Abstract
Ambient air pollution is a known public health hazard that negatively impacts non-cutaneous organs; however our knowledge regarding the effects on skin remains limited. Current scientific evidence suggests there are four mechanisms by which ambient air pollutants cause adverse effects on skin health: (a) generation of free radicals, (b) induction of inflammatory cascade and subsequent impairment of skin barrier, (c) activation of the aryl hydrocarbon receptor (AhR), and (d) alterations to skin microflora. In this review, we provide a comprehensive overview on ambient air pollutants and their relevant sources, and highlight current evidence of the effects on skin.
Keywords: Ozone, traffic related pollutants, outdoor air pollutants, inflammatory skin reaction, oxidative stress, skin aging, cutaneous microflora
Introduction
It has long been recognized that environmental exposures affect the health of skin. Ultraviolet (UV) radiation from sunlight has been the most studied environmental hazard and its consequences on skin are well established.1–3 Another potentially serious, yet less recognized, environmental exposure is ambient (outdoor) air pollution. In 2014, the US Environmental Protection Agency (EPA) reported that over 142 million Americans live in areas where the air quality fails to meet the National Ambient Air Quality Standards.4 In the same year, the World Health Organization attributed seven million premature deaths to air pollution exposure and designated air pollution as the “world’s largest single environmental health risk.”5 Numerous studies have unmasked the deleterious effects of air pollutants on internal organs.6–8 However, our knowledge regarding the effects on skin health remains limited. In this review, we provide an overview on ambient air pollution and highlight current evidence that suggests air pollution may have adverse effects on skin health.
Ambient air pollutants and sources
Outdoor air pollution is comprised of organic and inorganic substances that are introduced into the atmosphere and pose a health hazard to humans and the ecosystem. Pollutants are derived from natural and anthropogenic sources. The EPA has designated six criteria pollutants (Table 1) that are routinely monitored: (a) ground-level ozone, (b) particulate matter (PM), (c) sulfur dioxide (SO2), (d) lead (e) carbon monoxide (CO), and (f) nitrogen oxides (NOx,) which includes nitric oxide (NO), nitrogen dioxide (NO2), and nitrous oxide (N2O).
Table 1.
Six criteria pollutants monitored in the United States by the National Ambient Air Quality Standard
| Ambient Air Pollutant |
Date of Most Recent Revision |
Averaging Time |
Standard Level* |
Source Type | Common Sources |
|---|---|---|---|---|---|
|
| |||||
| Carbon Monoxide42 | Aug 31, 2011 | 1-hour | 40 mg/m3 (35 ppm) | Anthropogenic |
|
|
| |||||
| 8-hour | 10 mg/m3 (9 ppm) | ||||
|
| |||||
| Lead43 | Nov 12, 2008 | Quarterly | 0.15 µg/m3 | Anthropogenic |
|
|
| |||||
| Nitrogen Dioxide44, 45 | Feb 9, 2010 | 1-hour | 188 µg/m3 (100 ppb) | Anthropogenic |
|
|
| |||||
| Oct 8, 1996 | Annual | 100 µg/m3 (53 ppb) | |||
|
| |||||
| Ozone46 | Mar 27, 2008 | 8-hour | 147 µg/m3 (75 ppb) | Natural/Anthropogenic |
|
|
| |||||
| PM2.547 | Dec 14, 2012 | 24-hour | 15 µg/m3** | Natural/Anthropogenic |
|
|
| |||||
| Annual | 35 µg/m3 | ||||
|
| |||||
| PM1048 | Dec 14, 2012 | 24-hour | 150 µg/m3 | Natural/Anthropogenic |
|
|
| |||||
| Sulfur dioxide49 | Jun 22, 2010 | 1-hour | 196 µg/m3 (75 ppb) | Natural/Anthropogenic |
|
ppm, parts per million; ppb, parts per billion; mg/m3, milligrams per cubic meter of air; µg/m3, micrograms per cubic meter of air;
Based on primary standards for “sensitive” populations, such as asthmatics, children and elderly, who may experience adverse health effects from pollutant concentrations above levels reported.50 O3
As of December 2014, the primary fine particulate standard is 12.0 µg/m3.51
In addition to the above mentioned pollutants, ambient air pollution contains other toxic compounds and can be classified into (a) gaseous pollutants, (b) persistent organic pollutants (POPs), (c) particulate matter, (d) heavy metals, and (f) traffic-related/other toxic pollutants.
