Abstract
Background
Regression of left ventricular hypertrophy (LVH) is feasible with more frequent hemodialysis. We aimed to ascertain pathways associated with regression of left ventricular mass (LVM) in patients enrolled in the Frequent Hemodialysis Network (FHN) trials.
Methods
This was a post hoc observational cohort study. We hypothesized LVH regression with frequent hemodialysis was associated with a different cardiovascular biomarker profile. Regressors were defined as patients who achieved a reduction of more than 10% in LVM at 12 months. Progressors were defined as patients who had a minimum of 10% increase in LVM at 12 months.
Results
Among 332 randomized patients, 243 had biomarker data available. Of these, 121 patients did not progress or regress, 77 were regressors and 45 were progressors. Mean LVM change differed between regressors and progressors by −65.6 (−74.0, −57.2) g, p < 0.001. Regressors had a median (interquartile range) increase in dialysis frequency (from 3.0 (3.0, 3.0) to 4.9 (3, 5.7) per week, p = 0.001) and reductions in pre-dialysis systolic (from 149.0 (136.0, 162.0) to 136.0 (123.0, 152.0) mmHg, p <0.001) and diastolic (from 83.0 (71.0, 91.0) to 76.0 (68.0, 84.0) mmHg, p<0.001) blood pressures. Klotho levels increased in regressors versus progressors (76.9 (10.5; 143.3) pg/ml, p = 0.024). Tissue inhibitors of metalloproteinase (TIMP) – 2 levels fell in regressors compared to progressors (−7853 (−14653; −1052) pg/ml, p = 0.024). TIMP – 1 and LogBNP levels also tended to fall in regressors. Changes in LVM correlated inversely with changes in Klotho (r = −0.24, p = 0.014).
Conclusions
Markers of collagen turnover and changes in klotho levels are potential novel pathways associated with regression of LVH in the dialysis population, which will require further prospective validation.
Keywords: Frequent Hemodialysis, Cardiac Biomarkers, Klotho, Markers of collagen turnover, Left ventricular hypertrophy, Copeptin, Brain natriuretic peptide
Introduction
Left ventricular hypertrophy (LVH) is prevalent in end-stage renal disease (ESRD) and contributes to the high annual mortality rate seen in these patients (15-20%). While conventional hemodialysis (CHD) [3 times per week, 3-4 hours per session] is the standard renal replacement therapy in North America, it does not correct abnormal left ventricular geometry1.
Recent studies have highlighted the salutary effects of increased frequency or duration of hemodialysis on left ventricular (LV) mass. Given that reduction of LVH is associated with decreased risk of cardiovascular events2, LV mass is a logical surrogate outcome of interest. Three randomized controlled trials in the field of intensive hemodialysis (HD) have included LV mass as a primary outcome3-5. Culleton et al. assigned 52 prevalent patients to 5-6 times per week nocturnal hemodialysis (NHD) or conventional hemodialysis (CHD). After 6 months, mean LV mass was −15.3 g (95% CI −29.6 to −1.0 g; P = .01) lower in the NHD group compared to controls. Similarly, the Frequent Hemodialysis Network Daily and Nocturnal Trials demonstrated a fall in LV mass with adjusted mean LV mass differences of −13.1 g (95% CI −21.3 g to −5.0 P=0.002) and −10.9 g (95% CI –23.7 to 1.8, p=0.09), respectively. Predictors of LV mass response to intensive HD included LVH at baseline and reduction in pre-dialysis systolic blood pressure6. It is important to note that changes in blood pressure accounted for less than 50% of the variability attributable to the changes in LV mass suggesting that other important pathways may play a role in the pathogenesis of LVH and its regression in ESRD.
This is a post hoc study using data from the Frequent Hemodialysis Network Trials. We aimed to explore potential pathways associated with LVH regression and hypothesized that patients who experienced LVH regression with frequent hemodialysis (short daily and/or nocturnal hemodialysis) would manifest different responses in a series of a priori selected cardiovascular biomarkers. Given that biomarkers are also influenced by baseline level of LVH, we have also examined the impact of LVH regression on biomarker changes amongst individuals with evidence of LVH at baseline.
Concise Methods
FHN Trials
The FHN Daily and Nocturnal Trials were multicenter, randomized, prospective trials of in-center daily hemodialysis and home nocturnal hemodialysis, respectively, sponsored by the National Institute of Health, National Institutes Diabetes, Digestive and Kidney Diseases (NIDDK) and the Center for Medicare and Medical Services (CMS). The designs, inclusion and exclusion criteria of both Daily and Nocturnal Trials have been described previously 7, 8. Patients were enrolled between March 2006 and May 2009 and the trials concluded in May 2010. Both trials were approved by the local Institutional Review Board at each participating site. An independent Data Safety Monitoring Board provided oversight of both trials.
Dialysis Intervention
Patients in the conventional arm of both trials remained on their usual three times per week hemodialysis prescription subject to a prescribed equilibrated Kt/Vurea >1.1, a standardized Kt/Vurea of >2.0 and a treatment time ≥2.5 hours/session. Patients randomized to the frequent arm (six times per week hemodialysis) of the Daily Trial were targeted to an equilibrated Kt/Vn, where Vn = 3.271 × V2/3, of 0.9 provided that the length of the session was between 1.5 and 2.75 hours. Patients randomized to the frequent arm of the Nocturnal Trial followed hemodialysis prescriptions subject to a standardized Kt/Vurea of ≥4.0 and a treatment time of ≥6 hours. (72 of 87 patients in the Nocturnal Trial received therapy at home, rather than in-center).
Cardiac Magnetic Resonance Imaging (CMRI)
We measured LV mass (LVM) and biventricular volumes by CMRI in all randomized patients at baseline and at 12 months where feasible. All CMRI images were analyzed centrally in a blinded manner. CMRI was performed on 1.5-T MRI systems (minimum gradient performance: peak strength ≥12 mT/m, slew rate ≥ 40 mTm/s) with dedicated surface coils. Sites were required to use standardized protocols utilizing breath-held, retrospective ECG-gated steady-state free precession imaging in contiguous short-axis views (8-mm slice thickness, 2 mm gap) that were carefully prescribed from localizer long-axis images. Imaging parameters were adjusted on each specific CMRI scanner to provide 20-25 cardiac phases with an in-plane spatial resolution superior of ≤2 mm and a temporal resolution <50 ms. Using validated software (Argus, Siemens medical Solutions, Erlangen, Germany), we measured myocardial volume on end- diastolic frames by manual tracing of endocardial and epicardial contours. We excluded papillary muscles from the calculation of myocardial mass. Subsequently, this volume was multiplied by the specific density of the myocardium (1.05 g/cm3) to obtain LVM 9. Similarly, we traced biventricular endocardial contours in end-diastole and end-systole to derive end-diastolic and end-systolic volumes. We used the formula of DuBois and DuBois to index LVM to body surface area 10. We calculated anthropometric volume using the Watson equation 11.
