Abstract
Activated platelet-rich plasma (PRP), also referred to as platelet-rich fibrin (PRF), has been used to augment numerous techniques of cartilage repair in the knee but does not always result in superior quality of repair tissue. One possible reason that PRF does not consistently result in excellent cartilage regeneration is the transiency of growth factor provision with PRF. The objective of this study was to compare the release of transforming growth factor (TGF)-β1 from PRF and from PRP combined with a novel chondroitin sulfate glycosaminoglycan (CS-GAG) gel. PRP was prepared from nine healthy dogs and split into two aliquots: one activated with bovine thrombin and calcium chloride (CaCl2) to form PRF and the other aliquot was used to rehydrate a lyophilized CS-GAG gel. Both PRF and the CS-GAG gels were incubated in media for 13 days and media were collected, stored, and replaced every 48 hours and the concentration of TGF-β1 quantified in the media using an enzyme-linked immunosorbent assay. Concentrations of TGF-β1 in the media were up to three times greater with the CS-GAG gels and were significantly (p < 0.05) greater than with PRF on days 3, 5, 7, 9, and 13. Furthermore, TGF-β1 elution was still substantial at day 13 with the use of the CS-GAG gels. Additional in vitro work is warranted to characterize TGF-β1 elution from this CS-GAG gel with human PRP and to determine whether the use of these CS-GAG gels can augment cartilage repair in vivo.
Keywords: transforming growth factor-β1, chondroitin sulfate glycosaminoglycan, platelet-rich plasma, hydrogels, platelet-rich fibrin
Articular cartilage lesions of the knee are common and can be a cause of pain and dysfunction as well as precipitate progressive osteoarthritis.1–3 Current therapies for focal cartilage defects include but are not limited to marrow stimulation techniques such as microfracture, autogenous chondrocyte implantation, or implantation of autologous stem cells.4–9 Each of these therapies relies upon proliferation of either local or transplanted cells and associated production of extracellular matrix. Positive outcomes have been noted with these procedures; however, suboptimal tissue repair can also occur and is a cause of surgical failure.10–13 As a result, several approaches have been used with these techniques in an effort to improve cellular proliferation and the quality of the extracellular matrix.14–18
Platelet-rich plasma (PRP) is one biological therapy that has been used to augment cartilage repair because it is an autologous source of anabolic growth factors that can ameliorate detrimental effects of inflammatory cytokines on chondrocyte gene expression and can also enhance chondrocyte proliferation in vitro.19–22 In addition, PRP can be activated to cause fibrin polymerization and form a platelet-rich fibrin (PRF) gel, which can be surgically placed into cartilage defects and provide both growth factors and a bioresorbable scaffold for tissue repair. Numerous case series have described the use of PRF as an augment for treating cartilage defects in the knee with positive results. Haleem et al first described the implantation of autologous culture-expanded bone marrow mesenchymal stem cells delivered in PRF, and stabilized under a periosteal flap, to treat cartilage defects of the femoral condyle in five patients.23 Subsequent study described augmentation of autologous matrix-induced chondrogenesis (AMIC) with PRF for treating cartilage defects in the patella.24 Another group published on use of drilling plus addition of a polyglycolic acid-hyaluronan scaffold soaked with PRP.25–27 In addition, another well-described approach involves the combination of bone marrow aspirate concentrate added to collagen or a hyaluronic acid membrane and then supplemented with PRF in a “one-step” procedure.6,28,29 Finally, the first controlled trial assessing the benefits of PRF as an adjunct to cartilage repair demonstrated that the combination of microfracture plus PRF provided superior clinical results to microfracture alone in the treatment of cartilage defects in the knee.30
Although each of the aforementioned studies demonstrates the feasibility and possible benefits of augmenting cartilage repair techniques with PRF, only one of those aforementioned studies is a controlled trial. Furthermore, not all results with the use of PRF are positive in all aspects.23,31–33 For example, although individuals with focal cartilage lesions of the patella had clinical improvement with PRF-augmented AMIC, 60% had incomplete tissue fill and had intralesional osteophytes based on magnetic resonance imaging.24 One possible explanation for suboptimal results with the use of PRF augmentation of cartilage repair is that PRF does not provide sustained release of anabolic growth factors. In vitro studies with human PRF demonstrate that the vast majority of all insulin-like growth factor-1, vascular endothelial growth factor, and platelet-derived growth factor-AB is released from PRF constructs within 3 days, with negligible growth factor release after 7 days.34,35 Similar studies with canine PRF provide comparable results, demonstrating that the majority of transforming growth factor (TGF)-β1 is released from canine PRF in as little as 24 hours with minimal amounts released after day 3.36 Cartilage repair is a relatively slow process that takes months.37 Consequently, the development of delivery mechanisms that facilitate the sustained delivery of anabolic growth factors from PRP may improve the efficacy of this adjunct to cartilage repair.
