Abstract
Synthetic phosphothioated (PTO) oligodeoxynucleotide (ODN) sequences are commonly used for a variety of applications that benefit from nuclease protection. The PTO modification is mainly implemented in anti-sense ODN, but also in ODN that were shown to activate members of the toll-like receptor (TLR) family members such as TLR3 (Poly I:C), TLR8 (ssRNA) and TLR9 (CpG). Cortical neurons isolated from E18 rat embryos are routinely plated on surfaces coated with either cationic substances such as: poly L-ornithine (PLO), poly(ethylenimine) (PEI), poly L-lysine or ECM components, such as: laminin, collagen or fibronectin. We found that PTO-ODN aimed at activating TLR9, induces a non-TLR9 specific detachment phenotype on cortical neurons plated on either laminin or PEI, but not on PLO. This phenotype was correlated with decreased viability and was partially inhibited when caspase-3 was I nhibited using Ac-DEVD-CMK. This finding suggests that the use of PTO-ODN can cause non-specific effects on cell adhesion that could compromise interpretation of data from experiments using PTO-ODN.
Keywords: phosphothioate, ODN, neurons, adhesion, toll-like receptors
Introduction
The phosphothioate (PTO) modification of oligonucleotides contains one sulfur atom in place of an oxygen atom in the internucleotide linkage of DNA or RNA. This modification is extremely useful when used for anti-sense oligodeoxynucleotide (ODN) mediated gene expression inhibition (Williams et al. 1997), since they are more resistant to nuclease degradation than natural DNA or RNA and still bind to complementary nucleic acid sequences. Another application that utilizes PTO-oligonucleotides is the activation of certain members of the toll-like receptor (TLR) family of receptors. TLRs are a family of pattern recognition receptors (PRR), which recognize molecules that are broadly shared by pathogens and are referred to as pathogen-associated molecular patterns (PAMPS). Each TLR is activated by ligands that originate in different classes of pathogenic microorganisms. Certain TLRs recognize foreign nucleic acids. TLR3 recognizes dsRNA and polyinosine-polycytidylic acid (POLY I:C). TLR7 and TLR8 both recognize ssRNA and TLR9 recognizes unmethylated bacterial or viral DNA or synthetic CpG oligonucleotides (Kawai and Akira 2007). The above mentioned ligands are commonly used to study different aspects of TLR3, TLR8 and TLR9 activation. These ligands are generally synthesized with a PTO modification in their backbone. This modification renders these ODN more protected against cellular nucleases allowing prolonged activation of the desired TLR. Full PTOs are more stable than partially modified oligonucleotides, but due to their extreme stability and their hydrophobic character they can also have toxic effects on living cells. The study of TLRs has extended beyond the limits of immune-related cells and TLRs are expressed on a variety of non-immune cells; for example: neurons (Tang et al. 2007), astrocytes and oligodendrocytes (Bsibsi et al. 2002). The role of TLRs in general and of TLR9 in particular has not been deciphered yet in post-mitotic neurons. CpG ODNs are synthetic DNA ODNs that contain CG motifs and are known to activate TLR9 in immune cells. It is considered to mimic unmethylated CG islands that exist in bacterial and viral DNA. Three types of CpG ODN (Type A, B and C) were found according to their ability to activate dendritic or B cells. Inhibitory sequences were also found according to their ability to counteract the activation of immune cells by CpG ODNs (Krieg et al. 1998). In this report, we provide evidence for a non TLR9-specific activity conferred by P-CpG ODNs in cortical neurons grown on different adhesion-promoting substances.
Materials and methods
Materials
Resazurin (Alamar blue, 500 uM stock concentration, dissolved in PBS), Poly(ethylenimine) (PEI) and Poly-L-Ornithine (PLO) were purchased from Sigma (St. Louis, MO, USA). E.coli DNA was purchased from Invivogen (San Diego, CA, USA). The following CpG ODNs were purchased from Invivogen: 1826 ODN: 5’-tccatgacgttcctgacgtt 3’, 1826 control ODN: 5’ tccatgagcttcctgagctt 3’, 1688 ODN: 5’ tccatgacgttcctgatgct 3’, 1688 control ODN: 5’ tccatgagcttcctgatgct 3’, 2088 inhibitory ODN: 5’ tcctggcggggaagt 3’. All ODNs were synthesized with full Phosphothioate backbone. Caspase-3 inhibitor III (Ac-DEVD-CMK) and wortmannin were purchased from Calbiochem (La Jolla, CA, USA). Neurobasal medium and the B27 medium supplement were purchased from Invitrogen (Carlsbad, CA, USA)
Neuronal cultures
Dissociated cell cultures of cortical fragments were established from 18-day Sprague–Dawley rat embryos as previously described (Mattson and Kater 1988). Neurons were plated on either PEI (0.005%), laminin (10 µg/ml) or PLO (15 µg/ml) coated 48 well plates and were maintained with Neurobasal medium supplemented with B27 (1:50 v:v). All treatments were incubated for 72 hrs unless otherwise indicated.