Regression of LVM (“regressors”) was defined as patients who achieved a reduction of more than 10% in LVM at 12 months. “Progressors” was defined as patients who had a minimum of 10% increase in LVM at 12 months. A 10% cut-off was used to define regression as London et al12 had previously demonstrated favourable outcomes with a 10% LVM decrease in hemodialysis patients. LVH was defined as LVM index (LVM/body surface area) > 84.1 g/m2 (male) or >76.4 g/m2 (female)13 according to Patel et al.
Cardiac Biomarkers Measurements
A priori, our consortium defined a select group of serum cardiac biomarkers which have been shown to be associated with various pathogenetic pathways leading to LVH development including: (1) brain natriuretic peptide (extracellular volume overload and ventricular stretch) (Millipore, St. Charles MO, USA), with minimum detectability 11.5 pg/mL, intra-assay coefficient of variation (CV) 8.3%, and inter-assay CV 8.4%; (2) copeptin (EISA Phoenix Pharmaceuticals Inc. Burlingame CA, USA), with intra-assay CV < 10% and inter-assay CV < 15% (neurohormonal activation); (3) matrix metaloproteinases (MMP) using a metallic bead kit enzyme-linked immunoassay (Millipore, St. Charles MO, USA), with MMP 2 inter-assay CV 18% and intra-assay CV 5.4%, MMP 7 inter-assay CV 7.1% and intra-assay CV 3.7%, MMP 9 inter-assay CV 9.0% and intra-assay CV 1.9%; (4) tissue inhibitors of metalloproteinases (TIMP, Matrix remodeling and collagen deposition); (5) highly sensitive C reactive protein (CRP, inflammation) using a Polychem nephelometric assay (Polymedco, Cortland Manor NY, USA), with assay range 0.08 −160 mg/L, intra-assay CV 2.73-5.17 and inter-assay CV 4.67-5.67; (5) fibroblast growth factor 23 (FGF23) using a sandwich ELISA assay (Millipore, St. Charles MO, USA) with inter-assay CV 2.45-11.31% and intra-assay CV 7.8-11.2%; and (6) Klotho (Immuno-Biological Laboratories Co., Ltd., Japan, with intra-assay CV 2.7-3.5% and inter-assay CV 2.9-11.4%) which has been shown to be a marker of myocardial fibrosis and LVH development in uremic animal models). In order to minimize variability between assays, all assays for time paired samples were carried out in duplicate on the same plate.
Data Analysis and Outcome Measures
Descriptive statistics for continuous variables were summarized using mean ± SD or median (interquartile range, IQR). Categorical variables were summarized using proportions. We compared groups by LVM response status using standard statistical methods, including Students t-test for continuous variables and chi-squared or Fisher’s exact test for categorical variables. The effects of LVM response on changes of biomarkers were estimated by applying a mixed effects model to baseline and 12-month values using an unstructured covariance matrix. We examined the association between changes in LVM and changes in cardiac biomarkers using Pearson correlations. In order to ascertain the effect of baseline LVH on biomarker evolution, the participants were further sub-classified according to LVH status. All analyses were conducted using SAS statistical software (version 9.2, Cary NC) and a p-value criterion of <0.05 was chosen as the threshold for statistical significance.
Results
Among the 332 patients randomized across both trials (245 Daily, 87 Nocturnal), of whom 243 patients had LVM measurements as well as adequate serum samples for cardiac biomarker analyses. Of these, there were 77 patients classified as regressors (Daily: N=25, 3×/week and N=36, 6×/week; Nocturnal: N=6, 3×/week and N=10, 6×/week) and 45 patients classified as progressors (Daily: N=18, 3×/week and N=11, 6×/week; Nocturnal: N=10, 3×/week, N=6, 6×/week), with the remaining 121 patients not fulfilling either inclusion criteria. Selected baseline and clinical variables are shown in Table 1A and 1B. Mean LVM change differed between regressors and progressors by −65.60 (95% confidence interval, CI −74.04, −57.15) g, p<0.001. Specifically, LVM increased in the progressor group from 120 ± 41.5 to 151 ± 55.7 g and decreased in the regressor group from 158 ± 56.6 to 123 ± 43.9 g. At the end of follow-up, LV ejection fraction increased by 2.98±10.6% in regressors and fell by −1.9±9.30% in progressors, p=0.01. Of note, patients who had LVM regression had higher ESRD vintage (p=0.009) and tended to have a higher proportion with congestive heart failure (p=0.045). There were no differences in the biomarker levels at baseline when comparing regressors and progressors (Table 1A). Regressors had a median (interquartile range) increase in dialysis frequency (from 3.0 (3.0, 3.0) to 4.9 (3.0, 5.7) per week, p = 0.001) and median (interquartile range) reductions in pre-dialysis systolic (from 149.0 (136.0, 162.0) to 136.0 (123.0, 152.0) mmHg, p <0.001) and diastolic (from 83.0 (71.0, 91.0) to 76.0 (68.0, 84.0) mmHg, p<0.001). (Table 2)
Table 1.