Negatively charged hydrogels have been created and used to bind to positively charged anabolic growth factors, thus resulting in more delayed release of such growth factors over time.38 Heparin-based hydrogels have been used for this purpose but have been shown to cause coagulopathy and are thus not ideal for in vivo use.39,40 Chondroitin sulfates are similar to heparin in that they are also negatively charged and can bind to positively charged anabolic growth factors. Accordingly, a bioresorbable chondroitin sulfate glycosaminoglycan (CS-GAG) hydrogel has been developed that consists of methacrylated chondroitin sulfate-A which is then photo-cross-linked to result in a hydrogel matrix. The sulfate groups on the chondroitin sulfate have a high affinity for positively charged anabolic growth factors and previous work has shown that the use of these gels resulted in sustained release of fibroblast growth factor-2 (FGF-2) and brain-derived neurotrophic factor (BDNF) over a period of 15 days.41,42 Although FGF-2 and BDNF are not anabolic growth factors typically associated with PRP or with cartilage repair, the relevant growth factors in PRP are also positively charged and might interact similarly with CS-GAG hydrogels. In turn, the concept of using biocompatible negatively charged hydrogels for providing sustained release of anabolic growth factors from PRP could be applicable to augmentation of cartilage repair in the knee.
The purpose of this study was to compare the elution of TGF-β1 from canine PRF made by activating PRP with calcium chloride (CaCl2) and thrombin, to that of canine PRP combined with a CS-GAG gel. We hypothesized that the CS-GAG gel would result in significantly greater elution of TGF-β1 than PRF after 3 days.
Materials and Methods
This study was approved by the clinical research committee of the University of Georgia.
Dogs
Nine dogs were recruited at the University of Georgia for the study. To be included in the study, dogs were required to weigh > 15 kg, be between 1 and 10 years of age, have a normal complete blood count, have no medical conditions other than a possible history of osteoarthritis, and have taken no medications beyond monthly parasiticides in the prior 30 days.
PRP preparation
Dogs were sedated with intravenous injections of 0.5 mg/kg nalbuphine and 5 μg/kg dexmedetomidine for the blood draw. For each dog, two 60 mL syringes were preloaded with 8 mL of ACD-A anticoagulant and sequentially filled with 52 mL of blood obtained via a 2″ 18-gauge intravenous catheter placed in a jugular vein. Syringes were manually inverted several times to mix the blood and anticoagulant and were subsequently placed on a rocker to achieve complete mixing. PRP was prepared with the Angel System and both the PRP and platelet-poor plasma (PPP) was collected during the PRP preparation process. The desired platelet concentration for the PRPs was ~1 × 1012/L and the white blood cell count was below ~5 × 109/L. PRPs that contained greater than 1.5 × 1012 platelets/L or greater than 7 × 109 WBC/L were diluted with PPP to the concentration range described earlier.
Gel preparation
CS-GAG hydrogels were synthesized as described previously.41 Briefly, 500 μL of 3% (w/v) of methacrylated CS-GAG in sterile deionized (DI) H2O containing 0.05% photoinitiator was dispensed into a sterile 5 mL transport vial and exposed to long-wave (365 nm) UV light for exactly 2.5 minutes. The hydrogels thus formed were rinsed three times with sterile DI water waiting 5 minutes between washes to remove any uncross-linked GAG and unused photoinitiator. The CS-GAG gels were then frozen overnight at −80°C and lyophilized to dryness the next day. Upon drying, the hydrogels were tightly capped and stored in desiccant at room temperature and maintained under vacuum until use.
