for meta-analysis of SE [13] and REM/SWS sleep [14]. Two studies did not have sufficient data for meta-analysis of SOL [13,14], and four studies did not have sufficient data for meta-analysis of REML [12–14,21].
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
3.2. Qualitative synthesis
The definitions and criteria used to define idiopathic hypersomnia varied among studies (Table 1). The nature of how bedtime and wake time were handled during polysomnographic recordings were often not defined, with only three studies clearly reporting the use of ad libitum/extended duration recordings [13,17,19]. The most consistent finding across investigations was increased TST in IH relative to healthy controls, with six out of 11 studies reporting a significant difference between groups [13,15,17–19,21]. Notably all three studies that definitively used ad libitum/extended duration recordings demonstrated a significant difference TST.
Table 1.
Table of evidence.
| Study | Study design (MINORS score) | Sample size/Age/Sex | Nosology/Criteria for IH | PSG logistics | Notes |
|---|---|---|---|---|---|
| Bové et al., 1994 | Case control with three groups (IH, narcolepsy, and healthy controls) (18) | IH: 10/38.6 ± 14.1 | ICSD-1 [23]; IH patients reported EDS>1 year and sleep time >8 h; MSLT MSL <10 min | PSG occurred prior to MSLT; unclear how bedtime/wake time established | Subjects on no medications that would affect sleep spindle generation; all right-handed; none with mental illness; sleep logs used to r/o sleep deprivation; PSG data reported individually for all participants; 1 HC and 1 IH participant excluded from analysis due to age <18 years |
| HC: 10/44.5 ± 11.5 | |||||
| Sex breakdown not reported for individual groups | |||||
| Dolenc et al., 1996 | Case-control with three groups (dysthymia with EDS, IH, and HC) (15) | IH: 12/median 33/5M, 7F | Not defined | 44 h study; accommodation night from 22:30 to 07:30 → 5 nap MSLT → Extended PSG recording with LOff at subjects discretion and LOn after 30 min uninterrupted waking after 07:00 | Neither TIB nor SPT not reported to calculate SE; All participants free of psychotropic medications for ≥3 weeks |
| HC: 12/median 36/5M, 7F | |||||
| Guilleminault and Faull, 1982 | Case control study of IH relative to HC(15) | IH: 8/33.9 (range 23–49)/7M, 1F | Not reported | After 1 adaptation night, IH and HC spent 2 days on 23:00 to 07:30 TIB from which PSG comparisons drawn; IH later allowed another ad libitum PSG after this study phase | No medications for 30 days prior to study; TIB, SOL, SE, REM sleep were not reported. SD/SEM not reported for TST or SWS. Estimates for SWS not reported. |
| HC6/27 (range 19–45)/6M, 0F | |||||
| Philip et al., 2013 | Case control study of MWT and driving impairment in multiple groups (narcolepsy, OSA, IH, and HC) (15) | IH: 10/40.1 ± 12.6/2M, 8F | ICSD-2 criteria, with sleep duration determined by self report | Bedtime and waketime not defined; HC had polygraphy at home prior to MWT; IH had in lab PSG | 7 IH patients on modafinil; one IH patient also with treated OSA; 6 IH patient with normal sleep time and 4 with prolonged sleep time |
| HC: 14/32.91/8M, 6F | |||||
| Pizza et al., 2013 | Case control study of PSG micro- and microarchitecture in IH relative to HC and narcolepsy (20) | IH: 19/46.0 ± 12.75/13M, 6F | ICSD-2 | Bedtime and waketime not defined; PSG used was preceded by accommodation night in laboratory and followed by 5-nap MSLT; sleep recording carried out during usual sleep time | All participants were free of psychotropic medications for ≥3 weeks; IH fulfilled ICSD-2 criteria for IH without long sleep time |
| HC: 13/44.9 ± 14.39/7M, 6F | |||||
| Sforza et al., 2000 | Case control study of EEG power during sleep in IH relative to HC (16) | IH: 10/25.4 ± 6.6/4M, 6F | IH had severe EDS for at last 1 year, SE > 90% on PSG, and MSL<8 min on MSLT | Bedtime and waketime not reported; TIB was at least 7 h | HC age and sex-matched to IH; SOL defined as LOff to first occurrence of 3 consecutive epochs of stage 1 sleep or any one epoch of any other sleep stage |