Gaseous Pollutants
NOx, CO, SO2, ozone, and volatile organic compounds (VOCs) are the most common gaseous pollutants.9 They are mainly derived from the combustion of fossil fuels (i.e., coal, petroleum and natural gas). Ozone is formed in the atmosphere from chemical reactions involving NO2, VOCs, and UV light. CO is an odorless, colorless gas produced from incomplete combustion of fossil fuels. Common sources of CO include fuel-burning devices, such as automobiles, power generators, and boilers. CO is also a well-known byproduct of tobacco smoke.10 SO2 is a highly reactive compound generated from the processing of sulfur containing materials such as crude oil and coal.9
Persistent Organic Pollutants
POPs are compounds resistant to environmental degradation that are capable of long-range transport and bioaccumulation in humans and animals. Common POPs include pesticides, dioxins and dioxin-like polychlorinated biphenyls. Dioxins are highly toxic compounds with an estimated half-life of 7–11 years.11 They are byproducts of industrial processes such as smelting, chlorine bleaching and manufacturing of herbicides and pesticides. They are also naturally produced in volcanic eruptions and forest fires. Over 400 types of dioxins-related compounds have been identified with 2,3,7,8-tetrachlorodibenzoparadioxin (TCDD) considered the most toxic.11 These compounds are widely distributed in the environment with highest concentration in soil and lower concentrations in the air. Airborne dispersion of dioxins may result in bioaccumulation in plants and food products by binding to lipids and lipid membranes.12
Particulate Matter
PM is a complex mixture of liquid and/or solid droplets suspended in gas. Coarse particles (PM10) are between 2.5µm–10µm in diameter. They are components of dust, soil, and dusty emission from various industries. Fine particles (PM2.5) measure <2.5µm in diameter and are emitted from open fires, power plants, and automobile exhaust.9 Ultrafine particles (PM0.1) measure <0.1µm in diameter and are associated with emission from modern-day diesel powered engines. Currently, regulatory standards for PM0.1 do not exist, although these particles are emerging as the most abundant particulate pollutants in urban and industrial settings.13 PM0.1 may pose health hazards by penetrating endothelial tight junctions thereby gaining direct access to the interstitium and vascular system. This may adversely affect the pulmonary and cardiovascular system.7, 13, 14 In addition to gaining systemic access, PM0.1 and other PMs are known to exert deleterious effects by acting as a “Trojan Horse” and carrying additional toxic compounds on their surface, including bacteria, carcinogens, acids, POPs, and metals.9
Heavy Metals
Heavy metals such as cadmium, lead and mercury are common air pollutants that pose health hazards due to bioaccumulation.9 Volcanoes, waste incineration, cement, iron and steel production are relevant sources of airborne cadmium and lead particles. Lead is also emitted from the combustion of leaded-gasoline, which has been banned in many developed countries, but continues to be used in certain developing countries.15 Mercury is a component of the Earth’s mantle that evaporates from the surface of the sea. Other sources of airborne mercury particles include combustion of coal and other fossil fuels.15
Traffic-Related/Other Toxic Pollutants
Traffic-related pollution (TRP) is a mixture of pollutants derived from the primary emission of gasoline- and diesel-fueled vehicles. TRP contains carbon dioxide (CO2), CO, NOx, VOCs, PM, lead and other toxic chemicals such as formaldehyde and 1, 3-butadiene.16 Urban communities and neighborhoods located near busy roads are exposed to high levels of TRP. Besides TRP, there is an extensive list of other toxic pollutants,17 which includes polycyclic aromatic hydrocarbons (PAHs), benzene, and asbestos.