FHN Combined Daily and Nocturnal Trials
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | LVM Progress or Regress | Not Progress or Regress | P-value | |||||
| Progress (N=45) | Regress (N=77) | P-value | (N=121) | |||||
| N | N(%) or Mean ± SD | N | N(%) or Mean ± SD | N | N(%) or Mean ± SD | |||
| Age | 45 | 51.5 ± 12.3 | 77 | 50.5 ± 13.4 | 0.68 | 121 | 52.4 ± 14.4 | 0.33 |
| Female | 45 | 23 (51.1%) | 77 | 27 (35.1%) | 0.082 | 121 | 44 (36.4%) | 0.51 |
| Race/Ethnicity | 45 | 77 | 0.69 | 121 | 0.58 | |||
| Non-Hispanic White | 14 (31.1%) | 19 (24.7%) | 37 (30.6%) | |||||
| Black (Hispanic or Non-Hispanic) | 18 (40.0%) | 36 (46.8%) | 46 (38.0%) | |||||
| All Other | 13 (28.9%) | 22 (28.6%) | 38 (31.4%) | |||||
| Years since ESRD | 45 | 3.30 ± 3.75 | 77 | 5.81 ± 6.76 | 0.009 | 121 | 0.79 | |
| (Vintage) | ||||||||
| < 2 years | 20 (44.4%) | 26 (33.8%) | 0.24 | 47 (38.8%) | ||||
| >= 2 years | 25 (55.6%) | 51 (66.2%) | 74 (61.2%) | |||||
| LVM (g) | 45 | 120 ± 41.5 | 77 | 158 ± 56.6 | < .0001 | 121 | 139 ± 51.8 | 0.45 |
| LVM Index (g/m2) | 45 | 62.0 ± 18.0 | 77 | 80.2 ± 30.4 | < .0001 | 120 | 72.0 ± 25.1 | 0.68 |
| LV Ejection Fraction (%) | 45 | 59.7 ± 9.77 | 77 | 55.3 ± 10.6 | 0.027 | 121 | 56.2 ± 12.4 | 0.64 |
| ALDOSTERONE (pg/mL) | 41 | 387 ± 484 | 74 | 276 ± 468 | 113 | 277 ± 345 | ||
| ALDOSTERONE Log (pg/mL) | 41 | 5.38 ± 1.05 | 74 | 5.07 ± 0.90 | 0.10 | 113 | 5.21 ± 0.81 | 0.80 |
| BNP (pg/mL) | 35 | 189 ± 197 | 61 | 334 ± 352 | 94 | 352 ± 372 | ||
| BNP Log (pg/mL) | 35 | 4.70 ± 1.12 | 61 | 5.14 ± 1.36 | 0.10 | 94 | 5.16 ± 1.39 | 0.36 |
| Copeptin (ng/mL) | 44 | 220 ± 129 | 74 | 234 ± 155 | 0.61 | 118 | 236 ± 127 | 0.69 |
| CRP (mg/dL) | 44 | 0.95 ± 1.68 | 76 | 1.07 ± 1.89 | 121 | 1.35 ± 2.30 | ||
| CRP Log (mg/dL) | 44 | −.95 ± 1.48 | 76 | −.86 ± 1.41 | 0.73 | 121 | −.65 ± 1.40 | 0.19 |
| FGF23 (pg/mL) | 43 | 3290 ± 3419 | 75 | 4121 ± 4323 | 116 | 3206 ± 3846 | ||
| FGF23 Log (pg/mL) | 43 | 7.29 ± 1.50 | 75 | 7.47 ± 1.60 | 0.53 | 116 | 7.22 ± 1.47 | 0.36 |
| Klotho (pg/mL) | 44 | 688 ± 297 | 75 | 648 ± 206 | 0.44 | 118 | 705 ± 290 | 0.23 |
| MMP-2 (pg/mL) | 43 | 129E3 ± 32566 | 73 | 137E3 ± 37099 | 113 | 136E3 ± 32814 | ||
| MMP-2 Log (pg/mL) | 43 | 11.7 ± 0.26 | 73 | 11.8 ± 0.28 | 0.28 | 113 | 11.8 ± 0.24 | 0.48 |
| MMP-7 (pg/mL) | 42 | 41046 ± 26560 | 73 | 39983 ± 29386 | 112 | 40167 ± 26353 | ||
| MMP-7 Log (pg/mL) | 42 | 10.4 ± 0.63 | 73 | 10.4 ± 0.66 | 0.61 | 112 | 10.4 ± 0.67 | 0.98 |
| MMP-9 (pg/mL) | 43 | 113E3 ± 67065 | 73 | 128E3 ± 78205 | 113 | 124E3 ± 76201 | ||
| MMP-9 Log (pg/mL) | 43 | 11.5 ± 0.57 | 73 | 11.6 ± 0.64 | 0.39 | 113 | 11.5 ± 0.68 | 0.86 |
| TIMP-1 (pg/mL) | 42 | 234E3 ± 65653 | 73 | 226E3 ± 59899 | 0.53 | 108 | 239E3 ± 53853 | 0.21 |
| TIMP-2 (pg/mL) | 41 | 125E3 ± 65761 | 70 | 113E3 ± 27096 | 0.27 | 104 | 12E4 ± 40344 | 0.70 |
| B | |||||
|---|---|---|---|---|---|
| Variables | LVM Progress (N=45) | LVM Regress (N=77) | P-value | ||
| N | N(%) or Mean±SD | N | N(%) or Mean±SD | ||
| Dialysis Std Kt/Vurea | 45 | 2.44 ± 0.39 | 76 | 2.45 ± 0.32 | 0.91 |
| Baseline Urine (L / 24 hrs.) | 45 | 0.27 ± 0.37 | 77 | 0.20 ± 0.33 | 0.22 |
| Residual Renal Urea Clearance (mL/min) | 45 | 77 | 0.39 | ||
| = 0 | 45 | 23 (51.1%) | 77 | 45 (58.4%) | |
| > 0 – 1 | 45 | 5 (11.1%) | 77 | 13 (16.9%) | |
| > 1 – 3 | 45 | 14 (31.1%) | 77 | 14 (18.2%) | |
| > 3 | 45 | 3 (6.7%) | 77 | 5 (6.5%) | |
| Hypertension | 45 | 42 (93.3%) | 77 | 70 (90.9%) | 0.64 |
| Predialysis Diastolic BP (mmHg) | 45 | 79.3 ± 13.5 | 77 | 82.7 ± 12.4 | 0.17 |
| Predialysis Systolic BP (mmHg) | 45 | 146 ± 19.9 | 77 | 150 ± 20.1 | 0.27 |
| Myocardial Infarction | 45 | 2 (4.4%) | 77 | 9 (11.7%) | 0.18 |
| Congestive Heart Failure | 45 | 4 (8.9%) | 77 | 18 (23.4%) | 0.045 |