Cell viability
Cell viability was measured using the resazurin dye (10 uM final concentration). Resazuring was added directly to the cultures for 30 min incubation in 37°C. fluorescence was then measured using a fluorescent microplate reader. Excitation wavelength was 540nm and emission wavelength was 595nm.
Statistical analysis
Student’s t-test was used to determine significance in all viability measurements.
Results
Phosphothioated CpG ODN cause detachment of neurons from PEI-coated surfaces
Cortical neurons incubated with 1826 CpG ODN [5 and 10 uM], a type-B CpG ODN, showed detachment phenotype (Figure 1a). This was characterized by clustering of neuronal cell bodies and axonal processes into thick bundles. This effect was dose-dependant and could be visible as early as 48 hrs (data not shown). A mild effect on viability was conferred by P-CpG ODN in doses of 5 uM and higher (Figure 1b). Incubation with the 1826 ODN control sequence (with a GC motif instead of CG) resulted in identical results. Moreover, incubation of the cells with 1688 CpG ODN (also type-B CpG ODN) and its control ODN resulted in the same detachment phenotype (Figure 2a). ODN 2088 was reported to inhibit the activity of both ODN 1826 CpG and ODN 1688 CpG. When ODN 2088 was added to neurons either with or without the 1826 or 1688 CpG ODN, the detachment phenotype was evident (Figure 2a). This implies an effect for P-CPG ODN on the cells which is not specific for TLR9 activation. Similarly to P-ODN 1826, all the different P-CpGs induced mild but significant decreases in cell viability (Figure 2b).
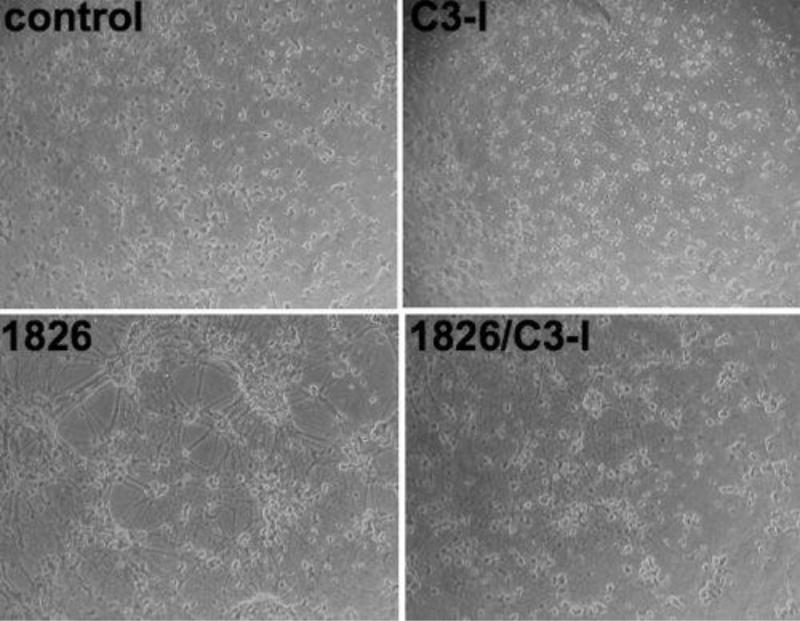
Figure 1. P-ODN induced detachment of cortical neurons plated on PEI.