Proposed mechanisms for direct skin toxicity
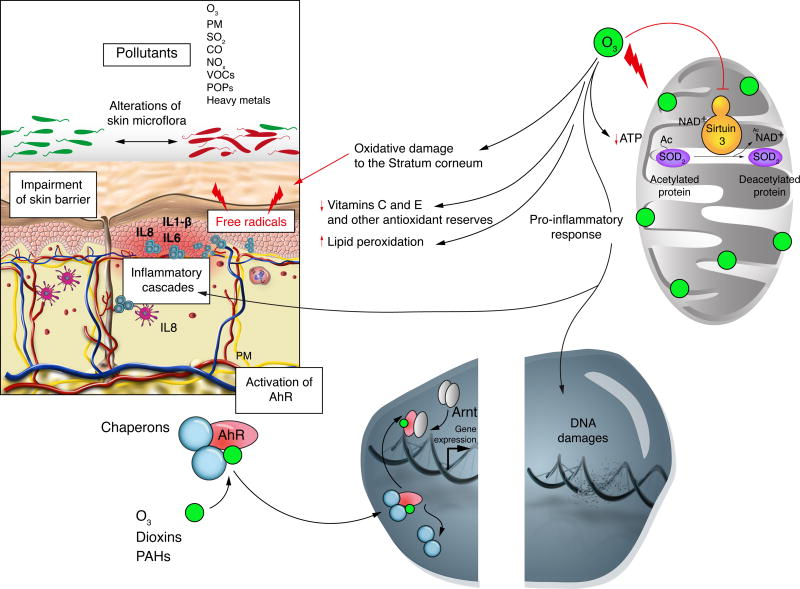
The exact mechanism by which ambient air pollutants cause skin damage has yet to be elucidated. Based on current evidence, there may be four potential mechanisms that account for the deleterious effects on skin: (a) generation of free radicals, (b) induction of inflammatory cascade and disruption of skin barrier, (c) activation of the aryl hydrocarbon receptor (AhR), and (d) alterations to skin microflora (Figure 1).
Figure 1.
Ambient air pollutants exert deleterious effects on the skin by generating free radicals, inducing cutaneous inflammatory cascades, activating AhR dependent mechanisms, and altering cutaneous microflora. Chronic exposure to ozone results in cumulative oxidative damage to the stratum corneum, ultimately generating free radical species. Furthermore, ozone depletes both enzymatic and non-enzymatic antioxidant reserves in the skin with notable effects on Vitamin C and E levels. In the mitochondria, ozone also depletes ATP and Sirtuin 3 levels, a protein involved in mitochondrial free radical scavenging. In addition to these effects, other pollutants are known to promote a pro-inflammatory environment in the skin resulting in increased levels of IL-1β, IL-6 and IL-8. These mediators activate granulocyte chemotaxis and phagocytosis. Ultimately, these combined processes result in direct and indirect toxicity to the skin.
O3, ozone; PM, particulate matter; SO2, sulfur dioxide; CO, carbon monoxide; NOx, nitrogen oxides; VOCs, volatile organic compounds; POPs, persistent organic pollutants; IL - interleukin; PAHs, polycyclic aromatic hydrocarbons; AhR, aryl hydrocarbon receptor; Arnt, AhR nuclear translocator; ATP, adenosine triphosphate; SOD2 - superoxide dismutase 2; NAD+, nicotinamide adenine dinucleotide.