| Atrial Fibrillation | 45 | 0 | 77 | 4 (5.2%) | 0.12 |
| Peripheral vascular disease | 45 | 5 (11.1%) | 77 | 9 (11.7%) | 0.923 |
| Cerebrovascular disease | 45 | 4 (8.9%) | 77 | 5 (6.5%) | 0.625 |
| Diabetes Mellitus | 45 | 21 (46.7%) | 77 | 29 (37.7%) | 0.329 |
| Chronic pulmonary disease | 45 | 1 (2.2%) | 77 | 4 (5.2%) | 0.424 |
| Liver Disease | 45 | 0 | 77 | 1 (1.3%) | 0.443 |
| Dialysis Access | 0.277 | ||||
| Fistula | 45 | 19 (65.5%) | 77 | 41 (67.2%) | |
| Graft | 45 | 8 (27.6%) | 77 | 10 (16.4%) | |
| Catheter | 45 | 2 (6.9%) | 77 | 10 (16.4%) | |
| Antihypertensives | 45 | 5 (11.1%) | 77 | 18 (23.4%) | 0.374 |
| ACEI | 45 | 16 (35.6%) | 77 | 29 (37.7%) | 0.816 |
| ARB | 45 | 10 (22.2%) | 77 | 17 (22.1%) | 0.985 |
| Dihydropyridine CCB | 45 | 18 (40.0%) | 77 | 39 (50.6%) | 0.248 |
| Non-Dihydropyridine CCB | 45 | 3 (6.7%) | 77 | 4 (5.2%) | 0.736 |
| Beta Blockers | 45 | 26 (57.8%) | 77 | 42 (54.5%) | 0.718 |
| Peripheral Alpha Blockers | 45 | 1 (2.2%) | 77 | 3 (3.9%) | 0.616 |
| Centrally Acting Agent | 45 | 9 (20.0%) | 77 | 16 (20.8%) | 0.918 |
| Non-Specific Vasodilators | 45 | 7 (15.6%) | 77 | 9 (11.7%) | 0.542 |
| Diuretic | 45 | 5 (11.1%) | 77 | 10 (13.0%) | 0.890 |
| Statin | 45 | 21 (46.6%) | 77 | 30 (39.0%) | 0.098 |
| Phosphorus (mg/dL) | 45 | 5.79 ± 1.47 | 77 | 6.26 ± 1.55 | 0.101 |
| Potassium (mmol/L) | 45 | 4.94 ± 0.83 | 77 | 4.95 ± 0.75 | 0.943 |
Table 2.
The Effect of Frequency of Dialysis and Blood Pressure between Progressors and Regressors
| Variable | Treatment Arm | Baseline Median (n) (interquartile range) | Month 12 Median (n) (interquartile range) | Mean Change from Baseline (95% CI) | LVM Response Effect (95% CI) | P-Value |
|---|---|---|---|---|---|---|
| Frequency of Dialysis (per week) | Progressors | 3.0 (45) (3.0, 3.0) |
3 (44) (3.0, 4.2) |
0.61 (0.23 to 0.99) | 0.80 (0.32 to 1.28) |
0.001 |
| Regressors | 3.0 (77) (3.0, 3.0) |
4.9 (76) (3.0, 5.7) |
1.41 (1.12 to 1.70) | |||
| Pre-dialysis Diastolic BP (mmHg) | Progressors | 77.0 (45) (69.8, 88.0) |
83.1 (44) (71.9, 91.0) |
2.46 (−0.49 to 5.40) | −7.94 (−11.52 to −4.36) |
< 0.001 |
| Regressors | 83.0 (77) (71.3, 90.7) |
75.6 (76) (67.6, 84.4) |
−5.48 (−7.77 to −3.20) | |||
| Pre-dialysis Systolic BP (mmHg) | Progressors | 148.8 (45) (132.3, 155.3) |
151.6 (44) (141.5, 164.2) |
4.97 (0.09 to 9.84) | −16.73 (−22.64 to −10.83) |
< 0.001 |
| Regressors | 149.0 (77) (136.0, 162.3) |
136.1 (76) (123.8, 152.4) |
−11.77 (−15.57 to −7.96) |
p-values indicate whether the LVM response effect from the mixed model for each variable is significant
Table 3 summarizes the differences in 12 month changes in all a priori defined biomarkers between progressors and regressors. Amongst the various cardiac biomarkers of interest, Klotho levels increased significantly in patients with LVM regression versus those who had LVM progression. FGF23 fell in both groups and did not differ between regressors and progressors. This was accompanied by a significant decrease in phosphorus in both groups. Similarly, TIMP-1 and TIMP-2 levels fell and logBNP levels tended to fall in patients who had LVM regression in comparison to LVM progression. Aldosterone levels increased among regressors and decreased in progressors. After adjustment for changes of potassium and blood pressure, the difference in (log) aldosterone change between regressors and progressors (10.8, 95% CI −20.6, 54.7) was not significant (p =0.54). Of note (Figures 1 and 2), changes in LVM correlated inversely with changes in Klotho (r = −0.24, p = 0.014) and changes in logBNP were associated with changes in LVM (r = 0.32, p = 0.013). Similarly, changes in pre- systolic and diastolic blood pressures correlated with changes in LVM (r = 0.52; p < 0.001, r = 0.47, p < 0.001, respectively).
Table 3.