Immediately after PRP acquisition, the PRP was manually invertedto thoroughly mix all cellularcomponents in the PRP. A 500 μL aliquot was then pipetted directly onto a 500 μL freeze-dried CS-GAG gel in a Petri dish and covered. The CS-GAG gels were incubated at room temperature for 5 minutes to allow for full absorption of the PRP by the CS-GAG gel. The CS-GAG gels and any excess PRP that did not absorbwere then transferred to six-well tissue culture plates.
Another 500 μL aliquot of PRP was used to form a PRF gel. To prepare the PRF, an activation solution was made by reconstituting 1 × 106 IU/L bovine thrombin with 5 mL of 10% CaCl2. Five μL of the bovine thrombin/CaCl2 solution was added to a 500 μL PRP aliquot and incubated for 5 minutes at room temperature to enable formation of a PRF gel. Gels and any liquid releasate were then transferred to a six-well tissue culture plate in the same manner as the CS-GAG gels (Fig. 1). All gels were covered with 3 mL of hanks balanced salt solution (HBSS) containing 1% antibiotic-antimycotic solution and 1% fetal bovine serum. Two wells in each tissue culture plate contained the HBSS culture solution alone as a control. Culture solution was aspirated completely and replaced at 24 hours and then every 48 hours for 13 days. Aspirates were frozen at −80°C until assayed.
Fig. 1.
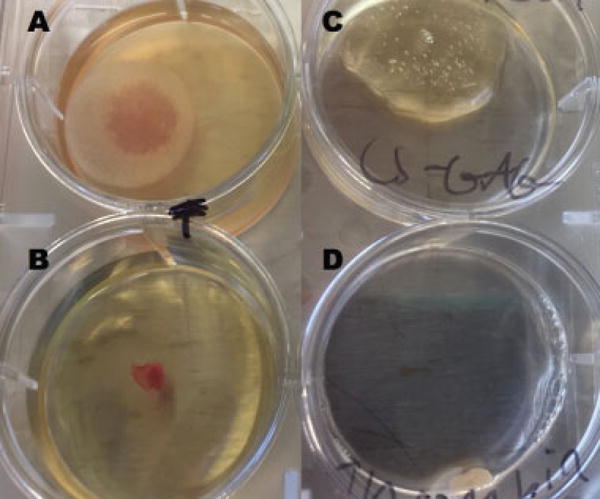
(A–D) Representative (B, D) PRF and (A, C) CS-GAG; gels from (A, B) days 1 and (C, D) 13. By day 13, only small portions of the PRF gels remained, while significant portions of the CS-GAG gels were still intact. CS-GAG, chondroitin sulfate glycosaminoglycan; PRF, platelet-rich fibrin.
Analysis
Samples were assayed for their TGF-β1 growth factor content with mouse/rat/porcine/canine Quantikine enzyme-linked immunosorbent assays (ELISAs) as described previously.43 Briefly, 40 μL of each sample was acid activated by addition of 10 μL of 1N HCl. Following a 10-minute incubation at room temperature, 10 μL of solution containing 1.2N NaOH and 0.5N HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid) was added to neutralize the reaction. Samples were assessed on 96-well ELISA plates in the following groupings: days 1 and 3, days 5 and 7, and days 9, 11, and 13. Samples from days 1, 3, 9, 11, and 13 were diluted 60-fold and samples from days 5 and 7 were diluted 1.5-fold because, based on previous data from other studies evaluating growth factor release from PRF, we expected substantially smaller concentrations of TGF-β1 in the media on these days.35,36 All samples were then run according to manufacturer instructions. ELISA results were analyzed with Prism 7 software by two-way analysis of variance. PRF and CS-GAG samples were then compared on each sample day using a paired sample Wilcoxon matched pairs signed-rank test.
Results
PRPs obtained in this study had a mean platelet concentration of 1.2 × 1012/L (±4 × 1011/L), a mean leukocyte concentration of 6.7 × 109/L (± 2.9 × 109/L), and a negligible hematocrit. In comparing the overall effect of treatment (i.e., CS-GAG gel or PRF gel) on TGF-β1 levels, CS-GAG gels released significantly more TGF-β1 than PRF gels (p = 0.0004; Fig. 2). There was also a significant effect of time on TGF-β1 elution (p < 0.0001). The interaction of time and treatment was not found to be significant (p = 0.52). When TGF-β1 elution from CS-GAG gels and PRF gels were compared for individual days, there was significantly (p < 0.05) greater release of TGF-β1 from CS-GAG gels on days 3, 5, 7, 9, and 13.
