Abstract
Rationale
Early reperfusion in patients experiencing acute ischemic stroke is effective in patients with large vessel occlusion. No randomized data are available regarding the safety and efficacy of endovascular therapy beyond 6 h from symptom onset.
Aim
The aim of the study is to demonstrate that, among patients with large vessel anterior circulation occlusion who have a favorable imaging profile on computed tomography perfusion or magnetic resonance imaging, endovascular therapy with a Food and Drug Administration 510 K-cleared mechanical thrombectomy device reduces the degree of disability three months post stroke.
Design
The study is a prospective, randomized, multicenter, phase III, adaptive, blinded endpoint, controlled trial. A maximum of 476 patients will be randomized and treated between 6 and 16 h of symptom onset.
Procedures
Patients undergo imaging with computed tomography perfusion or magnetic resonance diffusion/perfusion, and automated software (RAPID) determines if the Target Mismatch Profile is present. Patients who meet both clinical and imaging selection criteria are randomized 1:1 to endovascular therapy plus medical management or medical management alone. The individual endovascular therapist chooses the specific device (or devices) employed.
Study outcomes
The primary endpoint is the distribution of scores on the modified Rankin Scale at day 90. The secondary endpoint is the proportion of patients with modified Rankin Scale 0–2 at day 90 (indicating functional independence).
Analysis
Statistical analysis for the primary endpoint will be conducted using a normal approximation of the Wilcoxon–Mann–Whitney test (the generalized likelihood ratio test).
Keywords: Acute ischemic stroke, clinical trial, endovascular, brain imaging, recanalization, imaging based selection
Introduction
Endovascular stroke therapy, the removal of blood clots with mechanical devices, is an effective treatment for acute stroke. The main advantage of endovascular therapy is that it has a high rate of recanalization. Blood flow can be restored with a success rate of up to 88% with modern thrombectomy devices.1,2 This is approximately twice as effective as intravenous tissue plasminogen activator (iv tPA) which has a recanalization rate of 10–50% depending on the location of the blood clot.3,4
The efficacy and safety of endovascular therapy has been established by a series of recent randomized studies.1,2,5–7 In these trials, endovascular therapy was initiated within 6 h of stroke onset in the vast majority of patients. This prompted new guidelines endorsing endovascular therapy up to 6 h after symptom onset.8 In these guidelines, the American Heart Association (AHA) indicates “Further randomized, controlled trials should be done to determine whether advanced imaging paradigms using computed tomography (CT) perfusion and magnetic resonance imaging (MRI) perfusion, computed tomography angiography (CTA), and diffusion imaging, including measures of infarct core, collateral flow status, and penumbra, are beneficial for selecting patients for acute reperfusion therapy who are beyond 6 hours from symptom onset.” DEFUSE 3 will address this new directive.
Recent data suggest that CT perfusion studies, processed with the RAPID automated software program, can identify the ischemic core with accuracy similar to MRI,9 and select patients who respond very favorably to endovascular reperfusion therapy in early time-windows.1,2 Recent studies also suggest that this approach using both MR and CT perfusion can be used to identify patients who respond favorably to reperfusion therapy at extended time-windows.10,11 Therefore, DEFUSE 3 will allow patient selection with both MRI and CT perfusion. Study enrollment is limited to patients with salvageable tissue (target mismatch patients) who we hypothesize are more likely to respond favorably to endovascular reperfusion in the 6–16 h window than standard medical therapy alone. Use of the latest generation Food and Drug Administration (FDA) cleared thrombectomy devices, coupled with strict qualification and oversight criteria for the neurointerventionalists, should result in high rates of reperfusion.
DEFUSE 3 is the first study to be funded through the NIH StrokeNet. The NIH StrokeNet was created to conduct clinical trials to advance acute stroke treatment, prevention, recovery, and rehabilitation following a stroke.12,13 This network of 25 Regional Coordinating Centers across the US involves more than 300 hospitals and is designed to serve as the infrastructure and pipeline for new stroke trials. DEFUSE 3 will be conducted at up to 45 sites within the StrokeNet and 3 Canadian sites.
