Abstract
A positive relationship between biodiversity and ecosystem stability has been reported in many ecosystems; however, it has yet to be determined whether and how spatial scale affects this relationship. Here, for the first time, we assessed the effects of alpha, beta and gamma diversity on ecosystem stability and the scale dependence of the slope of the diversity–stability relationship.
By employing a long-term (33 years) dataset from a temperate grassland, northern China, we calculated the all possible spatial scales with the complete combination from the basic 1-m2 plots.
Species richness was positively associated with ecosystem stability through species asynchrony and overyielding at all spatial scales (1, 2, 3, 4 and 5 m2). Both alpha and beta diversity were positively associated with gamma stability.
Moreover, the slope of the diversity–area relationship was significantly higher than that of the stability–area relationship, resulting in a decline of the slope of the diversity–stability relationship with increasing area.
Synthesis. With the positive species diversity effect on ecosystem stability from small to large spatial scales, our findings demonstrate the need to maintain a high biodiversity and biotic heterogeneity as insurance against the risks incurred by ecosystems in the face of global environmental changes.
Keywords: asynchrony, biodiversity, community stability, ecosystem function and services, homogenization, metacommunity, primary productivity, spatial insurance, species richness, steppe
1. Introduction
Direct and indirect impacts of anthropogenic activities, which include land use change, nutrient enrichment, greenhouse gas emissions and climate change, tend to reduce the complexity of our planet’s natural ecosystems (Fahrig, 2003; Gossner et al., 2016; Holland, Clarke, & Bennett, 2017; Vitousek & Howarth, 1991). We are facing a crisis due to the world’s biodiversity being degraded at an alarming rate (Gonzalez et al., 2016; Naeem, Duffy, & Zavaleta, 2012; Plotnick, Smith, & Lyons, 2016). According to recent estimates, current rates of extinction may be comparable to the Cretaceous extinction of the dinosaurs, casting us as a metaphorical asteroid (Cafaro, 2015; Plotnick et al., 2016).
Biodiversity underpins the ecosystem functioning and services on which humans largely rely (Cardinale et al., 2012; Isbell et al., 2011; McCallum, 2015). Communities with greater diversity are expected to have a more stable functioning, because a greater number of ecological interactions among its components should facilitate its resistance and resilience (Isbell et al., 2015; Lehman & Tilman, 2000). Based on extensive theoretical and empirical studies, ecologists have made major advances towards understanding how biodiversity affects ecosystem productivity and stability (Loreau, 2010; Tilman, Isbell, & Cowles, 2014; Turnbull, Isbell, Purves, Loreau, & Hector, 2016). Many studies have demonstrated that species richness enhances ecosystem stability (as measured by the inverse of the temporal variability in its aggregate properties) in both experimental and natural communities at small spatial scales (Bai, Han, Wu, Chen, & Li, 2004; Hautier et al., 2015; Hector et al., 2010; Jiang & Pu, 2009). They show that species richness promotes the stability of productivity at local scales, mainly through the insurance effect (species asynchrony or compensation) (Bai et al., 2004; Loreau & de Mazancourt, 2013; Yachi & Loreau, 1999; Zhang, Loreau, He, Zhang, & Han, 2017b) and overyielding (Hector et al., 2010; Tilman, Reich, & Knops, 2006).
Understanding scaling relationships is critical to foster the development of predictive tools and better mechanistic models in ecology (Allen et al., 2016; Aragón, Oesterheld, Irisarri, & Texeira, 2011; Chalcraft, 2013; Loreau, Mouquet, & Gonzalez, 2003; Wang & Loreau, 2014, 2016; Wang et al., 2017; Wiens, 1989). Theory predicts that diversity should be positively associated with ecosystem stability at all spatial scales (Lehman & Tilman, 2000; Wang & Loreau, 2016), but diversity should have a stronger stabilizing effect at the regional scale, at least in absolute terms, with contributions from both alpha (α) and beta (β) diversity (Wang & Loreau, 2014, 2016). To date, few experiments have been conducted to test this hypothesis (Aragón et al., 2011; Chalcraft, 2013). Theory also predicts that ecosystem stability increases with area (Wang & Loreau, 2014; Wang et al., 2017), just as species richness increases with area (Storch, 2016). What is still unknown, however, is whether the slope of the diversity–stability relationship increases or decreases with spatial scale.
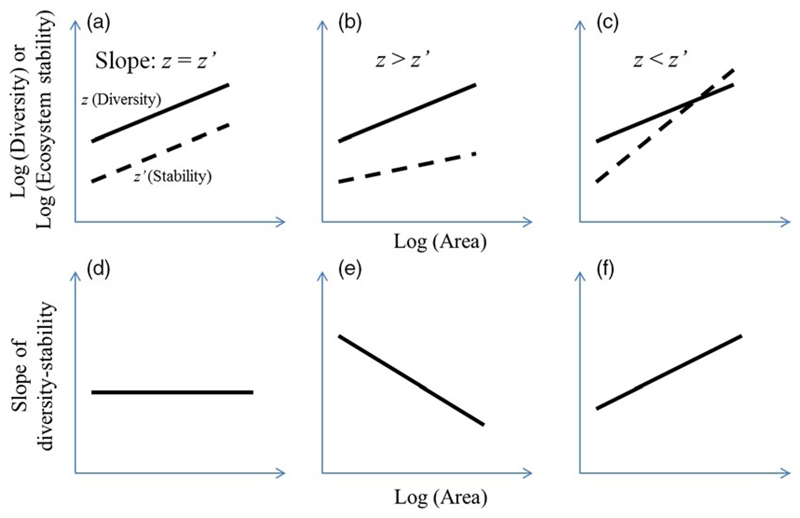
Three alternative scenarios are possible for how the slope of the diversity–stability relationship changes with spatial scale (Figure 1). If the diversity–area (with slope z) and stability–area (with slope z′) relationships have similar slopes (i.e., z = z′; Figure 1a), the corresponding slope of the diversity–stability relationship should remain constant with increasing area (Figure 1d). If z > z′ (Figure 1b), the slope of the diversity–stability relationship should decline with increasing area (Figure 1e). In contrast, if z < z′ (Figure 1c), the slope of the diversity–stability relationship should increase with increasing area (Figure 1f).