Fig. 2.
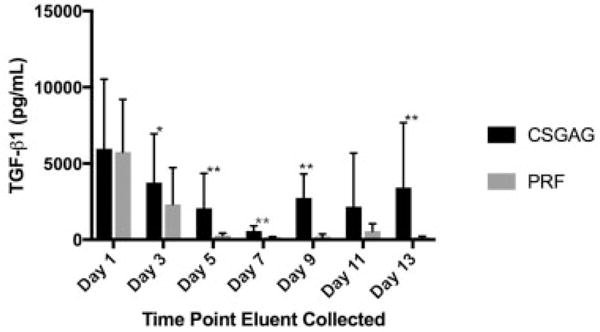
Mean TGF-β1 concentrations released from CS-GAG hydrogels (n = 9) and PRF gels (n = 9) over 13 days. Asterisks denote a significant difference in TGF-β1 concentration between groups at the indicated time points (*p < 0.05; **p < 0.01). CS-GAG, chondroitin sulfate glycosaminoglycan; PRF, platelet-rich fibrin; TGF, transforming growth factor.
Discussion
The results of this study demonstrate that most of the TGF-β1 content was eluted from PRF by day 3, with virtually negligible release seen at later time points. This finding is consistent with previous studies in both humans and dogs that similarly describe an initial burst release of anabolic growth factors, a rapid decline in growth factor release over the next 24 to 72 hours, and virtually negligible growth factor release after 7 days.35,36,44 The consistency in results from multiple studies regarding elution of TGF-β1 from canine and human PRF increases confidence in the repeatability of these results and the general conclusion that growth factor elution from either canine or human PRF is short lived.
In contrast to the PRF, the platelet-rich CS-GAG gels released a substantial amount of TGF-β1 through day 13 after gel creation. We hypothesize that this likely resulted from binding of the anabolic growth factors to the sulfate groups on the CS-GAG gels followed by their gradual release. However, another possible reason for the differences between the PRF and CS-GAG gels is that the PRF gel preparation involved the intentional exogenous activation of the PRP to initiate the clotting cascade for fibrin formation. Conversely, platelets in the PRP used to reconstitute the CS-GAG gels were not activated. The activation process causes the platelet α granules to degranulate and release their growth factors.45,46 Hence, the PRF is likely a reservoir for growth factors that have already been released from the platelets, while the CS-GAG hydrogel may be a reservoir for platelets that have not yet released their growth factors.47,48 As a result, it is difficult to conclude whether the difference in TGF-β1 elution was from the differential TGF-β1–binding capacity of the two gels or from the difference in intentional platelet activation. Although these data do not enable answering this question, it is somewhat clinically irrelevant because activation of PRP is required for creation of PRF and so enhancing sustained elution of growth factor from PRF without activating the platelets is not possible. On the contrary, even greater elution of growth factors might be possible with the use of the CS-GAG gels if the PRP is activated prior to its combination with the CS-GAG gel. In such case, platelets would degranulate and release anabolic growth factors that we hypothesize would bind to the sulfate groups of the CS-GAG gels and result in sustained release of such growth factors.
Even without activation of platelets, these data demonstrate a substantial improvement in temporal release of TGF-β1 in comparison to PRF. In turn, such sustained elution could equate to superior cartilage regeneration and greater clinical benefit with the use of PRP plus the CS-GAG gel in comparison to the use of PRF. Numerous reports detail PRF augmentation of surgical techniques for cartilage repair in the knee.23,24,26–28 Theoretically, this CS-GAG gel could be combined with PRP and used in lieu of PRF to augment these surgical techniques, providing growth factor supplementation for a more extended period of time than if PRF were used. However, prior to clinical application in people, it would be ideal if in vitro investigation were performed to characterize the growth factor elution profile from human platelets in conjunction with this CS-GAG gel. Likewise, controlled studies in animal models would ideally be performed to determine whether the use of the CS-GAG gel results in functional improvement or improved biochemical, biomechanical, or histologic quality of the repair tissue in comparison to the use of PRF.