Methods
Objective
The DEFUSE 3 study aims to demonstrate that endovascular therapy in the 6 to16-h time-window benefits patients with acute ischemic stroke due to an ICA or middle cerebral artery (MCA) occlusion and a target mismatch on multimodal CT or MR imaging.
Design
DEFUSE 3 is a multicenter, phase III, prospective, randomized, open-label, blinded outcome (PROBE) trial of endovascular therapy plus medical therapy versus medical therapy alone for patients in the extended time-window. An overview of the schedule of study events is shown in Figure 1.
Figure 1.
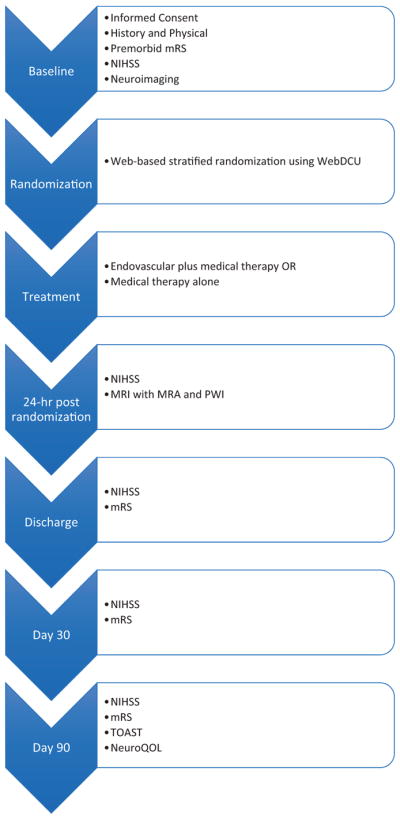
Study flow diagram.
Patient population
Patients who are evaluated for acute stroke are screened for study eligibility. This includes both patients who are directly admitted to the study site and patients who are transferred from an outside hospital. Patients who meet the clinical criteria (Table 1) are informed about the study and they are asked to consent for enrollment. Consent may also be obtained from a legally authorized representative.
Table 1.
Clinical inclusion and exclusion criteria
| Clinical inclusion criteria | |
|---|---|
| 1. | Signs and symptoms consistent with the diagnosis of an acute anterior circulation ischemic stroke |
| 2. | Age 18–90 years |
| 3. | Baseline NIHSS score is ≥ 6 and remains ≥ 6 immediately prior to randomization |
| 4. | Endovascular treatment can be initiated (femoral puncture) between 6 and 16 h of stroke onset. Stroke onset is defined as the time the patient was last known to be at their neurologic baseline (wake-up strokes are eligible if they meet the above time limits) |
| 5. | Modified Rankin Scale less than or equal to 2 prior to qualifying stroke (functionally independent for all ADLs) |
| 6. | Patient/Legally authorized representative has signed the informed consent form |
| Clinical exclusion criteria | |
| 1. | Other serious, advanced, or terminal illness (investigator judgment) or life expectancy is less than 6 months |
| 2. | Pre-existing medical, neurological or psychiatric disease that would confound the neurological or functional evaluations |
| 3. | Pregnancy |
| 4. | Inability to undergo a contrast brain perfusion scan with either MRI or CT |
| 5. | Known allergy to iodine that precludes an endovascular procedure |
| 6. | Treated with tPA >4.5 h after time last known well |
| 7. | Treated with tPA 3–4.5 h after last known well and any of the following; age >80, current anticoagulant use, history of diabetes and prior stroke, NIHSS score >25 |
| 8. | Known hereditary or acquired hemorrhagic diathesis, coagulation factor deficiency; recent oral anticoagulant therapy with INR >3 (recent use of one of the new oral anticoagulants is not an exclusion if estimated GFR >30 ml/min) |
| 9. | Seizures at stroke onset if it precludes obtaining an accurate baseline NIHSS |
| 10. | Baseline blood glucose of <50 mg/dl (2.78 mmol) or >400 mg/dl (22.20 mmol) |
| 11. | Baseline platelet count <50,000/μl |
| 12. | Severe, sustained hypertension (defined as systolic blood pressure >185 mmHg or diastolic blood pressure >110 mm Hg) |
| 13. | Current participation in another investigational drug or device study |
| 14. | Presumed septic embolus or suspicion of bacterial endocarditis |
| 15. | Clot retrieval attempted using a neurothrombectomy device prior to 6 h from symptom onset |
| 16. | Any other condition that, in the opinion of the investigator, precludes an endovascular procedure or poses a significant hazard to the subject if an endovascular procedure was performed |
GFR: glomerular filtration rate; NIHSS: National Institutes of Health Stroke Scale; tPA: tissue plasminogen activator; ADL: activities of daily living.
