Abstract
Polyomavirus-associated nephropathy (PVAN) is common in patients who have undergone kidney transplantation and has been reported in hematopoietic stem cell (HSC) transplant recipients. Aside from reduction of immunosuppression, few therapeutic options exist for treatment of PVAN. We report a case of PVAN in a severely immunocompromised allogeneic HSC transplant recipient that was treated with brincidofovir without reduction of immunosuppression. We review our institutional experience of PVAN in HSC transplantation and discuss the potential use of brincidofovir for treatment.
INDEX WORDS: Polyomavirus-associated nephropathy (PVAN), BK virus, BK viremia, kidney failure, hematopoietic stem cell (HSC) transplantation, brincidofovir
BK virus, a member of the Polyomavirus genus, is a known cause of transplant dysfunction in up to 10% of kidney transplant recipients.1,2 Polyomavirus-associated nephropathy (PVAN) also has been reported in native kidneys of nonrenal solid-organ transplant recipients and hematopoietic stem cell (HSC) transplant recipients.3–8 Kidney biopsy remains the gold standard for the diagnosis of PVAN; the presence of intranuclear BK virus inclusion bodies, which stain positive for the large T antigen, is pathognomonic for PVAN.9
BK viremia is associated with the development of PVAN in kidney transplants and thus kidney transplant recipients are screened routinely for BK viremia.2 However, BK viremia is not monitored routinely after HSC transplantation because the prevalence of PVAN and the relative contribution of BK virus to reduced kidney function after HSC transplantation are not well defined. Further, multiple other factors are likely to contribute to reduced kidney function after HSC transplantation, including radiation, calcineurin inhibitors, and infections. Even so, BK viremia has been implicated in kidney failure and PVAN after HSC transplantation in at least 2 studies.10,11 In another study in HSC transplant recipients, 3 of 20 kidney biopsy specimens showed evidence of PVAN, a sign that this complication might be more prevalent than previously thought.12 All 3 patients had BK viremia, and the pathology findings showed diffuse interstitial inflammation and tubulitis. Marked nuclear enlargement and intranuclear inclusions were seen in many tubular cell nuclei. The presence of BK virus was confirmed by positive nuclear immunohistochemical staining using antibodies against the large T antigen of the closely related polyomavirus simian virus 40 (SV40; antibodies to SV40 large T cross-react with the analogous antigen of BK virus).12
Although the decrease in maintenance immunosuppression may prevent the progression of PVAN in kidney transplant recipients,2 the majority of reported cases of PVAN in HSC transplant recipients required hemodialysis due to progressive kidney failure.4
Brincidofovir is an orally bioavailable lipid acyclic nucleoside phosphonate that undergoes intracellular conversion to cidofovir diphosphate.13 Brincidofovir has been shown to have activity against BK virus in renal tubular cells, and in vivo animal distribution studies have demonstrated high concentrations of total drug-related material in the kidney after oral administration of radiolabeled brincidofovir.13–15 However, unlike cidofovir, brincidofovir is not a substrate for the organic anion transporter, which is located in proximal renal tubules. Consequently, brincidofovir is not concentrated in proximal tubules and has not been reported to cause kidney toxicity in preclinical or clinical trials.13,15,16
We report a case of PVAN in a severely immunocompromised HSC transplant recipient that was treated with brincidofovir without reduction of immunosuppression. We also review our institutional experience of PVAN in HSC transplantation and discuss the potential use of brincidofovir for treatment.
CASE REPORT
A 58-year-old white man with a history of diffuse large B-cell lymphoma received a T-cell–depleted HSC transplant from a matched unrelated donor. Twenty-one days posttransplantation, the patient was enrolled in a randomized trial of brincidofovir for the prevention of cytomegalovirus (CMV) infection (trial CMX001-201; ClinicalTrials.gov identifier, NCT00942305). He received 200 mg of brincidofovir per week for 10 weeks. While enrolled in the study, BK viremia and viruria were monitored through week 24 post–HSC transplantation; the patient had persistent asymptomatic BK viruria, and BK viremia was undetectable with the exception of transiently low levels on 2 occasions. CMV viremia resolved at 12 months posttransplantation. The patient never developed Epstein-Barr or adenovirus viremia.