The aforementioned results and conclusions should be considered in light of some study limitations. One limitation of our study is that we only evaluated the release of TGF-β1. TGF-β1 is one of the most commonly investigated growth factors associated with PRP and has been considered a sentinel of growth factor release from PRP.36,49 However, there are numerous other growth factors associated with PRP that may function synergistically to benefit chondrogenesis.22,50–53 We hypothesize that other growth factors in PRP would interact similarly with the CS-GAG gel based on their net charge, a hypothesis that is also supported by previous study demonstrating sustained release of FBF and BDNF with the use of this CS-GAG gel. However, we do not have data to test this hypothesis and ideally future study would evaluate the temporal release of additional anabolic growth factors when PRP is combined with this CS-GAG gel.
Another shortcoming of this study is that we evaluated TGF-β1 elution for 13 days. This timeline was established a priori and was based on previous studies demonstrating that growth factor elution from PRF is negligible by 7 days. However, at day 13, there was still notable TGF-β1 elution from the CS-GAG gels. Ideally, the temporal release of anabolic growth factors from this CS-GAG gel would be quantified until the growth factors concentrations drop below the lower limit of quantification of their respective ELISAs.
Finally, we diluted media samples from days 5 and 7 1:1.5 with diluent, while samples from days 1, 3, 9, 11, and 13 were diluted 1:60 prior to quantifying TGF-β1 using an ELISA. We only diluted samples 1:1.5 on days 5 and 7 because previous data with human and canine PRF demonstrate a dramatic decrease in TGF-β1 concentration by day 5, and we were concerned that dilution of samples 1:60 would have resulted in undetectable concentrations of TGF-β1 in those samples. The measured concentrations of the TGF-β1 for either or both the CS-GAG and PRF gels are lower on days 5 and 7 than at all other time points, including later days. Manufacturer instructions recommend a 60-fold dilution with this ELISA, and therefore, we hypothesize that the lower dilution of samples on days 5 and 7 may have resulted in greater matrix interference with nontarget proteins during performance of the ELISA, thus decreasing its efficiency and therefore reducing the measured TGF-β1 concentrations.43 However, it should be noted that sample optical densities on days 5 and 7 were still within the range of the standard curve run on those same days. Furthermore, PRF and CS-GAG samples from days 5 and 7 were run on the same ELISA plate so we believe that relative comparison between the two treatments is viable. Likewise, the finding that TGF-β1 release was greater from the CS-GAG gels on days 5 and 7 was consistent with the findings that TGF-β1 release was significantly greater from the CS-GAG gels on days 3, 9, and 13 and for which 60-fold dilution of samples was performed. Hence, despite this limitation, we still conclude that TGF-β1 elution is greater from the CS-GAG gels 3, 5, 7, 9, and 13 days after gel creation.
Footnotes
Conflict of Interest
None.
References
- 1.Turkiewicz A, Petersson IF, Björk J, et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthritis Cartilage. 2014;22(11):1826–1832. doi: 10.1016/j.joca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Akkiraju H, Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol. 2015;3(04):177–192. doi: 10.3390/jdb3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis. 2010;15(05):631–638. doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 4.Studer D, Cavalli E, Formica FA, et al. Human chondroprogenitors in alginate-collagen hybrid scaffolds produce stable cartilage in vivo. J Tissue Eng Regen Med. 2016 doi: 10.1002/term.2203. [DOI] [PubMed] [Google Scholar]
- 5.Freitag J, Bates D, Boyd R, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet Disord. 2016;17:230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 7.Boushell MK, Hung CT, Hunziker EB, Strauss EJ, Lu HH. Current strategies for integrative cartilage repair. Connect Tissue Res. 2016:1–14. doi: 10.1080/03008207.2016.1231180. [DOI] [PubMed] [Google Scholar]