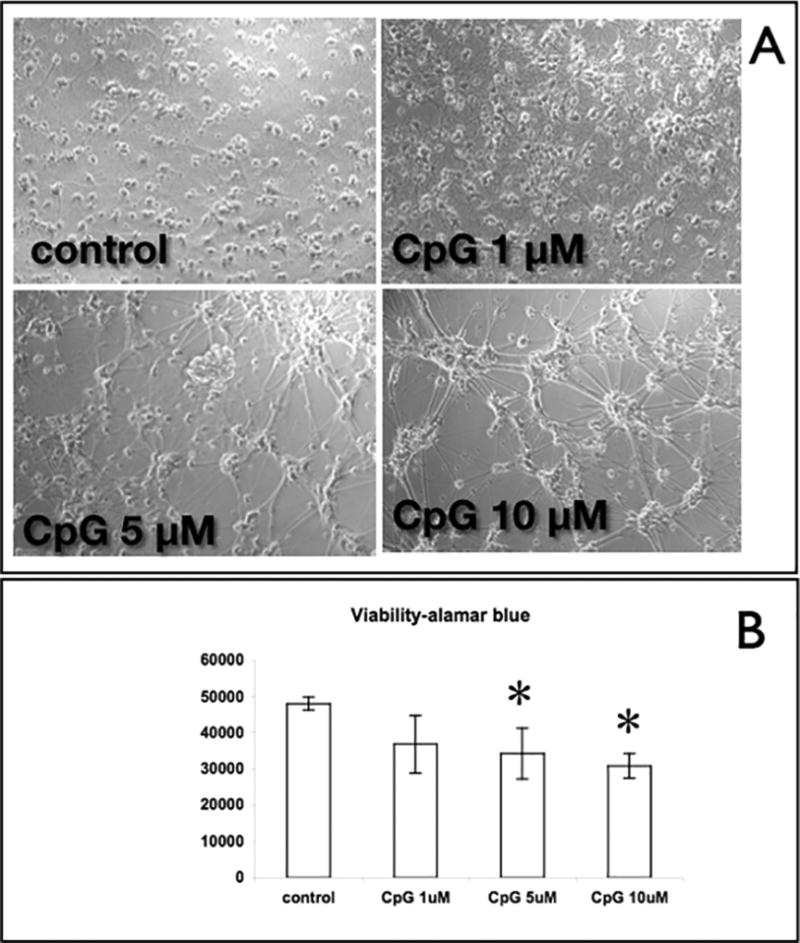
(a) Cortical neurons were plated on PEI coated dishes. At DIV7, cells were treated with increasing doses of P-ODN 1826 (1,5 and 10 µM) and after 72 hrs, cells were imaged. (b) Viability measurements of P-ODN 1826 were conducted using the viability dye resazurin. *: P<0.05 vs. control.
Figure 2. Detachment phenotype of P-ODN is not sequence specific.
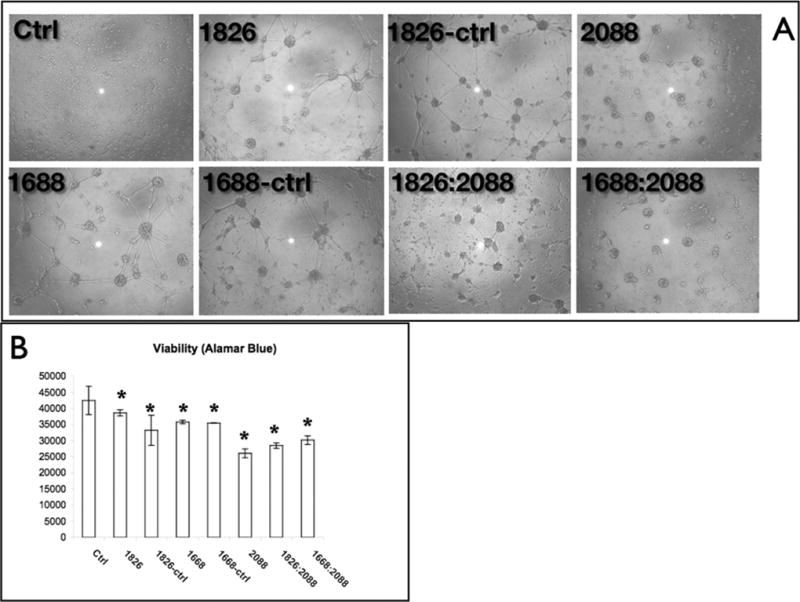
Cortical neurons were plated on PEI coated dishes. At DIV7, cells were treated with different p-CpG ODNs and their control sequences (10 µM for all the ODNs): 1826, 1688, 1826-ctrl, 1688-ctrl, the inhibitory ODN 2088 and combinations of 2088 with either 1826 or 1688. After 72 hrs, cells were imaged. (b) Viability measurements of P-ODN were conducted using the viability dye resazurin. *: P<0.05 vs. control.