Figure 1.
Three scenarios leading to changes in diversity– and stability–area relationships, and the corresponding changes in the slopes of diversity–stability relationship. As both diversity (slope: z) and ecosystem stability (slope: z′) theoretically increase with area, three possible scenarios exist (i.e., constant, decrease, and increase) for the pattern of the diversity effect on ecosystem stability with increasing area. (a) Diversity has a similar slope to that of ecosystem stability (i.e., z = z′); (b) Diversity has a larger slope than that of ecosystem stability (i.e., z > z′); (c) Diversity has a smaller slope than that of ecosystem stability (i.e., z < z′). (d) The constant scenario: as shown in (a) (z = z′), the corresponding slopes of the diversity–stability relationship remain relatively constant; thus, the diversity–stability relationship is not influenced by an increase in area; (e) The decrease scenario: as shown in (b) (z > z′), the corresponding slopes of the diversity–stability relationship decrease as the area increases; thus, the effect of diversity on ecosystem stability weakens as the area increases; (f) The increase scenario: as shown in (c) (z < z′), the corresponding slopes of the diversity–stability relationship increase with increasing area; thus, the effect of diversity on ecosystem stability is enhanced with increasing area [Colour figure can be viewed at wileyonlinelibrary.com]
Using a long-term (33-year; 1982–2014) field dataset in a temperate steppe of Inner Mongolia, China, here we explore whether species richness promotes ecosystem stability at multiple spatial scales. We also test whether α and β diversity are positively associated with γ stability (i.e., ecosystem stability in the largest area) in real natural communities. In addition, we test our alternative hypotheses as to how the slope of the positive diversity–stability relationship changes with increasing area.
2. Materials and Methods
2.1. Study site
This study was conducted near the Inner Mongolia Grassland Ecosystem Research Station (116°14′E, 43°13′N), which was located in the Xilin River Basin, Inner Mongolia, China. C3 perennial rhizome grass (i.e., Leymus chinensis) and C3 perennial bunchgrass (i.e., Stipa grandis) were co-dominant (Bai et al., 2004). The soil was classified as Haplic Calcisols and Calcic-Orthic Aridisol by the Food and Agriculture Organization of the United Nations (FAO) and the USA soil classification system, respectively. The long-term (1982–2014) mean annual air temperature was 0.97°C, with mean monthly temperature ranging from −21.4°C in January to 19.7°C in July. The mean annual precipitation was 345.2 mm, with approximately four-fifths falling during the growing season (i.e., from May to September). In 1979, 2 sites of 25-ha each in the grassland (separated by a spatial distance of 25 km) were fenced to keep out large animal grazers. At each site, an east–west transect of 200 × 100 m2 was established with five equal-sized replicate blocks (Figure S1; 40 × 100 m2 for each block). More details can be found in Bai et al. (2004).
The above-ground net primary productivity (ANPP) of the community was estimated from the above-ground plant biomass, which is an accepted approximation for ANPP in this region, because above-ground plant tissues die during the winter season. Although the field experiments were established in 1979, biomass was sampled five times (at monthly intervals) across the growing season (i.e., May, June, July, August, and September) from 1982 to 2014 (Figure S1). Plant above-ground biomass was measured by clipping all plants above the soil surface in a 1 × 1 m2 quadrat, without spatial overlap. Hence, each biomass measure was a proxy of community ANPP from budding to the sampling month. All living vascular plants were sorted to the species level, oven-dried at 65°C for 48 hr to a constant weight, and then weighed. The number of plant species per m2 was recorded in the same quadrat used to measure above-ground biomass. In total, more than 20,000 plant above-ground biomass values were measured in 1,650 basic 1-m2 quadrats, i.e., 5 plots per sampling time per site × 2 sites × 5 sampling times per year throughout the growing season × 33 years (Figure S1).
2.2. Spatial scales
Each site contained five blocks, and each block contained five plots that were dispersed, but relatively closely connected, in space (Figure S1). Within each block, a different plot was sampled each month. With the combination of five 1-m2 plots at each sampling date in each site, we assessed five spatial scales, i.e., 1, 2, 3, 4, and 5 m2. Since the ANPP of each species (i.e., population ANPP) was measured in each 1-m2 plot, data on both species richness and ANPP can be easily obtained at the five spatial scales (Zhang, He, Loreau, Pan, & Han, 2017a).
We treated each 1-m2 plot as an individual community (hereafter, termed small scale, i.e., 1 m2). The five plots sampled at the same date in each site (i.e., the five same colored 1-m2 plots in Figure S1) were regarded as a metacommunity (hereafter, termed large scale, i.e., 5 m2). Since different plots were sampled each month, we collected data for five metacommunities in each site, which makes 10 metacommunities in total in the two sites (Figure S1).
2.3. Alpha, beta and gamma diversity
Alpha diversity was measured as the average species richness of the 1-m2 plots (i.e., the smallest scale in the study), while γ diversity was measured as the species richness of the combination of the five 1-m2 plots in each metacommunity. Then, both α and γ diversity were averaged over the 33 years. Beta diversity was calculated as β diversity = γ diversity − α diversity (Tuomisto, 2010; Wang & Loreau, 2014), i.e., as the regional diversity excess.
2.4. Alpha and gamma stability
Ecosystem stability (Lehman & Tilman, 2000) was defined as S = μ/σ, where μ and σ are the inter-annual mean and standard deviation of ecosystem ANPP over the 33 years. The α (i.e., ecosystem stability at the 1-m2 scale) and γ stability (i.e., ecosystem stability at the 5-m2 scale) were used as proxies of ecosystem stability at the smallest and the largest scales, respectively. Just as with α diversity, α stability was averaged across the 1-m2 plots.