- 8.de Windt TS, Vonk LA, Slaper-Cortenbach IC, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(01):256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 9.Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(08):1316–1319. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 10.Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(03):455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Chahla J, Piuzzi NS, Mitchell JJ, et al. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: a systematic review of the literature and study quality analysis. J Bone Joint Surg Am. 2016;98(18):1511–1521. doi: 10.2106/JBJS.15.01495. [DOI] [PubMed] [Google Scholar]
- 12.Kaul G, Cucchiarini M, Remberger K, Kohn D, Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2315–2324. doi: 10.1007/s00167-011-1853-x. [DOI] [PubMed] [Google Scholar]
- 13.Gomoll AH. Microfracture and augments. J Knee Surg. 2012;25(01):9–15. doi: 10.1055/s-0031-1299654. [DOI] [PubMed] [Google Scholar]
- 14.Madeira C, Santhagunam A, Salgueiro JB, Cabral JM. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015;33(01):35–42. doi: 10.1016/j.tibtech.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Itokazu M, Wakitani S, Mera H, et al. Transplantation of scaffold-free cartilage-like cell-sheets made from human bone marrow mesenchymal stem cells for cartilage repair: a preclinical study. Cartilage. 2016;7(04):361–372. doi: 10.1177/1947603515627342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(01):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milano G, Sanna Passino E, Deriu L, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(07):971–980. doi: 10.1016/j.joca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Chen WH, Lin CM, Huang CF, et al. Functional recovery in osteoarthritic chondrocytes through hyaluronic acid and platelet-rich plasma-inhibited infrapatellar fat pad adipocytes. Am J Sports Med. 2016;44(10):2696–2705. doi: 10.1177/0363546516651822. [DOI] [PubMed] [Google Scholar]
- 20.van Buul GM, Koevoet WL, Kops N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362–2370. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 21.Elder S, Thomason J. Effect of platelet-rich plasma on chondrogenic differentiation in three-dimensional culture. Open Orthop J. 2014;8:78–84. doi: 10.2174/1874325001408010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23(09):2459–2474. doi: 10.1007/s00167-013-2743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haleem AM, Singergy AA, Sabry D, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1(04):253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhollander AA, De Neve F, Almqvist KF, et al. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19(04):536–542. doi: 10.1007/s00167-010-1337-4. [DOI] [PubMed] [Google Scholar]
- 25.Siclari A, Mascaro G, Gentili C, Cancedda R, Boux E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin Orthop Relat Res. 2012;470(03):910–919. doi: 10.1007/s11999-011-2107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siclari A, Mascaro G, Gentili C, Kaps C, Cancedda R, Boux E. Cartilage repair in the knee with subchondral drilling augmented with a platelet-rich plasma-immersed polymer-based implant. Knee Surg Sports Traumatol Arthrosc. 2014;22(06):1225–1234. doi: 10.1007/s00167-013-2484-1. [DOI] [PubMed] [Google Scholar]
- 27.Siclari A, Mascaro G, Kaps C, Boux E. A 5-year follow-up after cartilage repair in the knee using a platelet-rich plasma-immersed polymer-based implant. Open Orthop J. 2014;8:346–354. doi: 10.2174/1874325001408010346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buda R, Vannini F, Cavallo M, et al. One-step arthroscopic technique for the treatment of osteochondral lesions of the knee with bone-marrow-derived cells: three years results. Musculoskelet Surg. 2013;97(02):145–151. doi: 10.1007/s12306-013-0242-7. [DOI] [PubMed] [Google Scholar]
- 29.Vannini F, Battaglia M, Buda R, Cavallo M, Giannini S. “One step” treatment of juvenile osteochondritis dissecans in the knee: clinical results and T2 mapping characterization. Orthop Clin North Am. 2012;43(02):237–244. vi. doi: 10.1016/j.ocl.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Papalia R, Diaz Balzani L, Torre G, et al. Intraoperative application Platelet rich fibrin, postoperative injections OF PRP or microfracture only for osteochondral lesions of the knee: a five-year retrospective evaluation. J Biol Regul Homeost Agents. 2016;30(04, Suppl 1):41–49. [PubMed] [Google Scholar]