E.coli DNA does not induce detachment of adult neurons
To verify that the detachment phenotype was not mediated through TLR9, we used E.coli DNA, the natural ligand for TLR9. Increasing doses of E.coli DNA of up to 30 µg/ml did not induce any morphological change to the cells (Figure 3a). Moreover, no effect on the viability of the cells was noticed (Figure 3b).
Figure 3. E.coli DNA does not induce detachment phenotype on cortical neurons plated on PEI.
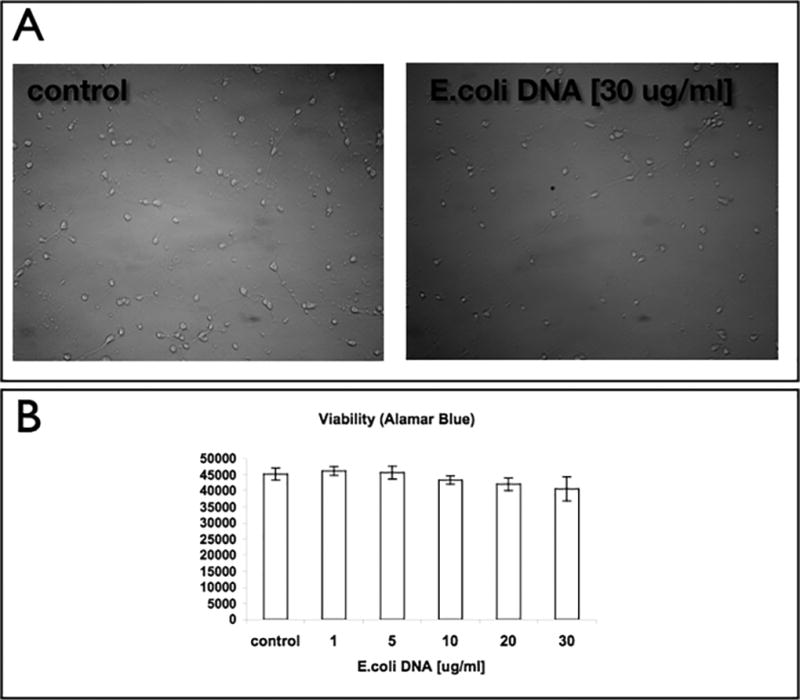
(a) Cortical neurons were plated on PEI coated dishes. At DIV7, cells were treated with 30 µg/ml E. coli DNA. Following 72 hrs, cells were imaged. (b) Viability measurements were conducted using the viability dye resazurin.
Caspase-3 inhibitor counteracts the effect conferred by 1826 CpG ODN
The decrease in viability following treatment of the cells with P-CpG suggested that an apoptotic process was involved. When a cell-permeable caspase-3 inhibitor (Ac-DEVD-CMK) was added 2 hours prior to P-ODN addition, the detachment phenotype was partially inhibited (Figure 4). This effect was not reversed however when wortmannin, a PI3K inhibitor was used (data not shown).
Figure 4. Inhibition of caspase-3 prevents the detachment effect of P-ODN on cortical neurons plated on PEI.
Cortical neurons were plated on PEI coated dishes. At DIV7, cells were treated with caspase-3 inhibitor (Ac-DEVD-CMK, 100 µM) for 2 hours prior to addition of P-ODN 1826 (10 µM). Following 72 hrs, cells were imaged.
The detachment effect is dependent on the coating substrate
Our results were in contrast to those of (Iliev et al. 2004) who recently described the effects of CpG ODN on neurons. In their study, the authors used PLO to facilitate neuronal attachment and did not encounter the detachment phenotype that we observed. We therefore investigated whether different coating substrates confer different effects. Indeed, when cortical neurons were grown on PEI or laminin substrates and treated with P-ODN CpG, the same detachment effects were observed (Figure 5). However, when neurons were cultured on PLO coated plates, no detachment effect was observed (Figure 5).
Figure 5. Detachment phenotype occurs in neurons plated on PEI and laminin but not PLO coated surfaces.