FHN Combined Daily and Nocturnal Trials Mixed Model Results (Percent Scale for Log Scale)
| Variable | Total # | Progress or Regress | LVM Response Effect | 95% Confidence Limits | P-Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| ALDOSTERONE Log (pg/ml) | 116 | Progress | −10.22 | −28.05 | 12.04 | 0.34 |
| Regress | 21.70 | 2.90 | 43.94 | 0.022 | ||
| Difference | 35.55 | 2.96 | 78.46 | 0.030 | ||
| BNP Log (pg/ml) | 114 | Progress | 11.62 | −32.73 | 85.21 | 0.67 |
| Regress | −32.11 | −53.24 | −1.43 | 0.042 | ||
| Difference | −39.18 | −66.40 | 10.08 | 0.10 | ||
| COPEPTIN (ng/ml) | 118 | Progress | 13.99 | −12.76 | 40.74 | 0.30 |
| Regress | 15.07 | −5.19 | 35.33 | 0.14 | ||
| Difference | 1.08 | −31.45 | 33.60 | 0.95 | ||
| COPEPTIN Log (ng/ml) | 118 | Progress | 9.89 | −1.33 | 22.40 | 0.086 |
| Regress | 6.53 | −1.76 | 15.53 | 0.13 | ||
| Difference | −3.06 | −15.07 | 10.65 | 0.64 | ||
| CRP Log (mg/dL) | 122 | Progress | −21.98 | −45.32 | 11.31 | 0.17 |
| Regress | −11.10 | −31.93 | 16.09 | 0.38 | ||
| Difference | 13.95 | −26.13 | 75.77 | 0.55 | ||
| FGF23 Log (pg/ml) | 120 | Progress | −25.33 | −51.37 | 14.65 | 0.18 |
| Regress | −41.49 | −57.67 | −19.13 | 0.001 | ||
| Difference | −21.64 | −53.17 | 31.11 | 0.35 | ||
| KLOTHO (pg/ml) | 119 | Progress | −21.79 | −75.16 | 31.57 | 0.42 |
| Regress | 55.08 | 15.37 | 94.78 | 0.007 | ||
| Difference | 76.87 | 10.48 | 143.26 | 0.024 | ||
| MMP-2 (pg/ml) | 117 | Progress | 1493.96 | −8694.73 | 11682.65 | 0.77 |
| Regress | −8113.96 | −15856.3 | −371.64 | 0.04 | ||
| Difference | −9607.92 | −21801.7 | 2585.81 | 0.12 | ||
| MMP-2 Log (pg/ml) | 117 | Progress | 0.68 | −6.82 | 8.79 | 0.86 |
| Regress | −5.93 | −11.29 | −0.24 | 0.041 | ||
| Difference | −6.57 | −14.87 | 2.54 | 0.15 | ||
| MMP-7 Log (pg/ml) | 117 | Progress | −12.63 | −23.05 | −0.80 | 0.037 |
| Regress | −17.92 | −25.44 | −9.65 | 0.000 | ||
| Difference | −6.06 | −19.05 | 9.01 | 0.41 | ||
| MMP-9 Log (pg/ml) | 117 | Progress | 6.51 | −13.80 | 31.60 | 0.56 |
| Regress | 0.42 | −14.85 | 18.42 | 0.96 | ||
| Difference | −5.72 | −26.56 | 21.03 | 0.64 | ||
| TIMP-1 (pg/ml) | 117 | Progress | −1785 | −15274 | 11703 | 0.79 |
| Regress | −16671 | −26776 | −6567 | 0.001 | ||
| Difference | −14886 | −30397 | 624 | 0.060 | ||
| TIMP-2 (pg/ml) | 114 | Progress | −5967 | −14983 | 3048 | 0.19 |
| Regress | −13820 | −22004 | −5636 | 0.001 | ||
| Difference | −7853 | −14653 | −1052 | 0.024 | ||
p-values indicate whether the LVM response effect from the mixed model for each variable is significant.
Figure 1.
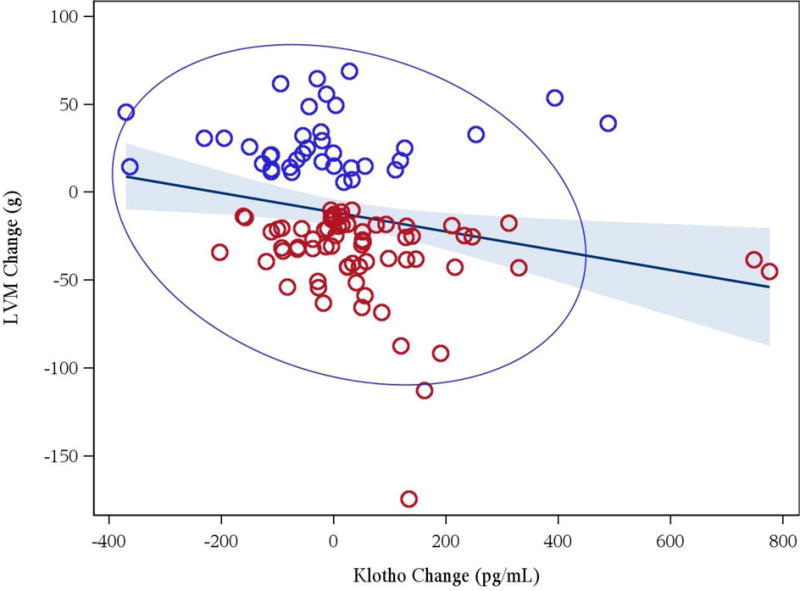
Association between changes in Klotho and changes in left ventricular mass (r = −0.24, p = 0.014) [red circles denote regressors, blue circles denote progressors]
Figure 2.
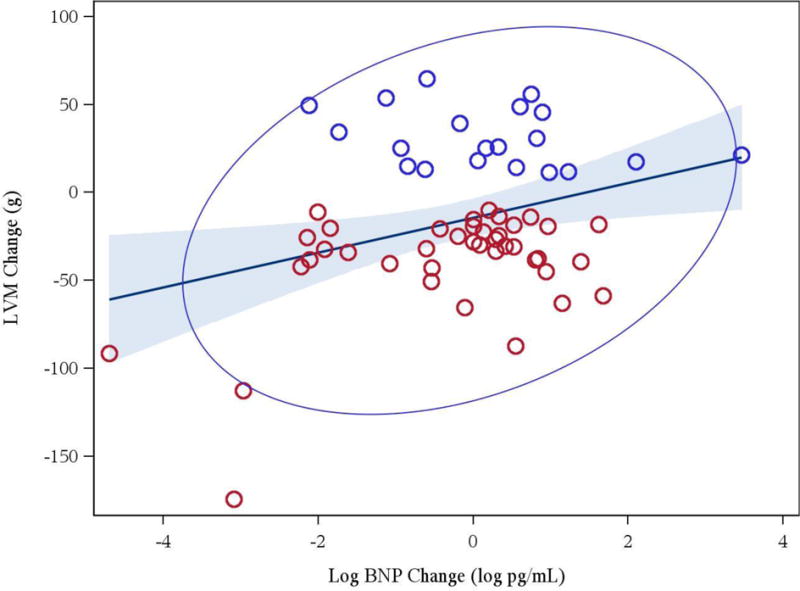
Association between changes in log of brain natriuretic peptide and changes in left ventricular mass (r = 0.32, p = 0.013) [red circles denote regressors, blue circles denote progressors]
Amongst those patients with either LVH progression or regression, 34 patients had LVH at baseline and 88 patients did not meet LVH criteria. Their demographics and biomarker results are described in Table 4. The proportion of patients with baseline LVH which regressed had higher ESRD vintage (81%) than those who progressed (43%). Among those with LVH at baseline, copeptin increased from baseline to 12 months in progressors and declined in regressors with a difference of −87.2 ng/ml (95% CI −178.8; 4.7, p =0.06).