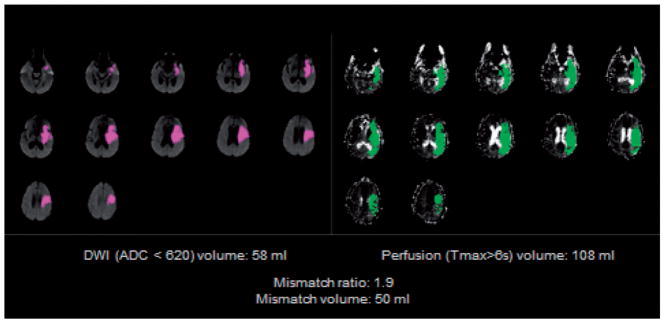
The neuroimaging eligibility criteria (Table 2) limit the study population to patients with a large artery occlusion and evidence of salvageable brain tissue (target mismatch) on multimodal CT or MR imaging. Target Mismatch criteria are assessed on automated maps that identify the volume and location of the ischemic core and critical hypoperfusion lesion (RAPID, iSchemaView, Menlo Park, CA, Figure 2). The RAPID neuroimaging platform was developed based on the data from DEFUSE 1 and was prospectively validated in DEFUSE 2.10,14 The agreement between local investigators and the Imaging Core Lab for identification of the mismatch profile in DEFUSE 2 was 97% (κ0·92; 95% CI 0.83–1). The accuracy of RAPID for identifying the size and location of perfusion and diffusion lesions has been established by extensive validation and testing on blood flow phantoms; the software received FDA 510K clearance for clinical use in 2013.
Table 2.
Neuroimaging inclusion and exclusion criteria.
| Neuroimaging inclusion criteria | |
|---|---|
| 1. | ICA or MCA-M1 occlusion (carotid occlusions can be cervical or intracranial; with or without tandem MCA lesions) by MRA or CTA |
| 2. | Target Mismatch Profile on CT perfusion or MRI (ischemic core volume is <70 ml, mismatch ratio is ≥1.8 and mismatch volume is ≥15 ml) |
| Alternative neuroimaging inclusion criteria if CTA or MRA is technically inadequate: | |
| 1. | Tmax >6 s perfusion deficit consistent with an ICA or MCA-M1 occlusion |
| 2. | Target Mismatch Profile on CT perfusion or MRI (ischemic core volume is <70 ml, mismatch ratio is ≥1.8 and mismatch volume is ≥15 ml) |
| Alternative neuroimaging inclusion criteria if MR perfusion is technically inadequate: | |
| 1. | ICA or MCA-M1 occlusion by MRA (or CTA, if MRA is technically inadequate and a CTA was performed within 60 min prior to the MRI) |
| 2. | DWI lesion volume <25 ml |
| Neuroimaging exclusion criteria | |
| 1. | ASPECT score <6 on non-contrast CT (if patient is enrolled based on CT perfusion criteria) |
| 2. | Evidence of intracranial tumor (except small meningioma), acute intracranial hemorrhage, neoplasm, or arteriovenous malformation |
| 3. | Significant mass effect with midline shift |
| 4. | Evidence of ICA dissection that is flow limiting or aortic dissection |
| 5. | Intracranial stent implanted in the same vascular territory that precludes the safe deployment/removal of the neurothrombectomy device |
| 6. | Acute symptomatic arterial occlusions in more than one vascular territory confirmed on CTA/MRA (e.g., bilateral MCA occlusions, or an MCA and a basilar artery occlusion). |
CTA: computed tomography angiography; DWI: diffusion weighted imaging; ICA: internal carotid artery; MCA: middle cerebral artery; MRA: magnetic resonance angiography.