- 31.Kazemi D, Fakhrjou A. Leukocyte and platelet rich plasma (L-PRP) versus leukocyte and platelet rich fibrin (L-PRF) for articular cartilage repair of the knee: a comparative evaluation in an animal model. Iran Red Crescent Med J. 2015;17(10):e19594. doi: 10.5812/ircmj.19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(04):528–535. doi: 10.1007/s00167-010-1238-6. [DOI] [PubMed] [Google Scholar]
- 33.Papalia R, Zampogna B, Russo F, et al. Comparing hybrid hyaluronic acid with PRP in end career athletes with degenerative cartilage lesions of the knee. J Biol Regul Homeost Agents. 2016;30(04, Suppl 1):17–23. [PubMed] [Google Scholar]
- 34.Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473(05):1635–1643. doi: 10.1007/s11999-015-4192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalowiec JM, D’Este M, Bara JJ, et al. An in vitro investigation of platelet-rich plasma-gel as a cell and growth factor delivery vehicle for tissue engineering. Tissue Eng Part C Methods. 2016;22(01):49–58. doi: 10.1089/ten.tec.2015.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visser LC, Arnoczky SP, Caballero O, Egerbacher M. Platelet-rich fibrin constructs elute higher concentrations of transforming growth factor-β1 and increase tendon cell proliferation over time when compared to blood clots: a comparative in vitro analysis. Vet Surg. 2010;39(07):811–817. doi: 10.1111/j.1532-950X.2010.00739.x. [DOI] [PubMed] [Google Scholar]
- 37.Lutianov M, Naire S, Roberts S, Kuiper JH. A mathematical model of cartilage regeneration after cell therapy. J Theor Biol. 2011;289:136–150. doi: 10.1016/j.jtbi.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Delplace V, Obermeyer J, Shoichet MS. Local affinity release. ACS Nano. 2016;10(07):6433–6436. doi: 10.1021/acsnano.6b04308. [DOI] [PubMed] [Google Scholar]
- 39.Seto SP, Casas ME, Temenoff JS. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell Tissue Res. 2012;347(03):589–601. doi: 10.1007/s00441-011-1265-8. [DOI] [PubMed] [Google Scholar]
- 40.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Karumbaiah L, Enam SF, Brown AC, et al. Chondroitin sulfate glycosaminoglycan hydrogels create endogenous niches for neural stem cells. Bioconjug Chem. 2015;26(12):2336–2349. doi: 10.1021/acs.bioconjchem.5b00397. [DOI] [PubMed] [Google Scholar]
- 42.Logun MT, Bisel NS, Tanasse EA, et al. Glioma cell invasion is significantly enhanced in composite hydrogel matrices composed of chondroitin 4-and 4, 6-sulfated glycosaminoglycans. J Mater Chem B Mater Biol Med. 2016;4(36):6052–6064. doi: 10.1039/C6TB01083K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birdwhistell K, Basinger R, Hayes B, Norton N, Hurley DJ, Franklin SP. Validation of commercial ELISAs for quantifying anabolic growth factors and cytokines in canine ACD-A anticoagulated plasma. J Vet Diagn Invest. 2017;29(02):143–147. doi: 10.1177/1040638717690186. [DOI] [PubMed] [Google Scholar]
- 44.Dohan Ehrenfest DM, Bielecki T, Jimbo R, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF) Curr Pharm Biotechnol. 2012;13(07):1145–1152. doi: 10.2174/138920112800624382. [DOI] [PubMed] [Google Scholar]
- 45.Textor JA, Tablin F. Activation of equine platelet-rich plasma: comparison of methods and characterization of equine autologous thrombin. Vet Surg. 2012;41(07):784–794. doi: 10.1111/j.1532-950X.2012.01016.x. [DOI] [PubMed] [Google Scholar]
- 46.Frelinger AL, III, Gerrits AJ, Garner AL, et al. Modification of pulsed electric field conditions results in distinct activation profiles of platelet-rich plasma. PLoS One. 2016;11(08):e0160933. doi: 10.1371/journal.pone.0160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrison S, Vavken P, Kevy S, Jacobson M, Zurakowski D, Murray MM. Platelet activation by collagen provides sustained release of anabolic cytokines. Am J Sports Med. 2011;39(04):729–734. doi: 10.1177/0363546511401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavallo C, Roffi A, Grigolo B, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. BioMed Res Int. 2016;2016:6591717. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sánchez-González DJ, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012;2012:532519. doi: 10.1155/2012/532519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Panseri S, Russo A, Cunha C, et al. Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg Sports Traumatol Arthrosc. 2012;20(06):1182–1191. doi: 10.1007/s00167-011-1655-1. [DOI] [PubMed] [Google Scholar]
- 53.Marcacci M, Filardo G, Kon E. Treatment of cartilage lesions: what works and why? Injury. 2013;44(Suppl 1):S11–S15. doi: 10.1016/S0020-1383(13)70004-4. [DOI] [PubMed] [Google Scholar]