Table 4.
Baseline Demographics of Patients with and without Left Ventricular Hypertrophy at Baseline in FHN Combined Daily and Nocturnal Trials
| LVH (N=34) |
Not LVH (N=88) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Progress (N=7) |
Regress (N=27) |
P-value | Progress (N=38) |
Regress (N=50) |
P-value | ||||
| N | N(%) or Mean±SD | N | N(%) or Mean±SD | N | N(%) or Mean±SD | N | N(%) or Mean±SD | |||
| Age | 7 | 41.9 ± 13.2 | 27 | 47.6 ± 13.3 | 0.32 | 38 | 53.3 ± 11.5 | 50 | 52.1 ± 13.3 | 0.66 |
| Female | 7 | 3 (42.9%) | 27 | 4 (14.8%) | 0.10 | 38 | 20 (52.6%) | 50 | 23 (46.0%) | 0.54 |
| Race/Ethnicity | 7 | 27 | 0.094 | 38 | 50 | 0.95 | ||||
| Non-Hispanic White | 7 | 4 (57.1%) | 27 | 5 (18.5%) | 38 | 10 (26.3%) | 50 | 14 (28.0%) | ||
| Black (Hispanic or Non-Hispanic) | 7 | 1 (14.3%) | 27 | 13 (48.1%) | 38 | 17 (44.7%) | 50 | 23 (46.0%) | ||
| All Other | 7 | 2 (28.6%) | 27 | 9 (33.3%) | 38 | 11 (28.9%) | 50 | 13 (26.0%) | ||
| Years since ESRD (Vintage) | 7 | 27 | 0.039 | 38 | 50 | 0.99 | ||||
| < 2 years | 7 | 4 (57.1%) | 27 | 5 (18.5%) | 38 | 16 (42.1%) | 50 | 21 (42.0%) | ||
| >= 2 years | 7 | 3 (42.9%) | 27 | 22 (81.5%) | 38 | 22 (57.9%) | 50 | 29 (58.0%) | ||
| ALDOSTERONE (ug/mL) | 7 | 331 ± 499 | 27 | 176 ± 173 | 34 | 399 ± 488 | 47 | 333 ± 568 | ||
| ALDOSTERONE Log (ug/mL) | 7 | 5.20 ± 1.04 | 27 | 4.87 ± 0.71 | 0.33 | 34 | 5.42 ± 1.06 | 47 | 5.19 ± 0.98 | 0.31 |
| BNP (pg/mL) | 6 | 361 ± 189 | 24 | 437 ± 436 | 29 | 154 ± 182 | 37 | 267 ± 271 | ||
| BNP Log (pg/mL) | 6 | 5.72 ± 0.68 | 24 | 5.61 ± 1.10 | 0.82 | 29 | 4.48 ± 1.08 | 37 | 4.83 ± 1.43 | 0.28 |
| Copeptin (ng/mL) | 7 | 185 ± 94.3 | 27 | 269 ± 205 | 0.30 | 37 | 227 ± 134 | 47 | 215 ± 116 | 0.65 |
| CRP (mg/L) | 7 | 0.54 ± 0.56 | 27 | 0.67 ± 1.10 | 37 | 1.03 ± 1.82 | 49 | 1.29 ± 2.18 | ||
| CRP Log (mg/L) | 7 | −1.2 ± 1.35 | 27 | −1.3 ± 1.48 | 0.85 | 37 | −.90 ± 1.52 | 49 | −.60 ± 1.31 | 0.32 |
| FGF23 (pg/mL) | 7 | 4134 ± 4037 | 27 | 5622 ± 4687 | 36 | 3126 ± 3327 | 48 | 3276 ± 3905 | ||
| FGF23 Log (pg/mL) | 7 | 7.67 ± 1.37 | 27 | 8.14 ± 1.16 | 0.36 | 36 | 7.21 ± 1.53 | 48 | 7.10 ± 1.70 | 0.75 |
| Klotho (pg/mL) | 7 | 909 ± 514 | 27 | 603 ± 192 | 0.17 | 37 | 646 ± 224 | 48 | 674 ± 211 | 0.55 |
| MMP-2 (pg/mL) | 7 | 128E3 ± 32069 | 27 | 136E3 ± 40610 | 36 | 129E3 ± 33111 | 46 | 137E3 ± 35340 | ||
| MMP-2 Log (pg/mL) | 7 | 11.7 ± 0.30 | 27 | 11.8 ± 0.30 | 0.68 | 36 | 11.7 ± 0.25 | 46 | 11.8 ± 0.27 | 0.29 |
| MMP-7 (pg/mL) | 7 | 27795 ± 13379 | 27 | 33724 ± 20310 | 35 | 43696 ± 27852 | 46 | 43657 ± 33258 | ||
| MMP-7 Log (pg/mL) | 7 | 10.1 ± 0.55 | 27 | 10.2 ± 0.65 | 0.65 | 35 | 10.5 ± 0.63 | 46 | 10.5 ± 0.66 | 0.73 |
| MMP-9 (pg/mL) | 7 | 85090 ± 42252 | 27 | 88928 ± 56033 | 36 | 118E3 ± 70077 | 46 | 151E3 ± 80644 | ||
| MMP-9 Log (pg/mL) | 7 | 11.3 ± 0.45 | 27 | 11.2 ± 0.60 | 0.91 | 36 | 11.5 ± 0.59 | 46 | 11.8 ± 0.57 | 0.04 |
| TIMP-1 (pg/mL) | 6 | 2E5 ± 32958 | 27 | 242E3 ± 78423 | 0.21 | 36 | 239E3 ± 68314 | 46 | 216E3 ± 43916 | 0.09 |
| TIMP-2 (pg/mL) | 6 | 103E3 ± 14950 | 25 | 12E4 ± 29988 | 0.20 | 35 | 129E3 ± 70385 | 45 | 109E3 ± 24891 | 0.12 |
Discussion
LVH is an important risk factor for cardiovascular morbidity and mortality in patients with ESRD. Our group has previously documented the effect size of LVM reduction with the use of frequent hemodialysis. We have also reported the association between changes in blood pressure and reduction in LVM. While hemodynamics alteration may represent an important component of the clinical benefits of frequent hemodialysis on LV remodelling, the impact of frequent hemodialysis on cardiovascular biomarkers and pathogenetic pathways have not been explored. In the present study, we aimed to generate new hypotheses and were able to demonstrate that markers of collagen turnover and changes in klotho levels are novel potential pathways, which may provide mechanistic insights into the development of LVH in patients with ESRD.