Generation of free radicals
Ozone in the stratosphere has protective effects by filtering solar UV radiation; however, in the troposphere ozone has toxic implications for skin. Ozone is a highly reactive compound that generates free radicals and depletes antioxidants in the epidermis.18 Initial studies examining the effects of ozone exposure on skin were carried out on SKH-1 hairless mice. Thiele et al.19 exposed hairless mice to varying levels of ozone, ranging from 0 parts per million (ppm) to 10ppm for 2-hours. After initial treatment, mice were further exposed to either 0ppm or 1ppm of ozone for an additional 6 days. Exposure to increasing doses of ozone resulted in a dose-dependent depletion of vitamin C and E, and formation of malondialdehyde, a marker for lipid peroxidation. Furthermore, low-level continuous exposure to ozone resulted in cumulative oxidative damage to the stratum corneum.19 Two similar studies conducted by this group further demonstrates that ozone depletes both enzymatic and non-enzymatic antioxidant reserves in the skin (i.e., uric acid, vitamin c, tocopherol, glutathione),20 and that topical application of antioxidants attenuates oxidative damage to cutaneous lipids.21
There were two main limitations in these studies. First, the investigators exposed mice to ozone levels that were higher than levels found in the environment; and second, mouse epidermis is significantly thinner than human epidermis. To address the first concern, McCarthy et al.22 exposed normal human epidermal keratinocytes (NHEKs) to environmentally relevant levels of ozone (e.g., 0.4 & 0.8ppm) for 30-minutes. Their results demonstrate that exposure to 0.8ppm of ozone causes DNA breakage and deplete ATP and Sirtuin 3 levels, which suggest that mitochondrial function may be compromised. Sirtuin 3 is mitochondrial NAD-dependent deacetylase important for activation of superoxide dismutase 2, an enzyme involved in free radical scavenging in the mitochondria.22, 23
With regards to differences in epidermal thickness, He et al.24 tested the effects of environmentally realistic levels of ozone (e.g., 0.8ppm) on human participants. The study assessed biochemical and clinical changes that occur in human skin after short-term, in-vivo exposure to ozone. There was a 70% decrease in endogenous vitamin E levels and a concomitant increase of 230% in lipid hydroperoxide in the stratum corneum. By comparison, higher doses of ozone (e.g., 5–10ppm for 2-hrs) were required to induce similar increases in lipid peroxidation markers in mouse skin, therefore suggesting that human stratum corneum may be biochemically more sensitive to the effects of ozone than mouse stratum corneum.19,24
Induction of inflammatory cascade
Several studies suggest that air pollution induces inflammation. Cytokines and interleukins are small proteins released from a wide-variety of cells involved in cellular signaling and induction of the inflammatory cascade. Interleukin 8 (IL-8) is a pro-inflammatory mediator of the innate immune system with two primary functions: induction of chemotaxis of neutrophils and other granulocytes and activation of phagocytosis.25 Pollutants such as diesel-exhaust particles (DEPs) have been shown to induce a strong inflammatory response in human skin cells, including a significant increase in IL-8 production.26 Ushio et al.26 incubated NHEKs for 24-, 48-, and 72-hours with varying concentrations of DEPs (0.4 to 20µg/ml). After 72-hours of incubation, there was a significant increase in the production IL-8 at low levels of DEP exposure. Also, there was a significant increase in IL-1β production after 48-hours of incubation with 20 µg/ml of DEP. Subsequent research27 further supports these findings by demonstrating that DEPs at concentrations relevant to human exposure increase expression of NF-kB, a transcription factor that regulates the expression of pro-inflammatory cytokines in mouse epidermal cells.
In addition to DEPs, dust particles containing a complex mixture of PMs and heavy metals have been shown to increase gene expression of pro-inflammatory cytokines in human epidermal cells. Choi et al.28 incubated NHEKs for 24-hours with sterilized dust particles obtained from three independent Asian dust storms occurring from 2004 to 2006. Epidermal cells showed a significant increase in mRNA expression of pro-inflammatory cytokines IL-6, IL-8, and granulocyte macrophage colony-stimulating factor. Furthermore, this study showed epidermal cells had significant expression of caspase-14, a protein found in terminally differentiated keratinocytes that is involved in regulation of skin hydration and cornification of the stratum corneum.28 Increases in caspase-14 suggest skin cells initiate a compensatory mechanism in response to exposure to dust particles.28 Altogether, these studies suggest that air pollution induces a pro-inflammatory state in the epidermis, which may alter epidermal differentiation and consequently affect the immunological barrier of the skin.
Activation of AhR
AhR is a cytosolic ligand-activated transcription factor found in various types of skin cells that regulate cellular proliferation, inflammation and melanogenesis.29 Ligand activation of AhR results in translocation to the nucleus where it complexes with the AhR nuclear translocator and binds to specific DNA consensus sites known as xenobiotic response element (XRE). Genes containing XREs include the cytochrome P450 detoxification enzymes (CYP).30 Activation of AhR may play a role in mediating toxic effects associated with xenobiotic exposure including air pollutants such as ozone, dioxins and PAHs.31
Several studies have examined the role of AhR activation in cutaneous mechanisms ranging from regulation of melanogenesis to the development of inflammatory skin lesions. Tauchi et al.32 engineered a line of transgenic mice that expressed an activated AhR in the absence of ligand stimulation. In these mice, activation of AhR led to the development of severe postnatal skin rash along with pruritus and inflammation resembling atopic dermatitis. Gene profile analysis of the mouse skin showed a significant upregulation of genes associated with inflammatory cascade reactions. These results imply that activation of AhR and AhR-targeted genes may be involved in the development of adverse skin reactions in response to AhR ligands.