The mismatch volume is determined by the RAPID software in real time based on the difference between the ischemic core lesion volume and the Tmax >6 s lesion volume. If both a CT perfusion and a multimodal MRI scan are performed prior to enrollment, later the 2 scans are assessed to determine eligibility. Only an intracranial MRA is required for patients screened with MRA; cervical MRA is not required. Cervical and intracranial CTA are typically obtained simultaneously in patients screened with CTA, but only the intracranial CTA is required for enrollment.
Figure 2.
RAPID mismatch map. The RAPID mismatch summary map allows investigators to quickly, accurately, and easily determine if the patient meets the imaging criteria for enrollment. The case shown here meets the Target Mismatch criteria: core volume is <70 ml, mismatch ratio is ≥1.8 and mismatch volume is ≥15 ml.
In some situations, RAPID generated CT or MRI maps may be obtained as part of standard care prior to DEFUSE 3 consent. In these instances, both clinical and neuroimaging selection criteria are considered when screening patients for study eligibility. In general, however, the investigator determines whether a patient meets the Target Mismatch criteria after consent has been obtained. Sequences from a CT perfusion or MRI scan are pushed to RAPID for post-processing. If a patient has undergone multiple imaging evaluations (both MRI and CT or multiple CTs or MRIs), the most recent imaging study determines if the patient meets the imaging criteria. The maps are emailed to investigators (protected health information is automatically removed) within 5 min after the images have been received by RAPID. Patients who meet the neuroimaging selection criteria are randomized.
Consented patients who do not meet neuroimaging selection or clinical selection criteria are not randomized. They receive standard therapy per local guidelines. Limited baseline data and information about stroke therapy received during the first 24 h are collected from these patients.
Randomization
Randomization takes place centrally on a web-based clinical trial management system (WebDCU™). A dynamic stratification system programmed into WebDCU™ ensures well-balanced subgroups. The randomization algorithm employs biased-coin minimization and the variance method with stratification weights.15 The strategy is to balance treatment assignment along the marginal distribution of each stratification factor. The stratification factors used and their hierarchy are: (1) ischemic core volume, (2) age, (3) time from symptom onset to enrollment, (4) National Institutes of Health Stroke Scale (NIHSS) score, and (5) study site. When a new patient is enrolled, the site enters the stratification factor values into the electronic case report form (eCRF) on WebDCU™. The dynamic randomization algorithm determines an imbalance measure for each treatment group. The treatment group associated with the smallest imbalance measure receives the largest probability of assignment in the biased-coin randomization. Optimal biased-coin acceptance region and stratification weights were determined prior to study via simulations and are listed in Table 3.
Table 3.
Dynamic randomization factors
| Factor | Levels | Weight |
|---|---|---|
| Ischemic core lesion volumea | <10, 10–25, 26–50, >50 | 3 (4) |
| Baseline NIHSS | 6–12, 13–18, >18 | 1 |
| Age | <55, 55–69, 70–79, >79 | 4 |
| Time from symptom onset to randomization | <9, 9–12, >12 | 2 |
| Study siteb | 8/1 |
NIHSS: National Institutes of Health Stroke Scale.
The weight for the core lesion volume factor increases to 4 for the >50 level.
The weight for the site factor is 8 for the first four subjects enrolled at the site, and then it changes to 1.
Treatment
Patients are assigned to either endovascular therapy plus medical therapy or to medical therapy alone (1:1 randomization). Crossover from medical to endovascular therapy is strictly prohibited; endovascular to medical therapy crossover is only allowed if an endovascular contraindication arises after randomization. The study sites are closely monitored for crossovers.