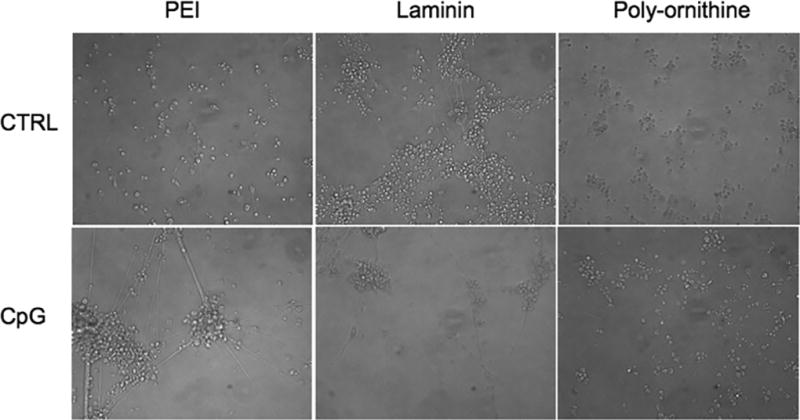
Cortical neurons were plated on either PEI (0.005%), laminin (10µg/ml) or PLO (15µg/ml) coated 48-well plates. At DIV7, cells were treated with P-ODN (10 µM). Following 72 hrs, cells were imaged.
Discussion
TLR were initially shown to be functional on immune-related cells. However, many cell types and tissue types have since emerged as sources for TLR expression and therefore potential targets for TLR activation by different ligands. The detachment phenotype, induced by PTO-ODN at concentrations of 5 µM and higher, was characterized by clustering of the cortical neuron cell bodies and bundling of their axons. This phenotype is typical for detachment of neurons due to their inability to attach to the surface. This effect was associated also with a mild but significant decrease in the viability of the cells. Intriguingly, caspase-3 is involved in the initiation of this process, as a caspase-3 inhibitor was able to significantly inhibit this phenotype. The ability of other CpG PTO-ODNs and their control sequences as well as inhibitory sequences to induce the same effect implied that the effect of PTO-ODN on post-mitotic neurons is non-specific. This was further demonstrated when E.coli DNA, the natural ligand for TLR9, did not induce the same effect. Bacterial DNA does contain CG motifs, but does not contain synthetically modified phosphothioates in the backbone of its sequence. A report by (Iliev et al. 2004) showed that at the same concentrations of PTO-CpG that we used, no such effect was observed. Since the only difference between the studies was the substrate used to induce attachment of the neurons, we compared between the two cationic substrates, PEI and PLO and laminin (a non-cationic substrate that is also used to facilitate neuronal attachment). Since the two cationic substances had opposite effects and since caspase-3 inhibition prevented the effect, charge effects between the negative-charged PTO-ODN and the positive-charged PEI are not likely the cause of this effect. It remains to be determined why PEI and PLO induce such opposite effects on neuronal cultures when PTO-ODN is introduced to the cultures.
The observation that PTO-ODNs confer a non-specific effect on PEI and laminin coated cultures is important because PEI and laminin are both widely used by many laboratories in the study of neuronal cultures. Anti-sense ODNs are widely used in the study of numerous cell types as well as neurons. TLRs in general are increasingly being studied in fields that are not related to the immune system. Synthetic CpG is not the only TLR ligand that is commercially synthesized using PTO nucleotides. Polyinosine-polycytidylic acid (POLY I:C) and dsRNA, both TLR3 ligands and ssRNA, a TLR7 and TLR8 ligands are also commercially synthesized using PTO-ODNs. It is therefore important to realize the interactions of PTO-ODN in general and PTO-TLR ligands in particular with different matrices used to prepare adhering cell cultures in general and neuronal cultures in particular.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging
References
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2000;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Iliev AI, Stringaris AK, Nau R, Neumann H. Neuronal injury mediated via stimulation of microglial toll-like receptor-9 (TLR9) Faseb J. 2004;18:412–414. doi: 10.1096/fj.03-0670fje. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Krieg AM, Wu T, Weeratna R, Efler SM, Love-Homan L, Yang L, Yi AK, Short D, Davis HL. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc Natl Acad Sci U S A. 1998;95:12631–12636. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Isolated hippocampal neurons in cryopreserved long-term cultures: development of neuroarchitecture and sensitivity to NMDA. Int J Dev Neurosci. 1988;6:439–452. doi: 10.1016/0736-5748(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Gillan ER, Knoppel E, Buzby JS, Suen Y, Cairo MS. Effects of phosphodiester and phosphorothioate antisense oligodeoxynucleotides on cell lines which overexpress c-myc: implications for the treatment of Burkitt's lymphoma. Ann Oncol. 1997;8(Suppl 1):25–30. [PubMed] [Google Scholar]