Aside from chronic inflammation, AhR has been shown to be a novel regulator of melanogenesis. Luecke et al.33 exposed melanocytes from human donors to 10nM of TCDD for 5 days and measured tyrosinase activity and melanin content in-vitro. After 3 days of TCDD exposure, tyrosinase activity was profoundly upregulated in melanocytes, which was abolished by co-treatment with oxy-4’-nitroflovane or α-napthoflavone, partial AhR antagonists. After 5 days of TCDD treatment, the melanocytes showed a high level of tyrosinase activity and a three-fold increase in total intracellular melanin content. A similar experiment conducted on human FM55 melanoma cells showed TCDD significantly increased melanin content, confirming that the melanogenic pathway was upregulated by AhR activation.33
AhR has also been identified as an ozone sensor. Afaq et al.34 exposed NHEKs to 0.3ppm of ozone over a 20-minute period and harvested epidermal cells immediately, 3-hours and 6-hours after exposure. In their study, ozone induced AhR nuclear translocation and increased AhR mRNA expression. Furthermore, ozone markedly increased protein and mRNA expression of CYP1 isoforms. CYP1 enzymes are known to metabolize compounds that are more toxic than the respective parent compounds and to activate many xenobiotic procarcinogens.34–37
Alterations to cutaneous microflora
Resident cutaneous microflora helps maintain homeostasis and prevents overgrowth of harmful microbes. Ambient air pollutants have been shown to alter skin microflora. He et al.24 showed that in-vivo human skin exposure to ozone led to a 50% reduction in resident skin microflora, suggesting that ozone has bacteriocidal effects. Sowada et al.38 investigated the relationship between skin microflora and PAH metabolism. These investigators isolated bacterial species on human skin that were capable of degrading benzo[a]pyrene (BaP), a prototypical PAH. The bacterial species isolated were capable of using BaP as its sole source of carbon and energy. These results raise the question whether skin bacteria exert a protective or harmful effect on skin with regards to PAH metabolism, as complete metabolism of BaP may be protective to human skin, but partial degradation may result in bioactivation and enhanced skin toxicity.
Epidemiologic evidence of adverse skin effects
Epidemiologic evidence focusing on the effects of outdoor air pollution on skin health remains scarce. Current evidence is limited to the impact on skin aging and atopic dermatitis. Two studies suggest air pollution exacerbates the development of atopic dermatitis. A cross-sectional study39 on 1273 German children found that children living in areas with high pollution were more likely to develop atopic dermatitis, compared to children who lived in areas with less pollution. Subsequent research40 followed a cohort of 3390 German children from birth until 6 years of age. These investigators studied the prevalence and incidence of eczema and respiratory allergic diseases and discovered that prevalence, not necessarily incidence of eczema, at age 6 is significantly associated to TRP and soot exposure, implying that the development of eczema may be associated with the duration of pollution exposure.
Air pollution also plays a role in accelerating extrinsic skin aging. Vierkötter et al.41 clinically assessed 400 Caucasian women aged 70–80 years who lived in urban or rural areas of Germany for signs of extrinsic skin aging. They found that air pollution was significantly associated with the presence of coarse wrinkles and pigmented spots. An increase in soot and TRP levels was associated with a 20% increase in pigmented spots on the forehead and cheeks.41 Furthermore, background particulate pollution, not directly attributed to traffic emissions, was found to positively correlate with pigmented spots on the face. These findings are strong evidence of the potential influence that ambient pollution may have on skin aging.