Endovascular therapy
In patients randomized to endovascular therapy, the femoral artery puncture is performed within 60 min (maximum 90 min) of the completion of the qualifying imaging. FDA-cleared thrombectomy devices (stent-retrievers) or suction thrombectomy systems are used in the treatment of thrombus removal in patients experiencing an acute stroke within 8 h of symptom onset. These devices are used up to 16 h following symptom onset in DEFUSE 3 based on an FDA investigational device exemption (IDE). The devices that are currently approved are the Trevo Retriever (Stryker Neurovascular, Fremont CA), the Solitaire Revascularization Device (Medtronic, Irvine, CA), Covidien MindFrame Capture Revascularization Device (Medtronic), and the Penumbra thrombectomy system (Penumbra, Alameda, CA). Additional devices may be added during the course of the study if they receive FDA clearance.
The individual investigators may use any of these devices or any combination of these devices to remove thrombus from the ICA, MCA M1 segment or, if needed, from M2 segments of the intracranial circulation. These are all approved anatomic locations for these devices. The thrombectomy devices should be used in accordance with the indications. If there is a severe stenosis in the common carotid artery or the proximal internal carotid artery (ICA), investigators may also use other FDA devices approved for angioplasty or FDA devices approved for stenting of the carotid artery as deemed appropriate. Adjuvant intra-arterial (IA) thrombolytic agents cannot be used in DEFUSE 3.
The study sites use local protocols for femoral access, sedation, heparin infusion, monitoring, etc. The interventionalist performs a cervical injection in the involved carotid circulation as a baseline angiogram. After the procedure, a post-treatment angiogram with a cervical injection of the involved carotid circulation is also obtained. Imaging covers the full region of the normal circulation in anterior-posterior (AP) and lateral projections at 2–3 films per second through the entire venous phase.
Standard medical therapy
All randomized patients received standard medical therapy based on current AHA guidelines. Based on the time-window for DEFUSE 3, it is anticipated that very few of the patients enrolled in DEFUSE 3 will have received iv tPA prior to randomization. For these patients, the study sites’ post-tPA protocol is followed. Non-tPA treated patients randomized to medical therapy are treated with aspirin, 325 mg on day 1, and 81–325 mg/day (at the discretion of the patient’s attending physician) on days 2–5, unless an indication for early anticoagulation is present (as determined by the patient’s attending physician). All patients received standard deep venous thrombosis (DVT) prevention therapy. Intravenous anticoagulants are prohibited (unless a clear indication for early anticoagulation is documented); dual antiplatelet therapy is prohibited unless carotid stenting was performed during the endovascular procedure or a clear indication for dual antiplatelet therapy is documented. The patient’s attending physician determines subsequent antithrombotic therapy.
Clinical and imaging evaluations
Randomized patients are followed clinically for 90 days and have an MRI/MRA/MR perfusion at 24 h (range 18–30 h) to assess infarct volume, recanalization, hemorrhage, and reperfusion (Table 4). If clinical worsening (defined as a ≥4-point increase on the NIHSS score) occurs prior to discharge, an additional CT scan or MRI is obtained as soon as possible. All brain imaging from stroke onset through hospital discharge, including the baseline MRI and CT, as well as angiographic images obtained for the diagnostic and therapeutic portions of the endovascular procedure, are transmitted to the core lab. The imaging core lab assesses the ASPECT score, ischemic core, and critically hypoperfused tissue volumes on baseline imaging, and final infarct volume, reperfusion, recanalization on the 24-h follow-up MRI scan. Intracranial hemorrhage is assessed on images obtained through 36 h post randomization. A thrombolysis in cerebral infarction (TICI) reperfusion score is assessed by the angiographic core lab on the digital subtraction angiography (DSA) images of patients randomized to endovascular therapy.
Table 4.