| HC: 10/25.2 ± 6.3/4M, 6F | |||||
| Sforza et al., 2016 | Case control study examining heart rate variability and arousal response in IH (15) | IH: 14/26.2 ± 7/3M, 11F | IH had complaint of EDS>1 year, sleep drunkenness upon morning awakening, sleep duration >10 h for 3 weeks conformed by sleep diary | All subjects had ad libitum 24 h PSG | Discrepancies between reported total (n = 19 and 14) for HC group in table and text versus number of men (n = 2) and women (n = 11) in HC group (total n calculated = 13) that could not be reconciled with the author; HC age-matched to IH; IH group was drug free |
| HC: 13/25.9 ± 6/11M, 2F | |||||
| Vanková et al., 2001 | Case control study of REM variables in IH relative to HC and narcolepsy (17) | IH: 10/37.5 ± 8.3/3M, 7F | “Polysymptomatic” form of IH; MSLT MSL<10 min | Not specified; PSG occurred after one accommodation night | Sedating and REM-suppressing drugs discontinued≥2 weeks prior to study. |
| HC: 28/29.4 ± 7.4/7M, 21F | |||||
| Vernet and Arnulf, 2009 | Case control study of IH relative to HC(20) | IH: 75/34.0 ± 12.5/27M, 48 F | Daily EDS occurring≥3 months; no improvement with increase in nighttime length for 15 days; MSL on MSLT<8 min or TST>660 min on long-term sleep monitoring | PSG conducted ad libitum after night of habituation followed by 5-nap MSLT | Psychotropic medications were withdrawn at least 10 half-lives prior to testing |
| HC: 30/38.6 ± 15.2/15M, 15F | |||||
| Vgontzas et al., 2000 | Case control study examining nocturnal and daytime sleep in primary and psychiatric hypersomnia and controls (18) | IH: 59/37.6 ±11.5 | DSM-IV criteria for primary hypersomnia (based on consensus between 2 physicians and structured interview) | Nocturnal PSG limited to 8 h TIB | Patients with SOREMs on PSG/naps or on psychotropic medications were excluded; 36% (21/59) of primary hypersomnia met criteria for a secondary psychiatric d/o (primarily mood d/o); TST derived assuming sleep time (%) was determined using both sleep latency and wake time after sleep onset. |
| HC: 50/43.2 ± 12.7 | |||||
| Sex breakdown not reported for individual groups | |||||
| Weinhold et al., 2011 | Case control study examining REM sleep transitions between IH, narcolepsy, and HC (13) | IH: 22/30 ± 2/10M, 12F | ICSD-2 | Not specified; PSG occurred after one accommodation night | One IH participant was taking stimulant and antidepressant medication, respectively |
| HC33/29 ± 2/16M, 17F |
CNS = central nervous system; DSM = Diagnostic and Statistical Manual; EDS = excessive daytime sleepiness; F = female; HC = healthy controls; ICSD = International Classification of Sleep Disorders; IH = idiopathic hypersomnia; LOff = lights off time; Lon = lights on time; M = male; MSLT = multiple sleep latency test; MSL = mean sleep latency; MWT = maintenance of wakefulness test; NR = not reported; PSG = polysomnography; SD = standard deviation; SE = sleep efficiency; SEM = standard error of the mean; SOL = sleep onset latency; SOREM = sleep onset rapid eye movement; SPT = sleep period time; TIB = Time in bed; TST = total sleep time.
Significant between group differences for other sleep architecture and staging variables were more variable, and were observed in a minority of studies. Three investigations demonstrated a significant reduction in SOL in IH relative to healthy controls [15,17,21]. Only one study reported a significant decrease in sleep efficiency among IH relative to HC [17], with no other studies reporting significant differences. Three studies reported significant increases in REM sleep as a percentage of TST [16,17,19], and reductions in SWS (quantity or percentage of TST) [16,19,22] in IH relative to healthy controls. No studies reported significant differences in latency to REM sleep between groups.