2.5. Species asynchrony
Community-wide species asynchrony (Loreau & de Mazancourt, 2008) was quantified as where is the variance of ecosystem ANPP. is the standard deviation of ANPP of species i in a community with N species over all the years. Community-wide species asynchrony ranges between 0 (perfect synchrony) and 1 (perfect asynchrony), regardless of spatial scale.
2.6. Overyielding effect
A positive relationship between species richness and ecosystem ANPP indicates an overyielding effect on ecosystem stability as biodiversity increases (Hector et al., 2010; Tilman et al., 2006).
2.7. Spatial scaling of species richness
Based on the positive relationship between species richness and area (MacArthur & Wilson, 1967; Storch, 2016), we used the followed equation to illustrate how spatial scaling affects diversity (Figure 1a–c):
| (1) |
where N, c, z, and A are the number of plants of species in the ecosystem, intercept, slope, and area, respectively. Parameter z represents the rate of change in the number of plant species with expanding sampling area, i.e., from α to γ diversity with increasing area.
2.8. Spatial scaling of ecosystem stability
Theoretically, ecosystem stability increases with spatial scale (Wang & Loreau, 2014; Wang et al., 2017). Here, we used:
| (2) |
where S, c′, z′, and A are the ecosystem stability, intercept, slope, and area, respectively, to illustrate how scaling affects ecosystem stability (Figure 1a–c). The parameter z′ represents the rate of increase in ecosystem stability with expanding sampling area, i.e., from α to γ stability with increasing area.
2.9. Spatial scaling of the slope of the diversity–stability relationship
We used linear regression to explore whether the slopes of the diversity–stability relationship changed with increasing area.
2.10. Statistical analysis
T tests were performed to test the difference between α and γ diversity and between α and γ stability. Moreover, two-way analysis of covariance was performed to show whether increases in the slope of the species–area relationship (z) differed from that of the ecosystem stability–area relationship (z′) using area as a continuous variable.
All statistical analyses were carried out using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Species richness promotes ecosystem stability
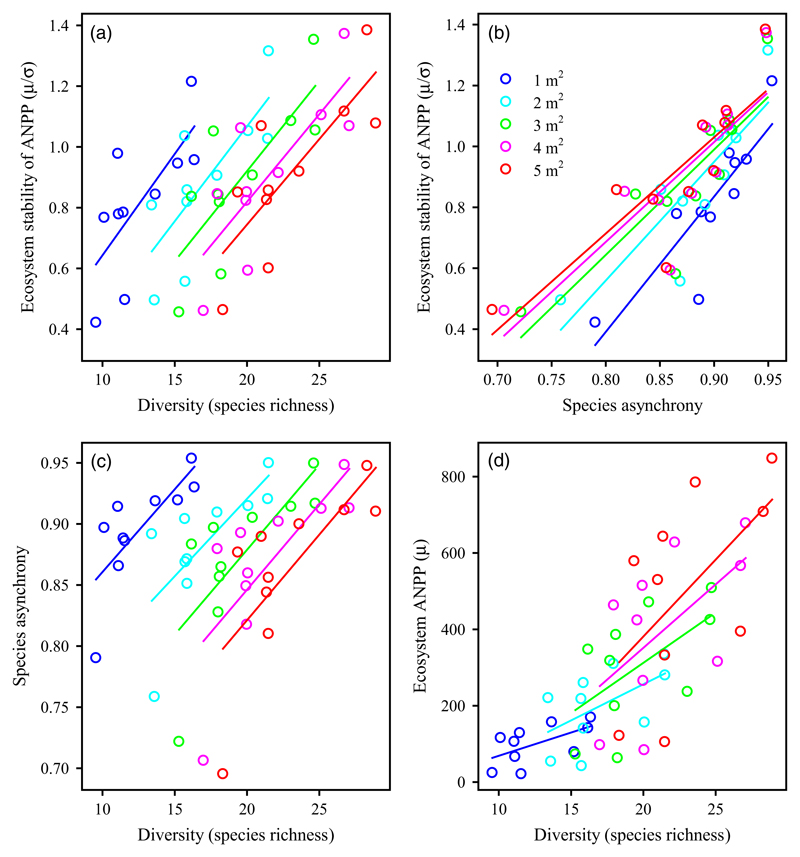
Species richness was positively associated with ecosystem stability at all spatial scales (Figure 2a; p < .05). Species asynchrony was also positively associated with ecosystem stability at all spatial scales (Figure 2b; p < .01), and species richness was positively correlated with species asynchrony (Figure 2c; p < .05). Species richness was positively associated with ecosystem ANPP (Figure 2d; p < .1). These positive relationships suggest that diversity promotes ecosystem stability at each scale through the effects of both species asynchrony and overyielding.
Figure 2.
Species richness promotes ecosystem stability at different spatial scales. (a) Species richness was positively associated with the temporal stability of ecosystem above-ground net primary productivity (ANPP) across years (1982–2014) at a given spatial scale (i.e., 1, 2, 3, 4, and 5 m2, respectively). (b) Species asynchrony was positively associated with ecosystem stability. (c) Species richness was positively associated with species asynchrony. (d) Relationships between species richness and ecosystem ANPP [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Effects of alpha and beta diversity on gamma stability
Species richness was smaller in the smallest areas (α diversity) compared to the largest areas (γ diversity) (t18 = −7.67, p < .0001). Thus, α diversity was significantly lower than γ diversity. Ecosystem stability was slightly higher, but not significant, in the largest area of the Inner Mongolia grassland (t18 = −0.88, p = .3910), suggesting that γ stability was not significantly greater than α stability.