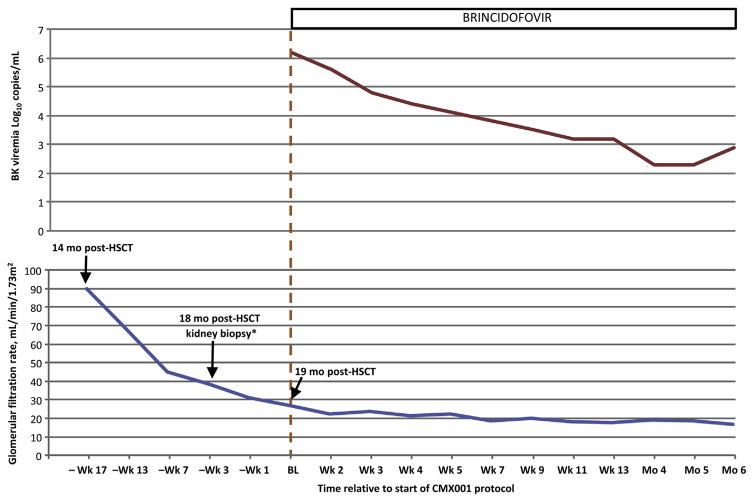
Approximately 6 months after transplantation, the patient experienced a relapse, which was treated with chemotherapy, donor leukocyte infusion, and lenalidomide. Approximately 10 months posttransplantation, he developed grade IV steroid-refractory acute graft-versus-host disease (GVHD) involving the gut and skin, which was treated with alemtuzumab and mycophenolate mofetil. Steroid dosage was tapered 15 months after transplantation. At 16 months, the patient developed acute kidney injury (Fig 117). A kidney biopsy was performed, revealing PVAN with severe BK virus–associated tubulointerstitial nephritis with diffuse interstitial inflammation, multifocal tubulitis, acute tubular injury, moderate tubular atrophy, and interstitial fibrosis. Immunostaining showed abundant SV40 large T-antigen–positive tubular cell nuclei involving 25% to 35% of cortical and medullary tubules sampled; adenovirus and CMV antigens were undetectable. The vast majority of infiltrating lymphocytes were CD3+ T cells.
Figure 1.
(Upper panel) Plasma BK viremia and (lower panel) estimated glomerular filtration rate (eGFR) in patient 1. *Time is not to scale; baseline (BL) is the first day of brincidofovir treatment in the open-label study CMX001-350. eGFR was calculated using the 4-variable isotope-dilution mass spectrometry–traceable MDRD (Modification of Diet in Renal Disease) Study equation.17 Abbreviation: HSCT, hematopoietic stem cell transplantation.
On day 572, based on biopsy-proven PVAN, the patient was enrolled in study CMX001-350, an open-label expanded-access study of brincidofovir in patients with serious diseases or conditions caused by infections with double-stranded DNA viruses (ClinicalTrials.gov identifier, NCT01143181). Brincidofovir was started orally at 100 mg twice weekly for 6 months. Figure 1 shows the patient’s plasma BK viral load and estimated glomerular filtration rate during treatment.
No drug-related adverse events occurred. Following completion of CMX001-350, the patient continued treatment with brincidofovir under emergency investigational drug application provisions for an additional 5 months until he died of bacterial sepsis 30 months post–HSC transplantation. During this time, he had stable kidney function and did not require dialysis. No repeat kidney biopsy or autopsy was performed to directly document improvement in PVAN on a tissue level. The clinical course and immunosuppressive regimen of patient 1 are described in Box 1.