3.3. Quantitative synthesis
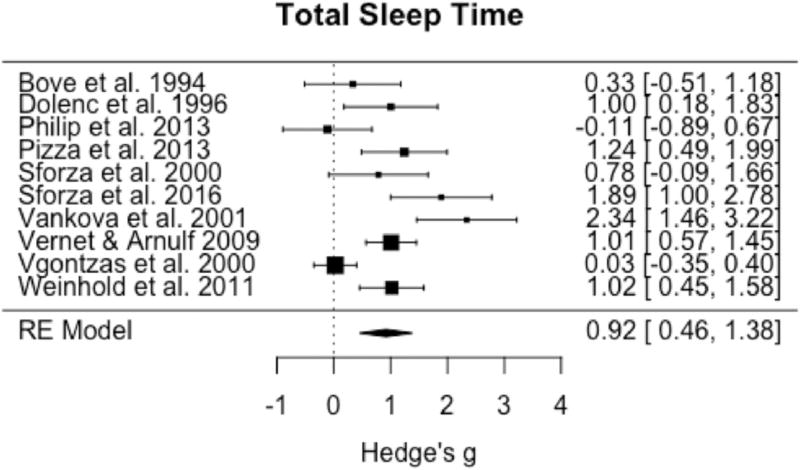
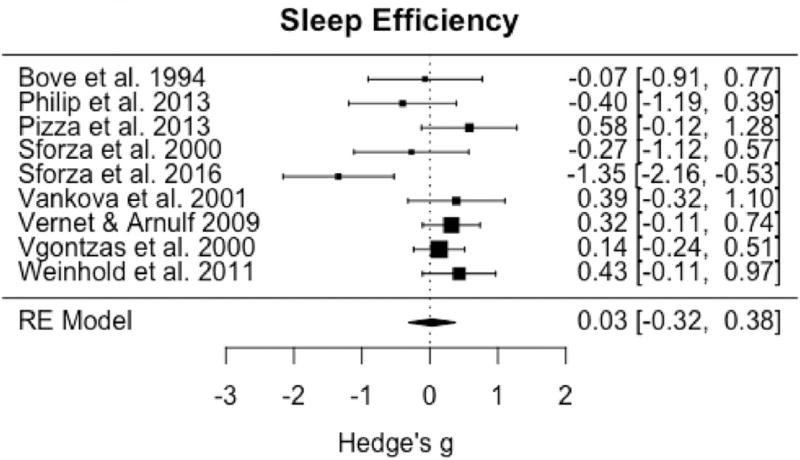
Ten studies met criteria for inclusion in meta-analysis of at least one sleep architecture or staging variable, with TST having all studies available for inclusion. Omnibus testing for TST demonstrated a significant increase in IH relative to healthy controls (pooled g = 0.92; 95% CI: 0.46 to 1.38, p < 0.0001) (Fig. 2). Significant and large heterogeneity among studies was observed (I2 = 79.3%; Q = 43.0, p < 0.0001). Omnibus testing including the 9 studies with sufficient data for analysis of SE demonstrated no significant difference between groups (pooled g = 0.03; 95% CI: −0.32 to 0.38, p = 0.86) (Fig. 3), with moderate to large and significant heterogeneity among studies (I2 = 64.0%; Q = 19.4, p = 0.01).
Fig. 2.
Forest plot of effect size (Hedge's g) for total sleep time in IH relative to healthy controls.
Fig. 3.
Forest plot of effect size (Hedge's g) for sleep efficiency in IH relative to healthy controls.