There is an emerging body of work that suggests pathological turnover of collagen is associated with LVH in the general population. Simplistically, excessive deposition of collagen may be controlled by overproduction of matrix, reduced removal or degradation of collagen or both. Indeed, biomarkers reflecting changes in extracellular matrix fibrillary collagen homeostasis was predictive of LVH and diastolic dysfunction in a cross sectional analysis in 144 patients without ESRD14. Further, the matrix metalloproteinases/tissue Inhibitors of metalloproteinases ratio was investigated in 103 general patients with hypertension and LVH. MMP/TIMP balance was suggested to play a role in predicting LV structure in the setting of hypertensive cardiac disease15. Additionally, a high level of TIMP was predictive of LVH and congestive heart failure in animal models and humans with hypertension15. The present observation suggests that MMP/TIMP balance is modifiable with the use of frequent hemodialysis. Specifically, regression in LVM was associated with a reduction in TIMP-2 levels resulting in a favourable MMP/TIMP ratio favoring extracellular matrix degradation. Whether the hypotensive effect of frequent hemodialysis or enhancement of solute or volume removal may affect MMP/TIMP in patients with ESRD is unknown. It is important to note that BNP tended to fall with LVH regression and correlated with changes in LVM. It is tempting to speculate that minimization of extracellular volume excess will lead to reduction in ventricular stretch, which is known to induce pathological extracellular matrix deposition16, 17. Our present data is consistent with the hypothesis that LVH regression in ESRD is dependent not only on changes in blood pressure alone; normalization of the MMP/TIMP may be a novel therapeutic target in patients with CKD and LVH.
Klotho is an anti-aging protein18 which beneficially regulates various cellular processes, such as senescence, inflammation, apoptosis, fibrosis, and calcium and phosphate metabolism19. Uremic solutes retention is associated with reduction in Klotho levels20. In 86 patients with chronic kidney disease, LVH was inversely associated with Klotho levels. In normal mice, intraperitoneal injection of indoxyl sulfate induced LVH was also accompanied by downregulation of Klotho. In vitro, Klotho inhibited cardiomyocyte hypertrophy by inhibiting p38 and extracellular signal regulated protein kinase 1 / 2 signaling pathways21. Restoration of Klotho is feasible through an enhancement of peroxisome proliferation-activated receptor gamma acetylation22 and is decreased through promoter hypermethylation23. Indeed, superTAG methylation has been demonstrated to be associated with uremia-induced epigenetic dysregulation of atherosclerosis-related genes24. The present observation extends the existing literature by describing the potential therapeutic impact of frequent hemodialysis on the augmentation of Klotho levels in patients with ESRD and LVH. It is tempting to speculate that frequent hemodialysis may modify epigenetic regulation of various genes25, which may result in an augmentation of klotho levels.
It is intriguing to note that LVH regression with frequent hemodialysis may occur in the setting of elevated levels of fibroblast growth factor 23 (FGF23)26. Given that FGF 23 has been suggested to induce LVH in in vitro and in vivo models27, the independent therapeutic effect of klotho on the heart requires additional prospective investigations. In our study, FGF23 levels declined in both progressors and regressors. FGF23 levels also declined significantly in all treatment groups, suggesting improvement in phosphate balance throughout the enrolled population. It is therefore unclear whether reduction in FGF23 may have played a permissive role among the population that responded to increased dialysis intensity.
It is interesting to note that there was a significant interaction between copeptin and LVH. Previously, our group has described a more pronounced reduction of LVM by frequent hemodialysis in patients with minimal residual renal function28. Using an observational study design, the MONDO investigators have also substantiated an independent association between pre-dialysis serum sodium and blood pressure variations29. Copeptin is part of the 164 amino acid precursor protein preprovasopressin together with vasopressin and neurophysin II. Recently, there is an emerging body of literature implicating the association between copeptin and cardiovascular mortality in patients with chronic kidney disease or end- stage renal disease 30. Taken together, it is unclear whether copeptin may provide additional insights into the volume regulation of patients with ESRD and its cardiac sequelae.
Although our study represents the largest cohort of ESRD patients undergoing frequent hemodialysis with cardiac MRI imaging and biomarker analyses, it is important to acknowledge the exploratory nature of the present work. Our sample size is limited to discern all potential pathways associated with LVH regression. In the future, additional biomarkers may be tested to enhance our present understanding of the biomarker profile of our patients with ESRD and LVH. We have also made multiple comparisons to explore potential mechanistic pathways. We also acknowledge that the present associative results cannot address causality but rather provide a novel trajectory of investigation. LVH is an important surrogate marker in ESRD. Using the Toronto nocturnal hemodialysis cohort, survival was demonstrated to be superior in patients with LVH regression31. We have illustrated two potentially important pathways contributing to LVM reduction in ESRD. Given the clinical impact of LVH regression in ESRD and the novel therapeutic potential of klotho and extracelullar matrix homeostasis, our results may provide new therapeutic and dialysis targets for patients with ESRD.
Acknowledgments
The Frequent Hemodialysis Network Trials (registered at ClinicalTrials.gov NCT00264758 and NCT00271999) were supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (U01 DK066579, U01 DK066597, U01 DK066480, U01 DK066481) and the Centers for Medicare and Medicaid Services and the National Institutes of Health Research Foundation (with contributions from Amgen, Baxter, DaVita, Dialysis Clinics, Inc., Fresenius Medical Care North America, Renal Research Institute and Satellite Healthcare). Measurements of additional ancillary biomarkers using stored samples from the Frequent Hemodialysis Network Trials at the NIDDK Repository were funded in part by the NIDDK at the National Institutes of Health (R01 DK DK091288 and DK076165). A list of members of the FHN Trial Group for each study has been published 25,26.