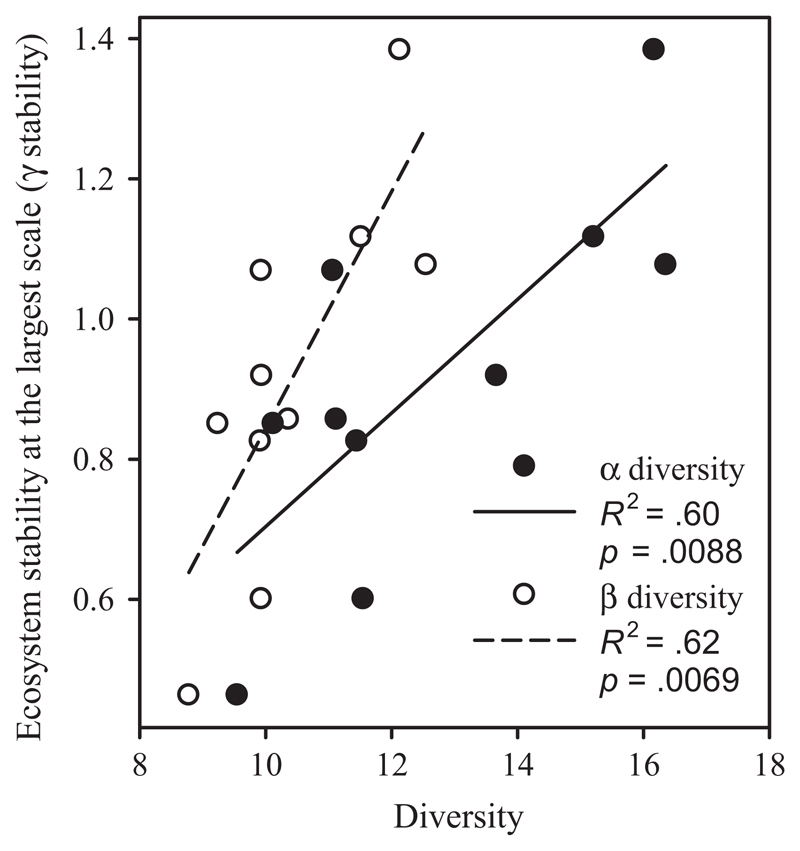
Alpha diversity was positively associated with γ stability (Figure 3; F1,8 = 11.8, p = .0088; R2 = .60). Beta diversity was also positively related with γ stability (Figure 3; F1,8 = 13.0, p = .0069; R2 = .62). Thus, both α and β diversity might contribute to γ stability.
Figure 3.
Alpha (α) and beta (β) diversity were associated with gamma (γ) stability. Both α and β diversity were positively associated with γ stability within the metacommunity. Solid and open symbols correspond to α diversity and β diversity, respectively
3.3. Spatial scaling of species richness and ecosystem stability
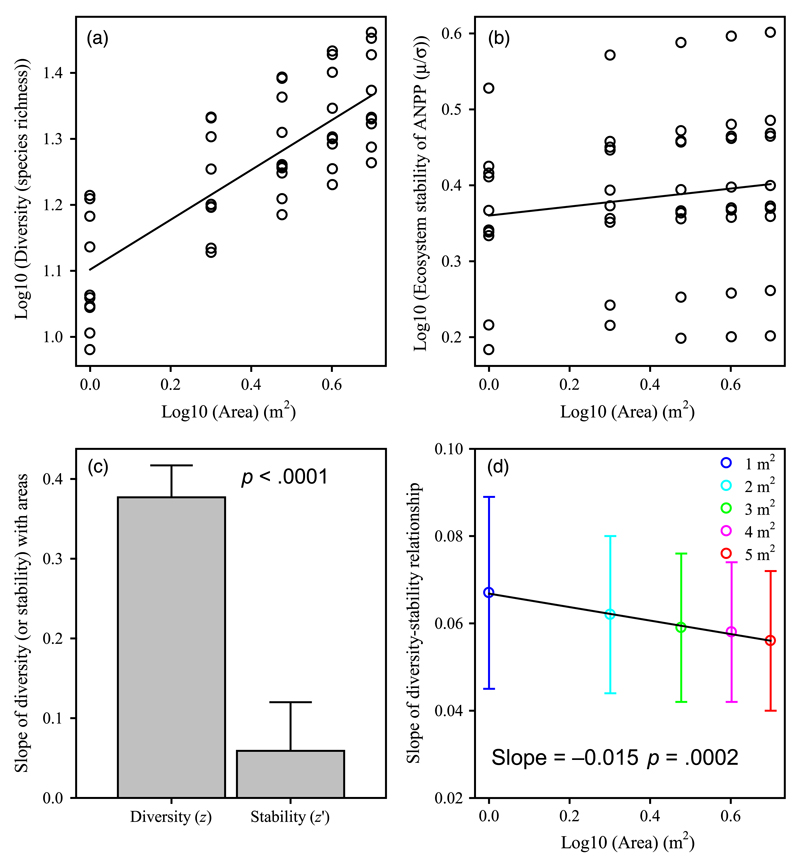
Species richness significantly increased with area (Figure 4a; F1,48 = 82.07, p < .0001; R2 = .63), but ecosystem stability did not (Figure 4b; F1,48 = 0.95, p = .3339; R2 = .02) The slope of the diversity–area relationship was significantly greater than that of the stability–area relationship (Figure 4c; z = 0.377, z′ = 0.059; F1,96 = 18.65, p < .0001). This result corresponds the scenario presented in Figure 1b (i.e., z > z′).
Figure 4.
Changes in the slopes between species richness and ecosystem stability with increasing area. (a) Species richness increased with areas. (b) Ecosystem stability did not alter with increasing area. (c) The slope of species richness vs. area (z = 0.377 ± 0.040) was significantly greater than that of ecosystem stability vs. area (z′ = 0.059 ± 0.061) (i.e., z > z′; F1,96 = 18.65, p < .0001; value of slope from a and b). (d) The slopes of species-richness–ecosystem-stability relationships declined with increasing area (data from Figure 2a; F1,3 = 441.00, p = .0002; R2 = .99), supporting our hypothesized decrease scenario (see Figure 1e); specifically, when z > z′, the corresponding slopes of the relationship between diversity and stability decrease with increasing area. When the area was not log-transformed, the linear regression was also significant (F1,3 = 36.21, p = .0092; R2 = .92; mean and 95% confidence intervals: −0.0030 [−0.0034 to −0.0026]). Error bars indicate 1 SE [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Spatial scaling of the slope of diversity–stability relationship
The slopes of the diversity–stability relationship declined significantly with area (Figure 4d; F1,3 = 441.00, p = .0002; R2 = .99; mean and 95% confidence intervals: −0.015 [−0.016 to −0.014]). This result was consistent with the hypothesized decrease scenario (i.e., the second scenario; Figure 1e), whereby, when z > z′, the slopes of the diversity–stability relationship decline with increasing area.