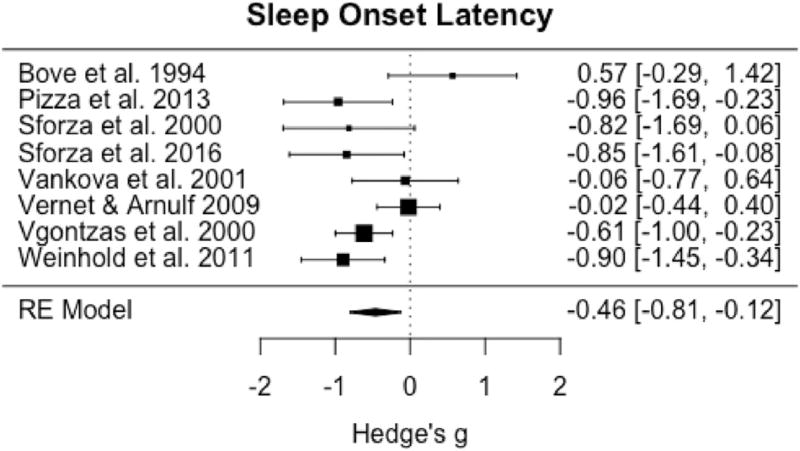
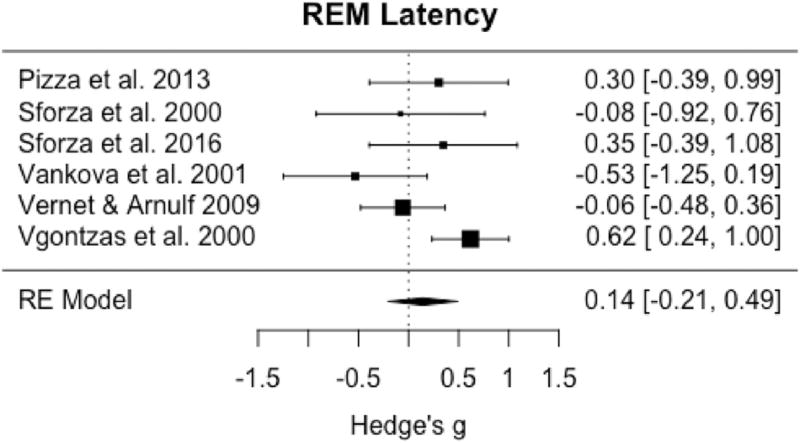
Among the eight studies with sufficient data available for meta-analysis of SOL, omnibus tests demonstrated significant reductions between IH and controls (pooled g = −0.46; 95% CI: −0.81 to −0.12, p = 0.009) (Fig. 4) with moderate to large and significant heterogeneity among studies (I2 = 60.3%; Q = 17.3, p = 0.02). Only six studies had sufficient data for omnibus testing of REML that demonstrated no significant differences in this variable between groups (pooled g = 0.14, 95% CI: −0.21 to 0.49, p = 0.42) (Fig. 5) with moderate heterogeneity among studies (I2 = 51.9%; Q= 10.7, p = 0.06).
Fig. 4.
Forest plot of effect size (Hedge's g) for sleep onset latency in IH relative to healthy controls.
Fig. 5.
Forest plot of effect size (Hedge's g) for REM latency in IH relative to healthy controls.
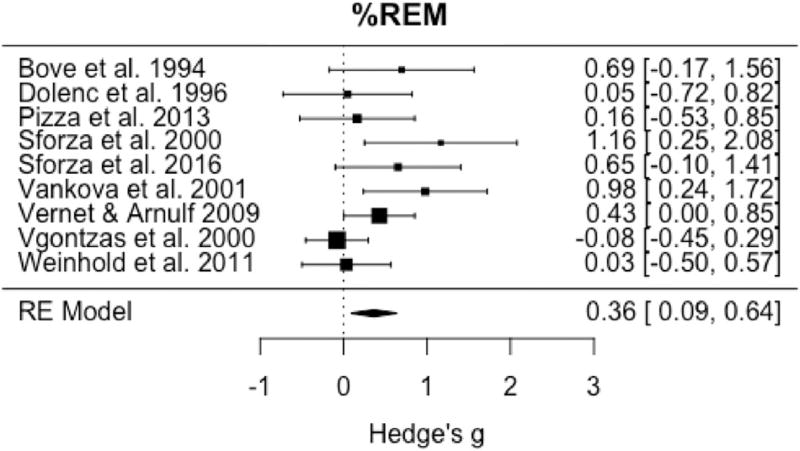
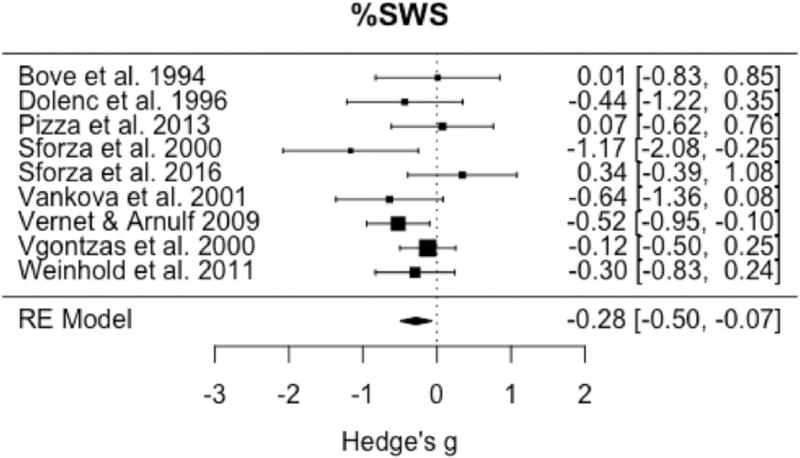
Omnibus testing of the nine studies available to evaluate the percentage of REM sleep demonstrated a significant increase in this variable in IH relative to controls (pooled g = 0.36, 95% CI: 0.09 to 0.64, p = 0.01) (Fig. 6) with small to medium heterogeneity (I2 = 43.2%; Q = 14.1, p = 0.08). Evaluation of