Footnotes
CONFLICT OF INTEREST: Joan Lo has received past research funding from Amgen and Sanofi unrelated to this study.
References
- 1.Foley RN. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial. 2003;16:111–7. doi: 10.1046/j.1525-139x.2003.160271.x. [DOI] [PubMed] [Google Scholar]
- 2.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65:1492–8. doi: 10.1111/j.1523-1755.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 3.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. Jama. 2007;298:1291–9. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocco MV, Lockridge RS, Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy CD, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin MF, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–91. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, Daugirdas JT, Kotanko P, Larive B, Levin NW, Mehta RL, Rocco M, Sanz J, Schiller BM, Yang PC, Rajagopalan S. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging. 2012;5:251–61. doi: 10.1161/CIRCIMAGING.111.969923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, Kliger AS. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. Am J Kidney Dis. 57:90–100. doi: 10.1053/j.ajkd.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, Beck GJ, Gassman JJ, Eggers PW, Star RA, Ornt DB, Kliger AS. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–59. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 9.Fieno DS, Jaffe WC, Simonetti OP, Judd RM, Finn JP. TrueFISP: assessment of accuracy for measurement of left ventricular mass in an animal model. J Magn Reson Imaging. 2002;15:526–31. doi: 10.1002/jmri.10107. [DOI] [PubMed] [Google Scholar]
- 10.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 11.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 12.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. Journal of the American Society of Nephrology. 2001;12:2759–67. doi: 10.1681/ASN.V12122759. [DOI] [PubMed] [Google Scholar]
- 13.Patel RK, Oliver S, Mark PB, Powell JR, McQuarrie EP, Traynor JP, Dargie HJ, Jardine AG. Determinants of left ventricular mass and hypertrophy in hemodialysis patients assessed by cardiac magnetic resonance imaging. Clin J Am Soc Nephrol. 2009;4:1477–1483. doi: 10.2215/CJN.03350509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zile MR, Desantis SM, Baicu CF, Stroud RE, Thompson SB, McClure CD, Mehurg SM, Spinale FG. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail. 2011;4:246–56. doi: 10.1161/CIRCHEARTFAILURE.110.958199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 16.Vanderheyden M, Goethals M, Verstreken S, De Bruyne B, Muller K, Van Schuerbeeck E, Bartunek J. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol. 2004;44:2349–54. doi: 10.1016/j.jacc.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino LS. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508–15. doi: 10.1681/ASN.V1271508. [DOI] [PubMed] [Google Scholar]
- 18.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 19.Hu MC, Kuro-o M, Moe OW. The emerging role of Klotho in clinical nephrology. Nephrol Dial Transplant. 2012;27:2650–7. doi: 10.1093/ndt/gfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, Wang S, Xiao T, Xu X, He T, Xia X, Wang J, Zhao J. Klotho Protects Against Indoxyl Sulphate-Induced Myocardial Hypertrophy. J Am Soc Nephrol. 2015;26:2434–46. doi: 10.1681/ASN.2014060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K, Nie L, Huang Y, Zhang J, Xiao T, Guan X, Zhao J. Amelioration of uremic toxin indoxyl sulfate-induced endothelial cell dysfunction by Klotho protein. Toxicol Lett. 2012;215:77–83. doi: 10.1016/j.toxlet.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Liu L, Lin W, Yin S, Duan A, Liu Z, Cao W. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017;91:144–156. doi: 10.1016/j.kint.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Wang X, Wang X, Jie P, Lu H, Zhang S, Lin X, Lam EK, Cui Y, Yu J, Jin H. Klotho is silenced through promoter hypermethylation in gastric cancer. Am J Cancer Res. 2011;1:111–119. [PMC free article] [PubMed] [Google Scholar]
- 24.Zawada AM, Rogacev KS, Hummel B, Grun OS, Friedrich A, Rotter B, Winter P, Geisel J, Fliser D, Heine GH. SuperTAG methylation-specific digital karyotyping reveals uremia-induced epigenetic dysregulation of atherosclerosis-related genes. Circ Cardiovasc Genet. 2012;5:611–20. doi: 10.1161/CIRCGENETICS.112.963207. [DOI] [PubMed] [Google Scholar]
- 25.Stenvinkel P, Ekstrom TJ. Does the uremic milieu affect the epigenotype? J Ren Nutr. 2009;19:82–5. doi: 10.1053/j.jrn.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raimann JG, Chan CT, Daugirdas JT, Depner T, Gotch FA, Greene T, Kaysen GA, Kliger AS, Kotanko P, Larive B, Lindsay R, Rocco MV, Chertow GM, Levin NW. The Effect of Increased Frequency of Hemodialysis on Volume-Related Outcomes: A Secondary Analysis of the Frequent Hemodialysis Network Trials. Blood Purif. 2016;41:277–86. doi: 10.1159/000441966. [DOI] [PubMed] [Google Scholar]
- 29.Raimann JG, Canaud B, Etter M, Kooman JP, Levin NW, Marcelli D, Marelli C, Power A, Duncan N, van der Sande FM, Carioni P, Thijssen S, Xu X, Usvyat LA, Wang Y, Kotanko P. Association between pre hemodialysis serum sodium concentration and blood pressure: results from a retrospective analysis from the international monitoring dialysis outcomes (MONDO) initiative. J Hum Hypertens. 2016;30:442–8. doi: 10.1038/jhh.2015.79. [DOI] [PubMed] [Google Scholar]
- 30.Fenske W, Wanner C, Allolio B, Drechsler C, Blouin K, Lilienthal J, Krane V. Copeptin levels associate with cardiovascular events in patients with ESRD and type 2 diabetes mellitus. J Am Soc Nephrol. 2011;22:782–90. doi: 10.1681/ASN.2010070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinh E, Chan CT. Intensive Home Hemodialysis Results in Regression of Left Ventricular Hypertrophy and Better Clinical Outcomes. Am J Nephrol. 2016;44:300–307. doi: 10.1159/000449452. [DOI] [PubMed] [Google Scholar]