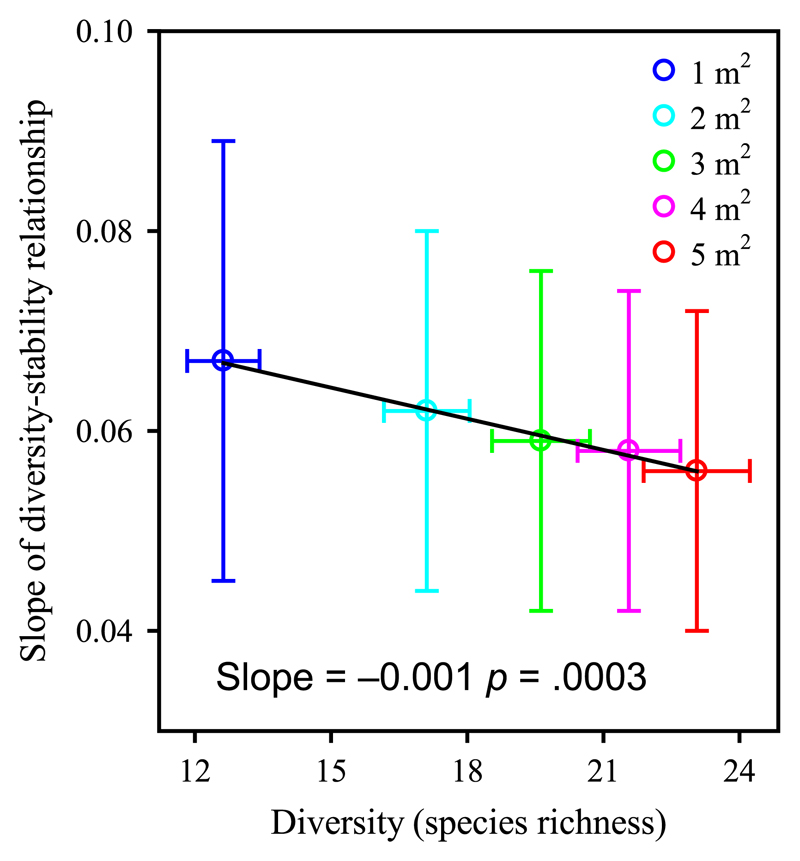
In addition, species richness was negatively associated with the slope of the diversity–stability relationship across areas (Figure 5; F1,3 = 378.91, p = .0003; R2 = .99).
Figure 5.
Relationship between species richness and the slope of diversity–stability with areas. Species richness was negatively related with the slope of diversity–stability with areas (data from Figure 2a), indicating that a higher species richness at larger area was associated with a lower value of the slope of diversity–stability relationship. The values of species richness were averaged each given spatial scale. Error bars indicate 1 SE [Colour figure can be viewed at wileyonlinelibrary.com]
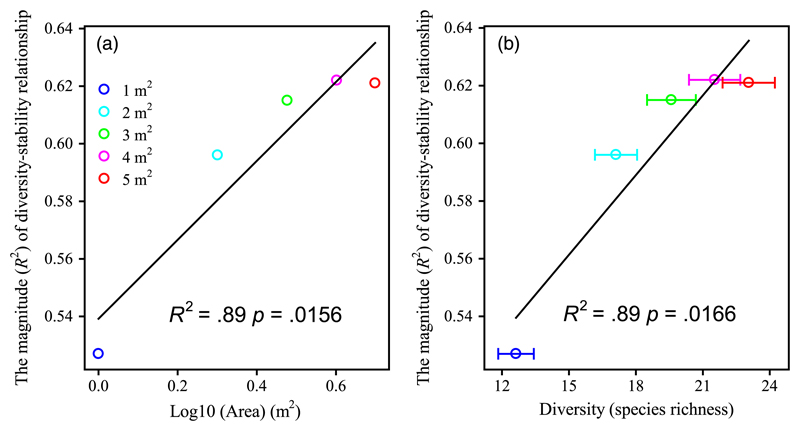
The magnitude (R2) of the diversity–stability relationship was increased with spatial scale (Figure 6a; F1,3 = 24.74, p = .0156; R2 = .89). Species richness was positively associated with the magnitude (R2) of the diversity–stability relationship across areas (Figure 6b; F1,3 = 23.69, p = .0166; R2 = .89).
Figure 6.
The magnitude of diversity–stability with areas. (a) The magnitude (R2) of diversity–stability relationship (data from Figure 2a) increases with spatial scales. (b) Species richness was positively related with the magnitude (R2) of diversity–stability with areas, suggesting that the diversity effect is more important at large spatial scale. The values of species richness were averaged each given spatial scale. Error bars indicate 1 SE [Colour figure can be viewed at wileyonlinelibrary.com]
4. Discussion
Our study tested whether diversity promotes γ stability, and the scale dependence of the diversity–stability relationship using long-term field data. It showed that species richness promoted ecosystem stability at the corresponding spatial scale, yielding positive diversity–stability relationships at all scales. Both α and β diversity were positively associated with γ stability, supporting a recent theoretical prediction by Wang and Loreau (2014, 2016). Furthermore, the slope of the diversity–area relationship was greater than that of the stability–area relationship, leading to a decline in the slope of the diversity–stability relationship with increasing area in agreement with our decrease scenario (Figure 1e).