Box 1. Clinical Course and Immunosuppressive Regimen of Patient 1.
| Days −10 to 0: Conditioning regimen of total-body irradiation (1,375 cGy with 600 cGy chest and 300 cGy testes boost), thiotepa, cyclophosphamide, rabbit ATG with KGF; CD34+-selected T-cell–depleted graft |
| Days 21 to 91: Brincidofovir, 200 mg, weekly (CMV prevention protocol) |
| Month 6: Recurrence of lymphoma; rituximab and dexamethasone weekly (3 doses) |
| Month 7: Donor lymphocyte infusion (5 × 106 CD3 cells per kilogram of body weight) and maintenance lenalidomide |
| Months 10 to 12: GVHD of skin, gut, and liver; high-dose steroids, MMF, alemtuzumab |
| Month 12: Maintenance MMF, 1 g, twice daily and prednisone, 10 mg/d |
| Month 15: MMF, 1 g, twice daily and prednisone taper completed |
| Month 16: Development of AKI |
| Month 17: MMF taper to 500 mg twice daily |
| Month 18: MMF discontinued and sirolimus started at 2 mg/d; kidney biopsy consistent with PVAN |
| Month 19: Brincidofovir, 100 mg, twice weekly |
| Month 20: Resume MMF, 1 g, twice daily for GVDH |
| Month 30: Death from bacterial sepsis |
Note: Times are relative; T-cell–depleted hematopoietic stem cell transplantation at day 0.
Abbreviations: AKI, acute kidney injury; ATG, antithymocyte globulin; CMV, cytomegalovirus; GVHD, acute graft-versus-host disease; KGF, keratinocyte growth factor; MMF, mycophenolate mofetil; PVAN, polyomavirus-associated nephropathy.
DISCUSSION
We report a case of PVAN treated with brincidofovir. A systematic review of autopsy and kidney biopsy records of both adults and children who underwent HSC transplantation at our institution from January 2004 through December 2012 identified 5 cases of PVAN in HSC transplant recipients. The demographics, medical and transplant histories, and key information related to PVAN for these 5 patients are summarized in Table 1. Notably, from 2004 to 2013, rates of kidney biopsy and autopsy in HSC transplant recipients were 1.4% and 10.4%, respectively.
Table 1.
Characteristics and Outcomes of BK Virus Nephropathy Cases
| Patient No. | Age (y)/Sex | Disease | Donor | GVHD Onset | HC Onset | Scr Doublinga | Time and Means of Diagnosis | Maximum BK Viremiab | Treatment | HD Start | Time and Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1c | 58/M | Lymphoma | MUD | On d 290 | No HC | On d 522 | Biopsy on d 555 | 2.3 × 107 copies/mL on d 565 | Brincidofovir orally, 100 mg, 2×/wk, started on d 572 (94 doses)d | No HD | On d 914 (GVHD, bacterial sepsis) |
| 2 | 64/M | Multiple myeloma | MRD | No GVHD | On d 61 (grade 3) | On d 311 | Autopsy on d 342 | 1.1 × 105 copies/mL on d 317 | Brincidofovir orally, 100 mg, 2×/wk, started on d 281 (9 doses)e | No HD | On d 342 (bacterial sepsis, hemolytic anemia, CMV encephalitis) |
| 3 | 7/M | AML | MMUD | On d 107 | No HC | On d 368 | Nephrectomy on d 326 | Not available | None | No HD | On d 439 (GVHD) |
| 4 | 27/M | Lymphoma | MMUD | No GVHD | On d 61 | On d 72 | Autopsy on d 122 | 1.3 × 104 copies/mL on d 107 | IV cidofovir, 0.5 mg/kg (×2) | On d 97 | On d 122 (bacterial pneumonia) |
| 5 | 59/F | AML | MMUD | On d 53 | On d 53 (grade 4) | On d 222 | Biopsy on d 270 | 2.2 × 106 copies/mL on d 258 | IV cidofovir, 5 mg/kg (×5), then 1 mg/kg (×2); intravesicular cidofovir, ciprofloxacin, leflunomide | On d 273 | On d 291 (bacterial pneumonia) |
Note: Day equals the number of days since stem cell infusion.
Abbreviations: AML, acute myelogenous lymphoma; CMV, cytomegalovirus; MRD, matched related donor; MMUD, mismatched unrelated donor; MUD, matched unrelated donor; GVHD, graft-versus-host disease; HC, hemorrhagic cystitis; HD, hemodialysis; IV, intravenous; Scr, serum creatinine.