the nine studies available for omnibus testing of percentage of SWS demonstrated a significant reduction in SWS in IH relative to controls (pooled g = −0.28, 95% CI: −0.50 to −0.07, p = 0.01) (Fig. 7) with little heterogeneity among studies (I2 = 13.7%; Q= 10.9, p = 0.21).
Fig. 6.
Forest plot of effect size (Hedge's g) for percentage of REM sleep in IH relative to healthy controls.
Fig. 7.
Forest plot of effect size (Hedge's g) for percentage of slow wave sleep in IH relative to healthy controls.
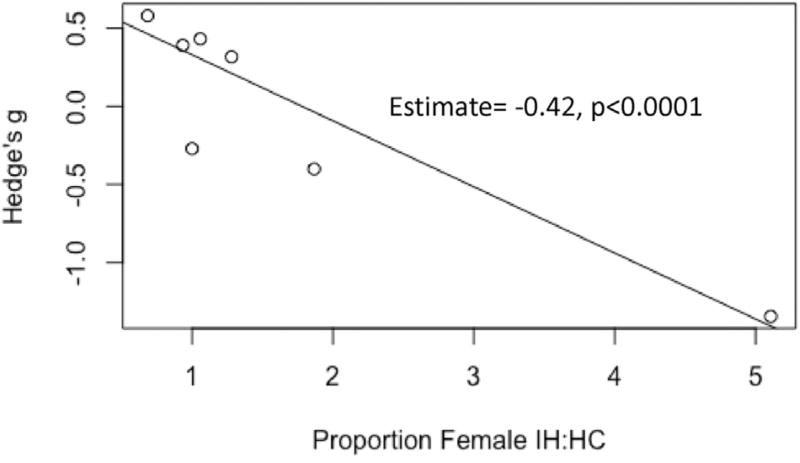
Moderator analyses demonstrated a significant effect of sex for sleep efficiency, such that increasing proportions of females to males in the IH relative to HC groups was associated with significantly reduced sleep efficiency (b1 = −0.42, p < 0.0001; b0 = 0.75) (Fig. 8). All other moderator analyses for a priori variables were not significant.
Fig. 8.
Scatterplot of moderator analysis demonstrating a significant effect of sex on sleep efficiency.
Since diagnostic criteria were variable across studies and could reflect an effect of evolving diagnostic criteria over time, post hoc moderator analyses using year of publication were also conducted, without significant association for any sleep architecture variable identified.