Conclusion
Our review demonstrates that ambient air pollution has an impact on skin health. Currently, our knowledge regarding the effects of air pollution on skin remains limited. Given the increasing trend of urbanization and rise of air pollution in cities around the world, urgent research is needed to understand the mechanisms by which air pollutants exert deleterious effects. Future studies should be designed to resemble real-life situation and to examine the short- and long-term effects of ambient air pollution on skin. Furthermore, future studies should address some of the methodological limitations to the models presented in this review. In particular, incubation of keratinocytes in submerged culture with particles is not physiological; and therefore, alternative exposure methods should be explored. The use of a three-dimensional cellular matrix or employing protocols that utilize an air-liquid interface or aerosol/air-droplet exposure method may be viable alternatives that more closely resemble in-vivo situations. Lastly, due to paucity of scientific evidence, there are no established guidelines currently available for protecting the skin against air pollution. Aside from reducing exposure, potential protection strategies should focus on repairing the skin barrier, replenishing antioxidant reserve, and reducing inflammation caused by air pollutants.
Acknowledgments
The authors have no acknowledgments.
Funding/Support: None
Abbreviations used
- AhR
aryl hydrocarbon receptor
- CO
carbon monoxide
- CO2
carbon dioxide
- CYP
cytochrome P450 enzymes
- DEPs
diesel-exhaust particles
- EPA
environmental protection agency
- N2O
nitrous oxide
- NHEKs
normal human epidermal keratinocytes
- NO
nitric oxide
- NO2
nitrogen dioxide
- NOx
nitrogen oxides
- PAH
polycyclic aromatic hydrocarbon
- PM
particulate matter
- PM0.1
ultrafine particulate matter
- PM2.5
fine particulate matter
- PM10
coarse particulate matter
- POPs
persistent organic pollutants
- ppb
parts per billion
- ppm
parts per million
- SO2
sulfur dioxide
- TCDD
2, 3, 7, 8 – tetrachlorodibenzoparadioxin
- TRP
traffic related pollutants
- UV
ultraviolet radiation
- VOCs
volatile organic compounds
- XREs
xenobiotic response elements
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–93. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher RP, Lee TK. Adverse effects of ultraviolet radiation: a brief review. Prog Biophys Mol Biol. 2006;92:119–31. doi: 10.1016/j.pbiomolbio.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 4.US Environmental Protection Agency. [Accessed: June 1, 2014];Air quality trends. Last updated: April 21, 2014 http://www.epa.gov/airtrends/aqtrends.html#airquality.
- 5.World Health Organization Media Centre. [Accessed on: July 1, 2014];7 million premature deaths annually linked to air pollution. 2014 Published on: March 25, 2014 http://www.who.int/mediacentre/news/releases/2014/air-pollution/en/
- 6.Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–16. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dockery DW, Pope CA. Acute respiratory effects of particulate air pollution. Annu Rev Public Health. 1994;15:107–32. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y-CT, Ghio AJ. Vascular effects of ambient pollutant particles and metals. Curr Vasc Pharmacol. 2006;4:199–203. doi: 10.2174/157016106777698351. [DOI] [PubMed] [Google Scholar]
- 9.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151:362–7. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Sterling T, Dimich H, Kobayashi D. Indoor byproduct levels of tobacco smoke: a critical review of the literature. J Air Pollut Control Assoc. 1982;32:250–59. [Google Scholar]
- 11.Matsumura Y, Ananthaswamy HN. Molecular mechanisms of photocarcinogenesis. Frontiers in bioscience: a journal and virtual library. 2002;7:765–83. doi: 10.2741/matsumur. [DOI] [PubMed] [Google Scholar]
- 12.Schecter A, Birnbaum L, Ryan JJ, Constable JD. Dioxins: an overview. Environ Res. 2006;101:419–28. doi: 10.1016/j.envres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Terzano C, Di Stefano F, Conti V, Graziani E, Petroianni A. Air pollution ultrafine particles: toxicity beyond the lung. Eur Rev Med Pharmacol Sci. 2010;14:809–21. [PubMed] [Google Scholar]
- 14.Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–60. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organization WH, Joint W. Health risks of heavy metals from long-range transboundary air pollution: World Health Organization Regional Office Europe. 2007 [Google Scholar]
- 16.Pollution. H E I P o t H E o T-R A. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects: Health Effects Institute. 2010 [Google Scholar]
- 17.US Environmental Protection Agency. [Accessed: October 1, 2014];The original list of toxic air pollutants. Last updated: August 08, 2013 http://www.epa.gov/ttn/atw/188polls.html.