Schedule of events
| Data collected | Screening | Enrollment | Baseline/Randomization | Endovascular procedure | 24 h (±6 h) | Hospital discharge | Day 30 (±7 days) | Day 90 (±14 days) |
|---|---|---|---|---|---|---|---|---|
| Screen failure log | X | |||||||
| Informed consent | X | |||||||
| Subject enrollment | X | |||||||
| Inclusion and exclusion criteria | X | |||||||
| MRI or CTP | X | Xb | ||||||
| Randomization | X | |||||||
| Medical history | X | |||||||
| Vital signs | X | |||||||
| NIH Stroke Scale | X | X | X | X | X | |||
| Modified Rankin Scale | Xc | X | X | X | ||||
| Baseline ASPECT Score | X | |||||||
| Baseline labsa | X | |||||||
| Endovascular therapy | X | |||||||
| 24-h labs | X | |||||||
| Hospital discharge | X | |||||||
| Adverse event assessment | X | X | X | X | X | |||
| NeuroQOL | X |
CBC: complete blood count; CTP: computed tomography perfusion; INR: international normalized ratio; MRI: magnetic resonance imaging; PTT: partial thromboplastin time.
Baseline laboratory evaluation includes CBC with platelets, creatinine, glucose, INR, activated PTT, and pregnancy test (if applicable). At 24-h follow-up only creatinine is required.
Patients will preferably undergo an MRI/MRA/MR perfusion at 24 h; if an MR cannot be performed, a CT/CTA/CTP can be substituted. For patients who are consented but not randomized, the schedule of events is limited to a summary of stroke therapies received within 24-h of stroke onset.
Historical mRS at baseline, then mRS/NIHSS to be performed by an NIHSS/mRS certified member of the research team who is blinded to treatment allocation at 30 and 90 days.
Primary outcome
The primary endpoint is the distribution of scores on the modified Rankin Scale (mRS) at day 90. The mRS score at 30 and 90 days must be performed by an mRS certified assessor who is blinded to treatment allocation. If an in-person visit is not possible, then the mRS should be performed by phone by an mRS certified assessor who is blinded to treatment allocation.
Secondary outcomes
The secondary clinical endpoint is the proportion of patients who are functionally independent at day 90, defined as a mRS score of 0–2.
DEFUSE 3 has three primary imaging endpoints: (1) infarct volume on diffusion weighted imaging (DWI) (or CT if DWI not feasible) at 24 ± 6 h after randomization; (2) lesion growth between the RAPID identified ischemic core on baseline imaging and the infarct volume at 24 h; and (3) reperfusion defined as the percentage reduction in Tmax > 6 s lesion between baseline and 24 h. The 24-h time-point is based on data demonstrating that assessment of infarct volume at 24 h captures the effect of reperfusion therapies on infarct growth and predicts the outcomes similarly to day 90 infarct volumes.16,17
The safety endpoints are (1) symptomatic intracranial hemorrhage within 36 h from randomization, defined as a ≥4-point worsening of the NIHSS associated with brain hemorrhage; (2) serious adverse events; and (3) 90-day mortality.
Adaptive design
DEFUSE 3 features a novel adaptive trial design that allows the study to focus on a subpopulation if interim or final analyses indicate futility in the overall population.18 The adaptive design, developed specifically for DEFUSE 3, is based on closed testing theory and the group sequential methods for the generalized likelihood ratio (GLR) statistic developed by Lai and Shih.19
In case of futility at either interim analysis (n = 200 and 340), the adaptive design allows reallocation of future accrual to the subgroup with the best prospects for showing efficacy. If a subgroup is chosen at an interim analysis, subsequent enrollment is limited to patients in that subgroup. Thus, this subgroup will become larger than it would have been in the absence of the adaptive design. The criterion for deciding which subgroup has the best chance of showing a benefit from endovascular therapy combines both the estimated size of the effect in the subgroup and the sample size of the subgroup.
For each interim analysis, an efficacy bound will be set to control the overall (one-sided) type I error rate at 2.5%. At each interim analysis, a futility bound will be set to decide if the study should continue recruitment in the overall group, shift accrual and testing to a subgroup, or stop in its entirety. When a subgroup is selected at an interim analysis, the maximum number of patients who can enter the final analysis is reduced by the number of randomized patients who are not included in that subgroup. This is because the maximum number of patients who can be randomized is fixed as 476. After subgroup selection, the futility boundary adapts to account for the reduced number of patients who can enter the final analysis. The final analysis is done after 476 randomized patients complete 90-day follow-up: If enrollment after one of the interim analyses is limited to a selected subgroup, the null will be tested in that subgroup only and efficacy or lack thereof will be declared.