Given the unanticipated finding of similar sleep efficiency between IH and controls occurring despite omnibus tests demonstrating significant reductions in SOL between groups, additional post hoc analyses were conducted to attempt to clarify whether the definition of sleep efficiency utilized in studies could have affected results. Specifically, whether sleep efficiency was calculated as TST divided by time in bed (e.g. from lights off to lights on) or sleep period time (e.g. from sleep onset to lights on) was of interest, since the former could be confounded by differences in SOL, while the latter would not. The majority of studies did not clearly state methods used to calculate sleep efficiency, however, using text descriptions or other data reported, three studies were identified that did not include sleep onset latency in reported sleep efficiency [12,16,17]. Of the remaining six studies utilized for omnibus sleep efficiency analyses, one study clearly reported values calculated using TST divided by time bed [15], two studies were presumed (based on other reported data and wording) to include sleep latency in estimates [20,21], and three studies did not report sufficient data to determine whether sleep latency was included in sleep efficiency calculations [14,18,19].
Moderator analysis considering whether sleep latency was excluded from sleep efficiency estimates demonstrated a significant effect (p = 0.006). The three studies that did not include sleep onset latency were associated with a non-significant reduction in sleep efficiency in IH relative to controls (pooled g = −0.57, 95% CI −1.35 to 0.21, p = 0.15, I2 = 62%), while the remaining six studies demonstrated a significant increase in sleep efficiency relative to controls (pooled g = 0.25, 95% CI 0.04 to 0.47, p = 0.02, I2 = 0%).
Finally, to help clarify whether total sleep duration might be related to findings for other sleep continuity and staging variables, bivariate correlations using effect sizes for total sleep time and effect sizes for all other variables of interest were performed, with no significant relationships identified.
4. Discussion
This investigation demonstrates that patients with idiopathic hypersomnia demonstrate several differences in nocturnal sleep architecture and staging relative to healthy controls. In particular, use of meta-analysis quantitatively demonstrates differences in sleep staging variables in IH that are not readily apparent using narrative review methodologies. These findings have important implications for nosological descriptions of IH, as well as research into potential pathophysiological mechanisms that underlie the disorder.
Consistent with increased sleep propensity and excessive nocturnal sleep duration that are observed clinically, meta-analysis demonstrated that IH subjects have significantly greater total sleep time and shorter nocturnal sleep onset latency relative to healthy controls. Although categorical moderator analysis assessing the effect of ad libitum/extended duration polysomnographic protocols did not demonstrate a significant effect on these variables, our results must be interpreted with caution given that the majority of studies did not report specifics regarding the nature of the sleep opportunity allowed to study participants. Since current nosological criteria allow for objectively quantified excessive sleep duration as a diagnostic criterion for IH [3], and all studies that explicitly used extended duration recordings demonstrated significant increases in TST in IH relative to controls, it would seem prudent for sleep laboratories to utilize ad libitum protocols whenever feasible, if IH is suspected clinically.
Increases in total sleep time occurred despite the absence of significant differences in sleep efficiency in IH relative to controls in omnibus tests. Interestingly, continuous moderator analysis demonstrated a significant effect of sex on this variable, such that there was a significant and negative association between the proportion of women in the sample of IH relative to HC and sleep efficiency. In addition, post hoc analysis suggested that whether sleep efficiency was calculated inclusive of sleep onset latency or not impacted results. To our knowledge, these are the first reports of an effect of gender or definition affecting sleep efficiency in IH relative to controls, which should be more carefully evaluated in future research protocols. However, these results must be interpreted with caution given the relatively low number of studies available for moderator analyses.
Meta-analysis also demonstrated a significant reduction in percentage of slow wave sleep in IH relative to HC. These findings contradict current nosological descriptions that report normal sleep staging in the disorder [3], and previous nosological descriptions of SWS being potentially increased in IH [24]. Although the magnitude of the reduction in SWS was modest, there was low heterogeneity among studies, strengthening the contention that the result was not due to some other unaccounted for moderator among studies. Given the connections between sleep slow waves and the restorative aspects of sleep [25], and possible reductions in slow wave activity in IH [16], future research examining sleep homeostatic function in IH may prove a fruitful area of research.