Relative to baseline concentration.
Quantitation of BK viremia was determined by a commercial polymerase chain reaction assay performed at Focus Diagnostics or Viracor-IBT Laboratories.
The subject of this case report; was treated with brincidofovir for BK virus nephropathy. Kidney biopsy was performed at an outside institution.
Patient 1 received 54 doses of brincidofovir on CMX001-350 study and 40 doses under emergency investigational drug (EIND) application provisions.
Patient 2 was treated with brincidofovir for CMV encephalitis.
Patient 2 was enrolled briefly in study CMX001-350 for CMV viremia and CMV encephalitis. He received brincidofovir, 100 mg, twice weekly for a total of 9 doses but, unable to tolerate oral medications, the patient discontinued brincidofovir treatment on day 313 after HSC transplantation. He developed acute kidney injury related to bacterial sepsis on day 311, but kidney function returned to baseline after sepsis resolved and remained stable until 1 week prior to his death.
In patient 3, PVAN was found incidentally when the patient underwent nephrectomy of a nonfunctional kidney on day 326 post–HSC transplantation for severe hypertension. A subsequent kidney biopsy specimen confirmed PVAN. The patient had known left hydronephrosis with hydroureter prior to HSC transplantation, and immunohistochemistry was positive for BK virus. At the time of nephrectomy, his kidney function was normal. The patient died on day 439 of multiple complications of HSC transplantation.
Patients 4 and 5 developed kidney failure and obstructive uropathy requiring bilateral percutaneous nephrostomies. Intravenous cidofovir was administered but ultimately both patients progressed to end-stage renal disease. Patient 4 died of sepsis 1 month after developing end-stage renal disease and autopsy revealed PVAN. Patient 5 underwent a kidney biopsy after her kidney function did not improve after the nephrostomies; the specimen was consistent with PVAN.
All patients identified to have PVAN were profoundly immunosuppressed due to T-cell–depleted HSC transplantation, treatment for advanced GVHD, and/or treatment with corticosteroids. Two of the patients had normal kidney function yet showed histologic evidence of PVAN. These findings correlate with the experience in the kidney transplant population, for which BK viremia with >1.0 × 104 copies/mL prompts kidney biopsy to rule out PVAN even in the absence of overtly decreased kidney function.2,9 In a study of kidney pathology at autopsy in patients who died after HSC transplantation, tubulitis was detected in 16 of 24 (67%) patients and renal tubular atypia was present in 19 of 26 (73%).18 Although the authors reported no histologic evidence of viral cytopathic effects to account for frequently observed tubular atypia, they did not perform staining specific for BK virus. These findings suggest that at least a portion of patients in this series may have had an unrecognized PVAN. It may be warranted to screen HSC transplant recipients for BK viremia even in the absence of overt reduced kidney function.
Low-dose cidofovir, ciprofloxacin, rapamycin, leflunomide, and intravenous immunoglobulin have been described as potentially effective therapies in cases of PVAN in kidney transplants; however, objective data regarding BK treatment are limited.9 At present, screening for BK viremia and subsequent reduction of immunosuppression remains the best strategy to treat and prevent PVAN in kidney transplants. 19 The data for treatment of PVAN in HSC transplant recipients are limited to case reports. Successful management of PVAN in one HSC transplant recipient with leflunomide has been reported,5 as has stabilization of kidney function after a brief course of cidofovir.6 Reducing immunosuppression usually is not feasible in these patients due to ongoing GVHD, and the majority of HSC transplant recipients reported to have PVAN eventually required dialysis.4,10
Patient 1 developed rapidly progressing reduction in kidney function before starting brincidofovir. While taking brincidofovir, his kidney function fluctuated, with transient deteriorations temporally related to recurrent episodes of sepsis. However, the patient was able to maintain adequate kidney function without requiring dialysis for 11 months until his death from unrelated causes. Brincidofovir was well tolerated without any myelosuppression. Adverse events included episodes of sepsis not related to brincidofovir. To our knowledge, this is the first report of PVAN managed successfully with brincidofovir without a corresponding reduction in immunosuppression. The 4-log decrease in BK virus viremia experienced by patient 1 while receiving brincidofovir (Fig 1) supports the candidacy of this compound as a potential treatment for PVAN, as well as the need for further study.