- 18.Cotovio J, Onno L, Justine P, Lamure S, Catroux P. Generation of oxidative stress in human cutaneous models following in vitro ozone exposure. Toxicol In Vitro. 2001;15:357–62. doi: 10.1016/s0887-2333(01)00036-4. [DOI] [PubMed] [Google Scholar]
- 19.Thiele JJ, Traber MG, Polefka TG, Cross CE, Packer L. Ozone-exposure depletes vitamin E and induces lipid peroxidation in murine stratum corneum. J Invest Dermatol. 1997;108:753–7. doi: 10.1111/1523-1747.ep12292144. [DOI] [PubMed] [Google Scholar]
- 20.Weber SU, Thiele JJ, Cross CE, Packer L. Vitamin C, uric acid, and glutathione gradients in murine stratum corneum and their susceptibility to ozone exposure. J Invest Dermatol. 1999;113:1128–32. doi: 10.1046/j.1523-1747.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- 21.Thiele JJ, Traber MG, Podda M, Tsang K, Cross CE, Packer L. Ozone depletes tocopherols and tocotrienols topically applied to murine skin. FEBS Lett. 1997;401:167–70. doi: 10.1016/s0014-5793(96)01463-9. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy JT, Pelle E, Dong K, Brahmbhatt K, Yarosh D, Pernodet N. Effects of ozone in normal human epidermal keratinocytes. Exp Dermatol. 2013;22:360–1. doi: 10.1111/exd.12125. [DOI] [PubMed] [Google Scholar]
- 23.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell metabolism. 2010;12:662–7. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 24.He QC, Tavakkol A, Wietecha K, Begum-Gafur R, Ansari SA, Polefka T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int J Cosmet Sci. 2006;28:349–57. doi: 10.1111/j.1467-2494.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 25.Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391–98. [PubMed] [Google Scholar]
- 26.Ushio H, Nohara K, Fujimaki H. Effect of environmental pollutants on the production of pro-inflammatory cytokines by normal human dermal keratinocytes. Toxicol Lett. 1999;105:17–24. doi: 10.1016/s0378-4274(98)00379-8. [DOI] [PubMed] [Google Scholar]
- 27.Ma C, Wang J, Luo J. Activation of nuclear factor kappa B by diesel exhaust particles in mouse epidermal cells through phosphatidylinositol 3-kinase/Akt signaling pathway. Biochem Pharmacol. 2004;67:1975–83. doi: 10.1016/j.bcp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Choi H, Shin DW, Kim W, Doh SJ, Lee SH, Noh M. Asian dust storm particles induce a broad toxicological transcriptional program in human epidermal keratinocytes. Toxicol Lett. 2011;200:92–9. doi: 10.1016/j.toxlet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–48. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- 30.Dupont E, Gomez J, Bilodeau D. Beyond UV radiation: a skin under challenge. Int J Cosmet Sci. 2013;35:224–32. doi: 10.1111/ics.12036. [DOI] [PubMed] [Google Scholar]
- 31.Agostinis P, Garmyn M, Van Laethem A. The Aryl hydrocarbon receptor: an illuminating effector of the UVB response. Sci STKE. 2007;2007:pe49. doi: 10.1126/stke.4032007pe49. [DOI] [PubMed] [Google Scholar]
- 32.Tauchi M, Hida A, Negishi T, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25:9360–8. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luecke S, Backlund M, Jux B, Esser C, Krutmann J, Rannug A. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigment Cell Melanoma Res. 2010;23:828–33. doi: 10.1111/j.1755-148X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 34.Afaq F, Zaid MA, Pelle E, et al. Aryl hydrocarbon receptor is an ozone sensor in human skin. J Invest Dermatol. 2009;129:2396–403. doi: 10.1038/jid.2009.85. [DOI] [PubMed] [Google Scholar]
- 35.Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000;114:328–33. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad N, Mukhtar H. Cytochrome p450: a target for drug development for skin diseases. J Invest Dermatol. 2004;123:417–25. doi: 10.1111/j.0022-202X.2004.23307.x. [DOI] [PubMed] [Google Scholar]