Our results also demonstrated an increase in percentage of nocturnal REM sleep in IH relative to HC, which occurred in the context of normal REM latency. Since REM sleep is mediated in part by enhanced cholinergic activity in the laterodorsal tegmental and pedunculopontine nuclei, and inhibited by the serotonergic dorsal raphe and noradrenergic locus coeruleus, although speculative, our results suggest the possibility of alterations in these, or other components the circuitry responsible for REM sleep regulation, in IH [26]. Notably, increases in REM sleep and reductions in SWS have also been demonstrated in meta-analyses of major depressive disorder [27,28]. Given the high rates of co-occurring depressive symptoms in IH [29], and data demonstrating that hypersomnolence increases the longitudinal risk of depression [30–32], future research that examines the links between these electroencephalographic sleep variables, mood disturbance, and excessive sleepiness are indicated.
There are limitations of this meta-analysis that merit discussion. First, although considerable efforts were made to include all available studies that would meet inclusion/exclusion criteria, it is possible that the systematic search strategy did not capture all relevant data. Second, it is possible that changes in sleep staging variables observed between IH and controls were driven in part by increased sleep duration, with the tendency for SWS to occur more prominently in early sleep, and REM sleep increasing during later portions of the sleep period. However, normalizing these variables as a percentage of total sleep time likely minimized the impact of this potential confound on results, and individual studies that did not demonstrate significant differences in TST also reported reductions in SWS and increases in REM sleep [16,22], suggesting these effects are likely not due exclusively to sleep duration. Moreover, post hoc analysis demonstrated effect sizes derived for both REM and SWS did not correlate significantly with effect sizes for TST, suggesting these findings are not solely the result of increased sleep duration in IH. Third, the identified studies consisted largely of young to middle-aged participants, and thus results cannot be generalized to geriatric and/or pediatric populations. Additionally, the available data are not able to provide additional clarity regarding the usual timing of sleep in IH, or correlations between polysomnographic variables and specific symptoms of IH, such as excessive sleep inertia. Also, given the aims of the study, the requirement of a healthy comparison group for inclusion in the meta-analysis excluded oft-cited case series that have helped shape descriptions IH as a disorder [33–35]. Finally, there were different numbers of studies available to analyze the aggregate effect sizes for specific sleep architecture and stating variables between groups, as well as moderator analyses, which may have influenced findings.
In summary, this systematic review and meta-analysis demonstrates that patients with IH demonstrate differences in several polysomnographic sleep architecture and staging variables relative to healthy controls. These findings suggest the potential role of altered homeostatic function and/or REM sleep in the pathophysiology of the disorder, as well as speculative links between other brain disorders with similar polysomnographic findings. Future research is required to clarify the clinical and biological significance of these findings. This investigation also suggests that current nosological descriptions of IH as having normal sleep staging may require revision in light of the available evidence base.
Supplementary Material
Acknowledgments
Dr. Plante is supported by NIMH (K23MH099234) and the American Sleep Medicine Foundation. None of the funding sources had any further role in the study design, data collection, analysis and interpretation of the data, and the decision to submit the paper for publication.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2017.10.005.