Power and sample size
The projected overall effect of endovascular therapy is based on (1) the observed 90-day modified Rankin Scale outcomes in DEFUSE 2 of target mismatch patients treated >6 h after symptom onset and (2) the assumption that early reperfusion will be achieved in 75% of the patients in the endovascular arm vs. 20% in the medical therapy arm.20–22 Using these data, we projected the distributions on the mRS at 90 days in the endovascular and control arms of DEFUSE 3 (Table 5).
Table 5.
Expected distribution of 90-day functional outcomes in DEFUSE 3
| mRS at day 90 | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Endovascular group | 18.0% | 11.5% | 19.6% | 11.5% | 16.4% | 11.5% | 11.5% | 100% |
| Medical group | 9.7% | 7.9% | 15.0% | 17.7% | 14.4% | 17.7% | 17.7% | 100% |
mRS: modified Rankin Scale.
This distribution corresponds to a standardized effect of 0.36 for the primary analysis. Based on these data, the fixed sample size for a non-adaptive design requires a total of 376 patients (188/arm) to have 90% power at an alpha of 5% (Wilcoxon–Mann–Whitney (WMW) test); 100 additional patients are included for the adaptive design to reach a maximum sample size of 476 for DEFUSE 3. The size of this increase is based on simulations and is selected to preserve the desired operating characteristics, while allowing shrinkage in effect size to 0.30, since the above estimate of 0.36 may be optimistic.
DEFUSE 3 is highly powered to demonstrate the differences in lesion growth. DEFUSE 2 demonstrated a substantial reduction in infarct growth among target mismatch patients treated in the 6–12 h time-window who achieved early reperfusion: median growth 0.5 ml (interquartile range (IQR): −2–10) with reperfusion (n = 23) vs. 39 ml (IQR: 18–121) without reperfusion (n = 13), p <0.001. These data have been extrapolated to DEFUSE 3 using the same assumptions described above; it anticipated an early reperfusion rate of 75% in the endovascular arm vs. 20% in the medical arm. This yields a sample size of 42 per group for 90% power.
Analysis of primary endpoint
The results of this study will be primarily expressed as whether an efficacy boundary was crossed at either one of the two interim analyses or at the final analysis. Crossing of the efficacy boundary will be considered evidence that endovascular therapy is beneficial, based on lower day-90 mRS scores in the endovascular group compared to controls (i.e. favorable shift on the mRS). The primary null hypothesis will be tested at the interim and final analyses using a normal approximation of the WMW test (the GLR test). The primary analysis will be intention to treat, unadjusted for covariates. The treatment effect, adjusted for study design, will be expressed as (1) the WMW measure of superiority, with its 95% confidence interval and p value; (2) the average number needed to treat (NNT) for benefit, with its 95% confidence interval, where NNT = 1/absolute risk difference (ARD); and (3) the common odds ratio with its 95% confidence interval and p value, calculated using a proportional odds model.
Discussion
DEFUSE 3 aims to shift the selection of patients for late reperfusion therapy to an objective decision based on scientific evidence. A central consideration in the optimization of patient selection for acute stroke therapies is the concept of the ischemic penumbra. The ischemic penumbra is defined as ischemic tissue that is potentially salvageable and is distinguished from the ischemic core that has already sustained irreversible injury. Clearly, the target of acute stroke therapies is salvage of the ischemic penumbra, preventing infarct growth, and, most importantly, improved functional outcome.