Acknowledgments
Support: None.
Financial Disclosure: Dr Momméja-Marin and Mr Chittick are employees of Chimerix, Inc (Durham NC), the developer of brincidofovir (CMX001). The other authors declare that they have no relevant financial interests.
References
- 1.Hirsch HH. BK virus: opportunity makes a pathogen. Clin Infect Dis. 2005;41(3):354–360. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 2.Kuypers DR. Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat Rev Nephrol. 2012;8(7):390–402. doi: 10.1038/nrneph.2012.64. [DOI] [PubMed] [Google Scholar]
- 3.Limaye AP, Smith KD, Cook L, et al. Polyomavirus nephropathy in native kidneys of non-renal transplant recipients. Am J Transplant. 2005;5(3):614–620. doi: 10.1046/j.1600-6143.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 4.Lekakis LJ, Macrinici V, Baraboutis IG, Mitchell B, Howard DS. BK virus nephropathy after allogeneic stem cell transplantation: a case report and literature review. Am J Hematol. 2009;84(4):243–246. doi: 10.1002/ajh.21358. [DOI] [PubMed] [Google Scholar]
- 5.Raval M, Gulbis A, Bollard C, et al. Evaluation and management of BK virus-associated nephropathy following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1589–1593. doi: 10.1016/j.bbmt.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verghese PS, Finn LS, Englund JA, Sanders JE, Hingorani SR. BK nephropathy in pediatric hematopoietic stem cell transplant recipients. Pediatr Transplant. 2009;13(7):913–918. doi: 10.1111/j.1399-3046.2008.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Pinto LN, Laskin BL, Jodele S, Hummel TR, Yin HJ, Goebel J. BK virus nephropathy in a pediatric autologous stem-cell transplant recipient. Pediatr Blood Cancer. 2011;56(3):495–497. doi: 10.1002/pbc.22860. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SG, Nickeleit V, Herlitz LC, et al. BK polyoma virus nephropathy in the native kidney. Nephrol Dial Transplant. 2013;28(3):620–631. doi: 10.1093/ndt/gfs537. [DOI] [PubMed] [Google Scholar]
- 9.Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment [published online ahead of print on February 25, 2014] Nephrol Dial Transplant. doi: 10.1093/ndt/gfu023. http://dx.doi.org/10.1093/ndt/gfu023. [DOI] [PubMed]
- 10.O’Donnell PH, Swanson K, Josephson MA, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant. 2009;15(9):1038–1048.e1. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1512–1519. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Chang A, Hingorani S, Kowalewska J, et al. Spectrum of renal pathology in hematopoietic cell transplantation: a series of 20 patients and review of the literature. Clin J Am Soc Nephrol. 2007;2(5):1014–1023. doi: 10.2215/CJN.01700407. [DOI] [PubMed] [Google Scholar]
- 13.Rinaldo CH, Gosert R, Bernhoff E, Finstad S, Hirsch HH. 1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells. Antimicrob Agents Chemother. 2010;54(11):4714–4722. doi: 10.1128/AAC.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis. 2010;202(10):1492–1499. doi: 10.1086/656717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciesla SL, Trahan J, Wan WB, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59(3):163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 16.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 18.El-Seisi S, Gupta R, Clase CM, Forrest DL, Milandinovic M, Couban S. Renal pathology at autopsy in patients who died after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9(11):683–688. doi: 10.1016/s1083-8791(03)00243-x. [DOI] [PubMed] [Google Scholar]
- 19.Anwar S, Brennan DC. Treatment of BK viremia after renal transplantation: are fluoroquinolones a false dawn? Clin J Am Soc Nephrol. 2014;9(3):445–447. doi: 10.2215/CJN.13001213. [DOI] [PMC free article] [PubMed] [Google Scholar]