References
- 1.Billiard M, Sonka K. Idiopathic hypersomnia. Sleep Med Rev. 2016;29:23–33. doi: 10.1016/j.smrv.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Khan Z, Trotti LM. Central disorders of hypersomnolence: focus on the narcolepsies and idiopathic hypersomnia. Chest. 2015;148:262–73. doi: 10.1378/chest.14-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. International classification of sleep disorders. 3. Darien, IL: 2014. [Google Scholar]
- 4.Kurth S, et al. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–9. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurth S, Olini N, Huber R, et al. Sleep and early cortical development. Curr Sleep Med Rep. 2015;1:64–73. doi: 10.1007/s40675-014-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slim K, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 7.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernet C, Arnulf I. Narcolepsy with long sleep time: a specific entity? Sleep. 2009;32:1229–35. doi: 10.1093/sleep/32.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bové A, Culebras A, Moore JT, et al. Relationship between sleep spindles and hypersomnia. Sleep. 1994;17:449–55. doi: 10.1093/sleep/17.5.449. [DOI] [PubMed] [Google Scholar]
- 13.Dolenc L, Besset A, Billiard M. Hypersomnia in association with dysthymia in comparison with idiopathic hypersomnia and normal controls. Pflugers Arch. 1996;431:R303–4. doi: 10.1007/BF02346389. [DOI] [PubMed] [Google Scholar]
- 14.Philip P, et al. Maintenance of wakefulness test scores and driving performance in sleep disorder patients and controls. Int J Psychophysiol. 2013;89:195–202. doi: 10.1016/j.ijpsycho.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Pizza F, et al. Polysomnographic study of nocturnal sleep in idiopathic hypersomnia without long sleep time. J Sleep Res. 2013;22:185–96. doi: 10.1111/j.1365-2869.2012.01061.x. [DOI] [PubMed] [Google Scholar]
- 16.Sforza E, Gaudreau H, Petit D, et al. Homeostatic sleep regulation in patients with idiopathic hypersomnia. Clin Neurophysiol. 2000;111:277–82. doi: 10.1016/s1388-2457(99)00242-4. [DOI] [PubMed] [Google Scholar]
- 17.Sforza E, Roche F, Barthélémy JC, et al. Diurnal and nocturnal cardiovascular variability and heart rate arousal response in idiopathic hypersomnia. Sleep Med. 2016;24:131–6. doi: 10.1016/j.sleep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Vanková J, Nevsímalová S, Sonka K, et al. Increased REM density in narcolepsy-cataplexy and the polysymptomatic form of idiopathic hypersomnia. Sleep. 2001;24:707–11. doi: 10.1093/sleep/24.6.707. [DOI] [PubMed] [Google Scholar]
- 19.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:753–9. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vgontzas AN, Bixler EO, Kales A, et al. Differences in nocturnal and daytime sleep between primary and psychiatric hypersomnia: diagnostic and treatment implications. Psychosom Med. 2000;62:220–6. doi: 10.1097/00006842-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Weinhold SL, Seek-Hirschner M, Nowak A, et al. Wake-REM sleep transitions for measuring REM sleep disturbance: comparison between narcolepsy, idiopathic hypersomnia and healthy controls. Sleep Biol Rhythms. 2011;9:172–7. [Google Scholar]
- 22.Guilleminault C, Faull KF. Sleepiness in nonnarcoleptic, non-sleep apneic EDS patients: the idiopathic CNS hypersomnolence. Sleep. 1982;5(suppl. 2):S175–81. doi: 10.1093/sleep/5.s2.s175. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. Rochester, NY: 1990. [Google Scholar]
- 24.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2. Westchester, IL: 2005. [Google Scholar]
- 25.Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5:S6–15. [PMC free article] [PubMed] [Google Scholar]
- 26.Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93:747–65. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol Psychiatry. 2011;70:912–9. doi: 10.1016/j.biopsych.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Benca RM, Obermeyer WH, Thisted RA, et al. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- 29.Barateau L, Lopez R, Franchi JA, et al. Hypersomnolence, hypersomnia, and mood disorders. Curr Psychiatry Rep. 2017;19:13. doi: 10.1007/s11920-017-0763-0. [DOI] [PubMed] [Google Scholar]
- 30.Jaussent I, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34:1103–10. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaGrotte C, et al. The relative association of obstructive sleep apnea, obesity and excessive daytime sleepiness with incident depression: a longitudinal, population-based study. Int J Obes (Lond) 2016 doi: 10.1038/ijo.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plante DT, Finn LA, Hagen EW, et al. Longitudinal associations of hypersomnolence and depression in the Wisconsin sleep cohort study. J Affect Disord. 2017;207:197–202. doi: 10.1016/j.jad.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassetti C, Aldrich MS. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;210:1423–35. doi: 10.1093/brain/120.8.1423. [DOI] [PubMed] [Google Scholar]
- 34.Roth B. Idiopathic hypersomnia: a study of 187 personally observed cases. Int J Neurol. 1981;15:108–18. [PubMed] [Google Scholar]
- 35.Anderson KN, Pilsworth S, Sharples LD, et al. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;10:1274–81. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.