- 37.Falk HL, Kotin P, Mehler A. Polycyclic hydrocarbons as carcinogens for man. Arch Environ Health. 1964;8:721–30. doi: 10.1080/00039896.1964.10663743. [DOI] [PubMed] [Google Scholar]
- 38.Sowada J, Schmalenberger A, Ebner I, Luch A, Tralau T. Degradation of benzo[a]pyrene by bacterial isolates from human skin. FEMS Microbiol Ecol. 2014;88:129–39. doi: 10.1111/1574-6941.12276. [DOI] [PubMed] [Google Scholar]
- 39.Schafer T, Vieluf D, Behrendt H, Krämer U, Ring J. Atopic eczema and other manifestations of atopy: results of a study in East and West Germany. Allergy. 1996;51:532–9. doi: 10.1111/j.1398-9995.1996.tb04665.x. [DOI] [PubMed] [Google Scholar]
- 40.Krämer U, Sugiri D, Ranft U, et al. Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J Dermatol Sci. 2009;56:99–105. doi: 10.1016/j.jdermsci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Vierkötter A, Schikowski T, Ranft U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130:2719–26. doi: 10.1038/jid.2010.204. [DOI] [PubMed] [Google Scholar]
- 42.US Environmental Protection Agency. [Accessed: June 1, 2014];Review of national ambient air quality standards for carbon monoxide; final rule. 40 CFR Parts 50, 53 and 58. Published: August 31, 2011 http://www.gpo.gov/fdsys/pkg/FR-2011-08-31/html/2011-21359.htmf.
- 43.US Environmental Protection Agency. [Accessed: June 1, 2014];Review of national ambient air quality standards for lead; final rule. 40 CFR Parts 50, 53 and 58. Published: November 12, 2008 http://www.gpo.gov/fdsys/pkg/FR-2008-11-12/html/E8-25654.htm.
- 44.US Environmental Protection Agency. [Accessed: June 1, 2014];Review of national ambient air quality standards for nitrogen dioxide; final rule. 40 CFR Parts 50 and 58. Published: February 9, 2010 http://www.gpo.gov/fdsys/pkg/FR-2010-02-09/html/2010-1990.htm.
- 45.US Environmental Protection Agency. [Accessed: June 1, 2014];Review of national ambient air quality standards for nitrogen dioxide; Final Rule. 40 CFR Parts 50. Published: October 8, 1996 http://www.gpo.gov/fdsys/pkg/FR-1996-10-08/html/96-25786.htm.
- 46.US Environmental Protection Agency. [Accessed: June 1, 2014];Review of national ambient air quality standards for ozone; final rule. 40 CFR Parts 50 and 58. Published: March 27, 2008 http://www.gpo.gov/fdsys/pkg/FR-2008-03-27/html/E8-5645.htm.
- 47.US Environmental Protection Agency. [Accessed: June 1, 2014];National primary and secondary ambient air quality standards for PM2.5. 40 CFR Section 50.7. Published: July 1, 2013 http://www.gpo.gov/fdsys/pkg/CFR-2013-title40-vol2/xml/CFR-2013-title40-vol2-sec50-7.xml.
- 48.US Environmental Protection Agency. [Accessed: June 1, 2014];National primary and secondary ambient air quality standards for PM10. 40 CFR Section 50.6. Published: July 1, 2013 http://www.gpo.gov/fdsys/pkg/CFR-2013-title40-vol2/xml/CFR-2013-title40-vol2-sec50-6.xml.
- 49.US Environmental Protection Agency. [Accessed: June 1, 2014];Review of national ambient air quality standards for sulfur dioxide; final rule. 40 CFR Parts 50, 53 and 58. Published: June 22, 2010 http://www.gpo.gov/fdsys/pkg/FR-2010-06-22/html/2010-13947.htm.
- 50.US Environmental Protection Agency. [Accessed: June 1, 2014];Air and Radiation: National ambient air quality standards. Last updated: December 14, 2012 http://www.epa.gov/air/criteria.html.
- 51.US Environmental Protection Agency. [Accessed: June 1, 2014];The National Ambient Air Quality Standards: Overview of EPA's revision to the air quality standards for particle pollution (particulate matter) Last updated: December 14, 2012 http://www.epa.gov/pm/2012/decfsoverview.pdf.