We found a positive relationship between species richness and ecosystem stability at all spatial scales. This positive biodiversity–stability relationship is consistent with the results obtained by both theoretical models (Lehman & Tilman, 2000; Loreau, 2010; Wang & Loreau, 2016) and experimental studies (Hautier et al., 2015; Jiang & Pu, 2009; Tilman et al., 2006; Zhu, van der Werf, Anten, Vos, & Evers, 2015). Species asynchrony (Hector et al., 2010; Isbell, Polley, & Wilsey, 2009; Zhu et al., 2015) and the overyielding effect (Hector et al., 2010; Tilman et al., 2006) are important mechanisms explaining the positive relationship between species richness and ecosystem stability in plant communities. Supporting previous studies (Hautier et al., 2014; Hector et al., 2010; Isbell et al., 2009), we also found that species richness was positively related with species asynchrony, which, in turn, was positively associated with ecosystem stability. Therefore, species asynchrony might help explain how positive diversity affects ecosystem stability at every spatial scale in the Inner Mongolia grassland. Species asynchrony stabilizes ecosystem stability at each spatial scale. Theoretically, species that coexist in the same ecosystem may respond asynchronously to environmental fluctuations (Lehman & Tilman, 2000; Loreau & de Mazancourt, 2013; Tredennick, de Mazancourt, Loreau, & Adler, 2017). Overall, our study not only supported the results from previous study that temporal complementarity between species enhances the temporal stability of ecosystem productivity at small scales in the Inner Mongolia grassland (Bai et al., 2004) but also verified these mechanisms at larger spatial scales.
Moreover, (α) and (β) diversity were positively related with γ stability. This occurred where species richness was higher at the larger scale but ecosystem stability was constant across scales. A higher β diversity represents a higher heterogeneity or spatial variability in species composition (Tuomisto, 2010; Wang & Loreau, 2014, 2016). One recent field study showed that a higher spatial variability promoted temporal stability at the regional scale in the Tallgrass Prairie Preserve of northeastern Oklahoma, USA (McGranahan et al., 2016). Another field study showed that a greater species compositional dissimilarity between microcosms (i.e., a higher β diversity) caused spatial synchrony to decline, promoting γ stability (France & Duffy, 2006). The results of the current study suggest that higher levels of species richness at the local scale enhance ecosystem stability at both small and large spatial scales. They also show that β diversity contributes to ecosystem stability at larger spatial scales. These results support Wang and Loreau’s (2014, 2016) theoretical predictions, and suggest that biodiversity has a stabilizing effect on γ stability via both local (Yachi & Loreau, 1999) and spatial (Loreau et al., 2003) insurance effects. Thus, maintaining local biodiversity and spatial biotic heterogeneity enhances both biodiversity and the stability of ecosystem properties at large spatial scales.
Beta diversity is associated with the spatial heterogeneity of species composition, and promotes stability at large scales (Aragón et al., 2011; France & Duffy, 2006). The physical environment and living organisms are rarely uniformly or randomly distributed. Instead, resources in natural ecosystems aggregate in patches, or form gradients, or other kinds of spatial structures. Larger areas likely contain more microhabitats than do smaller areas, and thus are spatially more heterogeneous. For instance, large areas might include habitat types or resources that are absent in small areas. Therefore, a higher spatial heterogeneity of habitats has the potential to maintain more species (Bergholz et al., 2017; Law & Dieckmann, 2000; Turner & Tjørve, 2005). In turn, such species should be able to exploit spatially heterogeneous resources more effectively, enhancing stability at a large scale through spatial niche complementarity (Loreau et al., 2003).
In contrast, the homogenization of environmental conditions and resources is likely to reduce species richness and ecosystem stability in natural ecosystems. Environmental homogenization may reduce γ diversity due to a decline in either α or β diversity, or both. Species predicted to go extinct are initially those that are least abundant as environments gradually homogenize. Such extinctions are associated with low growth rates (e.g., due to poor habitat quality), high variance in growth rates (environmental stochasticity) or small population size, due to low carrying capacity or other factors (Sodhi, Brook, & Bradshaw, 2009). Recent theory (Wang & Loreau, 2014, 2016), a recent empirical study (McGranahan et al., 2016), and our own study (Figure 3) all indicate that temporal stability at large scale benefits from both a high local diversity and a greater spatial turnover in species composition. Less abundant species, particularly rare species that may be critical to ecosystem multifunctionality (Lyons & Schwartz, 2001; Soliveres et al., 2016), may be subject to stronger environmental stochasticity and a higher possibility of extinction when exposed to habitat homogenization under most global change factors (Gossner et al., 2016; Holland et al., 2017; Vitousek & Howarth, 1991). The loss of such species would then lead to a decline in regional stability.
Interestingly, the slope of the diversity–stability relationship, however, decreased with species richness (Figure 5). Since a higher species richness is also associated with a larger area, this phenomenon might partly explain why the slope of the diversity–stability relationship decreases with increasing area in natural ecosystems.
An important new result of our study is that the slope of the diversity–area relationship was significantly larger than that of the stability–area relationship, leading to a decline in the slope of the diversity–stability relationship as area increases. We re-analysed simulation data from Wang and Loreau’s (2016) model metacommunities, and found the same result (Figure S2a; F1,59996 = 13.74, p = .0002). In the model metacommunities, the slope of the diversity–variability relationship was significantly more negative at small spatial scales (−0.0021) than that at large spatial scales (−0.0015) (Figure S2b; t58 = −2.26, p = .0278) across all parameter values. Interestingly, the difference between the slopes at small and large scales increased with the strength of the interaction between the patch- and species-specific environmental responses (i.e., value = 8; t28 = −3.62, p = .0011). When the strength of the interaction between the patch- and species-specific environmental responses increases, the association between β diversity and γ stability probably decreases (Wang & Loreau, 2016), thereby weakening the correlation between γ diversity and stability. In contrast, the slope of the diversity–stability relationships did not show any significant difference between the two spatial scales for a given set of parameter values in the simulated metacommunities, whether the between-patch environmental correlation (i.e., −0.8, −0.4, 0, 0.4 and 0.8, respectively) or the between-species environmental correlation (i.e., −0.05, 0 and 0.4, respectively) was varied. These results suggest that the effect of spatial scale on the slope of the diversity–stability relationship occurred mainly through the interaction between patch- and species-specific environmental responses. Overall, these metacommunity model simulations agree with our findings on the changes in the slope of the diversity–stability relationship with scale.