Acute stroke trials should therefore ideally be limited to patients with an ischemic penumbra. MRI-based studies, such as DEFUSE 1 and 2, and CT perfusion studies such as CRISP, EXTEND-IA, and SWIFT-PRIME suggest that MRI and/or CT perfusion can be used to identify these patients.1,2,10,11,14
The DEFUSE 2 study utilized automated mismatch analysis software (RAPID) to prospectively establish MRI profiles in a consecutive cohort of patients who then underwent endovascular therapy.10,23 DEFUSE 2 confirmed the concepts originally demonstrated in DEFUSE and EPITHET;14,24 target mismatch patients who achieved early reperfusion therapy had less infarct growth and more favorable clinical outcomes.10 No association between reperfusion and favorable outcomes or infarct growth was present in patients without target mismatch. Furthermore, the positive association between reperfusion, favorable clinical response, and attenuation of infarct growth did not diminish in DEFUSE 2 patients with target mismatch who were treated up to 6–12 h after symptom onset.25
Many factors affect the evolution of the ischemic penumbra into the ischemic core, and the rate of progression of irreversible injury is highly variable between individuals. This variability is likely mediated by the adequacy of collateral blood flow as well as the metabolic milieu of individual stroke patients. The individuality of penumbral evolution among stroke patients implies that identifying the extent of the ischemic core and penumbra is useful for making treatment decisions.
DEFUSE 3 enrollment is limited to patients with salvageable tissue (target mismatch patients) who, despite relatively late treatment, are likely to respond favorably to reperfusion. Use of modern thrombectomy devices by experienced neuro-endovascular therapists should result in high rates of reperfusion. DEFUSE 3 has the potential to substantially expand the treatment window for large vessel ischemic stroke which could lead to a considerable reduction in stroke morbidity.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The DEFUSE 3 study is funded by the National Institutes of Health U01NS092076. ClinicalTrials.gov Identifier: NCT02586415.
Footnotes
DEFUSE 3 is using RAPID software for patient selection.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs Gregory W Albers and Roland Bammer have equity interest and are consultants for iSchemaView.
References
- 1.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 3.Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992;42:976–982. doi: 10.1212/wnl.42.5.976. [DOI] [PubMed] [Google Scholar]
- 4.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414905. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 7.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 9.Cereda CW, Christensen S, Campbell BC, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a dwi standard. J Cereb Blood Flow Metab. 2016;36:1780–1789. doi: 10.1177/0271678X15610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (defuse 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lansberg MG, Christensen S, Kemp S, et al. Main results of the CTP to predict response to recanalization in ischemic stroke project (CRISP) Stroke. 2016;47:A57. [Google Scholar]
- 12.Broderick JP, Palesch YY, Janis LS. The national institutes of health strokenet: a user’s guide. Stroke. 2016;47:301–303. doi: 10.1161/STROKEAHA.115.011743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mott M, Janis S, Koroshetz WJ. Strokenet takes off: National Institute of Neurological Disorders and Stroke Organizational update. Stroke. 2016;47:e51–52. doi: 10.1161/STROKEAHA.115.012063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 15.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 16.Campbell B, Purushotham A, Christensen S, et al. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab. 2012;32:50–56. doi: 10.1038/jcbfm.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell BC, Tu HT, Christensen S, et al. Assessing response to stroke thrombolysis: validation of 24-hour multimodal magnetic resonance imaging. Arch Neurol. 2012;69:46–50. doi: 10.1001/archneurol.2011.232. [DOI] [PubMed] [Google Scholar]
- 18.Lai TL, Lavori PW, Liao OY. Adaptive choice of patient subgroup for comparing two treatments. Contemp Clin Trials. 2014;39:191–200. doi: 10.1016/j.cct.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai TL, Shih M-C. Power, sample size and adaptation considerations in the design of group sequential clinical trials. Biometrika. 2004;91:507–528. [Google Scholar]
- 20.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (trevo 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the merci retriever in patients with acute ischaemic stroke (swift): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 22.Pereira VM, Gralla J, Davalos A, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke. Stroke. 2013;44:2802–2807. doi: 10.1161/STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (epithet): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 25.Lansberg MG, Cereda CW, Mlynash M, et al. Response to endovascular reperfusion is not time-dependent in patients with salvageable tissue. Neurology. 2015;85:708–714. doi: 10.1212/WNL.0000000000001853. [DOI] [PMC free article] [PubMed] [Google Scholar]