5. Conclusions
Our study provides strong empirical support for recent theory (Wang & Loreau, 2014, 2016) predicting that biodiversity promotes ecosystem stability at large spatial scales, through effects of both α and β diversity. Our study also extends this theory by predicting that the slope of the diversity–stability relationship declines with increasing area because diversity depends more strongly on spatial scale than does ecosystem stability. Future empirical and theoretical studies are needed to test and extend these findings and predictions, which will greatly improve our understanding of the effects of global changes and environmental homogenization.
Supplementary Material
Acknowledgements
We thank Shu Jiang, Zuozhong Chen, Dehua Huang, Shiping Wang, Yongfei Bai, and the others for their contributions to the long-term data collection. We also thank Shaopeng Wang for providing the data on simulated metacommunities from Wang and Loreau (2016). This study was supported by the National Natural Science Foundation of China (NSFC: 31570469) and the International Postdoctoral Exchange Fellowship Program (20170070) to Y.Z., the National Key R&D Program of China (2016YFC0500202) to N.H., the TULIP Laboratory of Excellence (ANR-10-LABX-41) and by the BIOSTASES Advanced Grant, funded by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (666971) to M.L., and the National Key R&D program of China (2016YFC0500700) and NSFC (31430016) to X.H.
Funding information
National Natural Science Foundation of China, Grant/Award Number: 31570469 and 31430016; International Postdoctoral Exchange Fellowship Program, Grant/Award Number: 20170070; National Key R&D Program of China, Grant/Award Number: 2016YFC0500202 and 2016YFC0500700; TULIP Laboratory of Excellence, Grant/Award Number: ANR-10-LABX-41; European Union’s Horizon 2020 research and innovation programme, Grant/Award Number: 666971
Footnotes
Authors’ Contributions
Y.Z., N.H., M.L. and X.H. designed the research; Y.Z., N.H. and Q.P. collected data used in the analysis; Y.Z. performed the analysis; Y.Z. wrote the paper with input from all authors.
The authors declare no competing interests.
Data Accessibility
Data deposited in the Dryad Digital Repository: https://doi.org/10.5061/dryad.58fv8 (Zhang et al., 2017a).
Orcid
Yunhai Zhang http://orcid.org/0000-0003-0613-3624
Nianpeng He http://orcid.org/0000-0002-0458-5953
References
- Allen CR, Angeler DG, Cumming GS, Folke C, Twidwell D, Uden DR. Quantifying spatial resilience. Journal of Applied Ecology. 2016;53:625–635. doi: 10.1111/1365-2664.12634. [DOI] [Google Scholar]
- Aragón R, Oesterheld M, Irisarri G, Texeira M. Stability of ecosystem functioning and diversity of grasslands at the landscape scale. Landscape Ecology. 2011;26:1011–1022. doi: 10.1007/s10980-011-9625-z. [DOI] [Google Scholar]
- Bai YF, Han XG, Wu JG, Chen ZZ, Li LH. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- Bergholz K, May F, Giladi I, Ristow M, Ziv Y, Jeltsch F. Environmental heterogeneity drives fine-scale species assembly and functional diversity of annual plants in a semi-arid environment. Perspectives in Plant Ecology, Evolution and Systematics. 2017;24:138–146. doi: 10.1016/j.ppees.2017.01.001. [DOI] [Google Scholar]
- Cafaro P. Three ways to think about the sixth mass extinction. Biological Conservation. 2015;192:387–393. doi: 10.1016/j.biocon.2015.10.017. [DOI] [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, et al. Naeem S. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- Chalcraft DR. Changes in ecological stability across realistic biodiversity gradients depend on spatial scale. Global Ecology and Biogeography. 2013;22:19–28. doi: 10.1111/j.1466-8238.2012.00779.x. [DOI] [Google Scholar]
- Fahrig L. Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics. 2003;34:487–515. doi: 10.1146/annurev.ecolsys.34.011802.132419. [DOI] [Google Scholar]
- France KE, Duffy JE. Diversity and dispersal interactively affect predictability of ecosystem function. Nature. 2006;441:1139–1143. doi: 10.1038/nature04729. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Cardinale BJ, Allington GRH, Byrnes J, Arthur Endsley K, Brown DG, et al. Loreau M. Estimating local biodiversity change: A critique of papers claiming no net loss of local diversity. Ecology. 2016;97:1949–1960. doi: 10.1890/15-1759.1. [DOI] [PubMed] [Google Scholar]
- Gossner MM, Lewinsohn TM, Kahl T, Grassein F, Boch S, Prati D, et al. Allan E. Land-use intensification causes multitrophic homogenization of grassland communities. Nature. 2016;540:266–269. doi: 10.1038/nature20575. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Seabloom EW, Borer ET, Adler PB, Harpole WS, Hillebrand H, et al. Hector A. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science. 2015;348:336–340. doi: 10.1126/science.aaa1788. [DOI] [PubMed] [Google Scholar]
- Hector A, Hautier Y, Saner P, Wacker L, Bagchi R, Joshi J, et al. Loreau M. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology. 2010;91:2213–2220. doi: 10.1890/09-1162.1. [DOI] [PubMed] [Google Scholar]
- Holland GJ, Clarke MF, Bennett AF. Prescribed burning consumes key forest structural components: Implications for landscape heterogeneity. Ecological Applications. 2017;27:845–858. doi: 10.1002/eap.1488. [DOI] [PubMed] [Google Scholar]
- Isbell F, Calcagno V, Hector A, Connolly J, Harpole WS, Reich PB, et al. Loreau M. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477:199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- Isbell F, Craven D, Connolly J, Loreau M, Schmid B, Beierkuhnlein C, et al. Eisenhauer N. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature. 2015;526:574–577. doi: 10.1038/nature15374. [DOI] [PubMed] [Google Scholar]
- Isbell FI, Polley HW, Wilsey BJ. Biodiversity, productivity and the temporal stability of productivity: Patterns and processes. Ecology Letters. 2009;12:443–451. doi: 10.1111/j.1461-0248.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- Jiang L, Pu ZC. Different effects of species diversity on temporal stability in single-trophic and multitrophic communities. The American Naturalist. 2009;174:651–659. doi: 10.1086/605961. [DOI] [PubMed] [Google Scholar]
- Law R, Dieckmann U. A dynamical system for neighborhoods inplant communities. Ecology. 2000;81:2137–2148. [Google Scholar]
- Lehman CL, Tilman D. Biodiversity, stability, and productivity in competitive communities. The American Naturalist. 2000;156:534–552. doi: 10.1086/303402. [DOI] [PubMed] [Google Scholar]
- Loreau M. From population to ecosystem: Theoretical foundations for a new ecological synthesis. Princeton, NJ and Oxford: Princeton University Press; 2010. [Google Scholar]
- Loreau M, de Mazancourt C. Species synchrony and its drivers: Neutral and nonneutral community dynamics in fluctuating environments. The American Naturalist. 2008;172:E48–E66. doi: 10.1086/589746. [DOI] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecology Letters. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- Loreau M, Mouquet N, Gonzalez A. Biodiversity as spatial insurance in heterogeneous landscapes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12765–12770. doi: 10.1073/pnas.2235465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KG, Schwartz MW. Rare species loss alters ecosystem function – Invasion resistance. Ecology Letters. 2001;4:358–365. doi: 10.1046/j.1461-0248.2001.00235.x. [DOI] [Google Scholar]
- MacArthur RH, Wilson EO. The theory of Island biogeography. Princeton, NJ: Princeton University Press; 1967. [Google Scholar]
- McCallum HI. Lose biodiversity, gain disease. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8523–8524. doi: 10.1073/pnas.1510607112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan DA, Hovick TJ, Dwayne Elmore R, Engle DM, Fuhlendorf SD, Winter SL, et al. Debinski DM. Temporal variability in aboveground plant biomass decreases as spatial variability increases. Ecology. 2016;97:555–560. [PubMed] [Google Scholar]
- Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336:1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- Plotnick RE, Smith FA, Lyons SK. The fossil record of the sixth extinction. Ecology Letters. 2016;19:546–553. doi: 10.1111/ele.12589. [DOI] [PubMed] [Google Scholar]
- Sodhi NS, Brook BW, Bradshaw CJ. Causes and consequences of species extinctions. In: Levin SA, editor. The Princeton guide to ecology. Princeton, NJ: Princeton University Press; 2009. pp. 514–520. [Google Scholar]
- Soliveres S, Manning P, Prati D, Gossner MM, Alt F, Arndt H, et al. Allan E. Locally rare species influence grassland ecosystem multifunctionality. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2016;371 doi: 10.1098/rstb.2015.0269. 20150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch D. The theory of the nested species–area relationship: Geometric foundations of biodiversity scaling. Journal of Vegetation Science. 2016;27:880–891. doi: 10.1111/jvs.12428. [DOI] [Google Scholar]
- Tilman D, Isbell F, Cowles JM. Biodiversity and ecosystem functioning. Annual Review of Ecology, Evolution, and Systematics. 2014;45:471–493. doi: 10.1146/annurev-ecolsys-120213-091917. [DOI] [Google Scholar]
- Tilman D, Reich PB, Knops JMH. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
- Tredennick AT, de Mazancourt C, Loreau M, Adler PB. Environmental responses, not species interactions, determine synchrony of dominant species in semiarid grasslands. Ecology. 2017;98:971–981. doi: 10.1002/ecy.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto H. A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33:2–22. doi: 10.1111/j.1600-0587.2009.05880.x. [DOI] [Google Scholar]
- Turnbull LA, Isbell F, Purves DW, Loreau M, Hector A. Understanding the value of plant diversity for ecosystem functioning through niche theory. Proceedings of the Royal Society B: Biological Sciences. 2016;283 doi: 10.1098/rspb.2016.0536. 20160536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WR, Tjørve E. Scale-dependence in species-area relationships. Ecography. 2005;28:721–730. doi: 10.1111/j.2005.0906-7590.04273.x. [DOI] [Google Scholar]
- Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- Wang SP, Loreau M. Ecosystem stability in space: α, β and γ variability. Ecology Letters. 2014;17:891–901. doi: 10.1111/ele.12292. [DOI] [PubMed] [Google Scholar]
- Wang SP, Loreau M. Biodiversity and ecosystem stability across scales in metacommunities. Ecology Letters. 2016;19:510–518. doi: 10.1111/ele.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Loreau M, Arnoldi J-F, Fang J, Rahman KA, Tao S, de Mazancourt C. An invariability-area relationship sheds new light on the spatial scaling of ecological stability. Nature Communications. 2017;8:15211. doi: 10.1038/ncomms15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JA. Spatial scaling in ecology. Functional Ecology. 1989;3:385–397. doi: 10.2307/2389612. [DOI] [Google Scholar]
- Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, He NP, Loreau M, Pan QM, Han XG. Data from: Scale dependence of the diversity–stability relationship in a temperate grassland. Dryad Digital Repository. 2017a doi: 10.5061/dryad.58fv8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Loreau M, He NP, Zhang G, Han XG. Mowing exacerbates the loss of ecosystem stability under nitrogen enrichment in a temperate grassland. Functional Ecology. 2017b;31:1637–1646. doi: 10.1111/1365-2435.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JQ, van der Werf W, Anten NPR, Vos J, Evers JB. The contribution of phenotypic plasticity to complementary light capture in plant mixtures. New Phytologist. 2015;207:1213–1222. doi: 10.1111/nph.13416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.