Abstract
Objective
To examine the association between risk factor burdens—categorized as optimal, borderline, or elevated—and the lifetime risk of atrial fibrillation.
Design
Community based cohort study.
Setting
Longitudinal data from the Framingham Heart Study.
Participants
Individuals free of atrial fibrillation at index ages 55, 65, and 75 years were assessed. Smoking, alcohol consumption, body mass index, blood pressure, diabetes, and history of heart failure or myocardial infarction were assessed as being optimal (that is, all risk factors were optimal), borderline (presence of borderline risk factors and absence of any elevated risk factor), or elevated (presence of at least one elevated risk factor) at index age.
Main outcome measure
Lifetime risk of atrial fibrillation at index age up to 95 years, accounting for the competing risk of death.
Results
At index age 55 years, the study sample comprised 5338 participants (2531 (47.4%) men). In this group, 247 (4.6%) had an optimal risk profile, 1415 (26.5%) had a borderline risk profile, and 3676 (68.9%) an elevated risk profile. The prevalence of elevated risk factors increased gradually when the index ages rose. For index age of 55 years, the lifetime risk of atrial fibrillation was 37.0% (95% confidence interval 34.3% to 39.6%). The lifetime risk of atrial fibrillation was 23.4% (12.8% to 34.5%) with an optimal risk profile, 33.4% (27.9% to 38.9%) with a borderline risk profile, and 38.4% (35.5% to 41.4%) with an elevated risk profile. Overall, participants with at least one elevated risk factor were associated with at least 37.8% lifetime risk of atrial fibrillation. The gradient in lifetime risk across risk factor burden was similar at index ages 65 and 75 years.
Conclusions
Regardless of index ages at 55, 65, or 75 years, an optimal risk factor profile was associated with a lifetime risk of atrial fibrillation of about one in five; this risk rose to more than one in three in individuals with at least one elevated risk factor.
Introduction
Advancing age is a prominent risk factor of atrial fibrillation.1 Ongoing improvements of health and medicine, such as public health efforts, medical treatments, and surgical interventions, have delayed senescence and postponed mortality.2 This extended longevity has increased the risk of age related diseases such as atrial fibrillation.2 Worldwide, a rapid upward trajectory of prevalence and incidence of atrial fibrillation is occurring,3 and in the United States, the number of individuals with atrial fibrillation has been projected to rise to about 12 to 15 million by 2050.4 5 Preventive strategies for atrial fibrillation are therefore highly important, owing to estimated death rates of 20% and 50% at one and five years after initial diagnosis in older adults, respectively.6
In addition to advancing age, several short term risk factors for incident atrial fibrillation have been identified. Established risk factors for developing atrial fibrillation within 10 years include cigarette smoking, alcohol misuse, hypertension, obesity, diabetes, myocardial infarction, and heart failure.1 7 8 9 Modifiable risk factors could be associated with increased risk of atrial fibrillation even if they were previously considered borderline—for example, systolic blood pressure of 130-139 mm Hg and overweight (body mass index 25-30).10 The elevation of one risk factor rarely occurs in isolation, because many people often have a mixture of borderline or elevated levels of risk factors.7 9 10 11 The prevalence of multimorbidity (defined as two or more chronic morbidities) is about 23%, and the proportion of individuals with multimorbidities increases with advancing age.11 The accumulation of risk factors imposes an additive risk for developing atrial fibrillation.8 9
Predictive risk scores have examined the risk of atrial fibrillation within timeframes of five or 10 years.8 9 Lifetime risk has proved to be a useful quantification of the absolute risk over a person’s lifetime, and is an essential addition to the use of short term relative risks in clinical practice. The lifetime risk of atrial fibrillation has been reported to range from 17% to 26% in men and from 21% to 23% in women aged 40 years or older.12 13 14 15 However, data are scarce with respect to the lifetime risk of atrial fibrillation in the presence of one or multiple risk factors. Moreover, knowledge is limited about the lifetime risk of atrial fibrillation with borderline-elevated risk factors; predictive risk scores primarily focus on elevated risk factors without accounting for borderline-elevated risk factors.8 9
By contrast with the relative risk of atrial fibrillation, lifetime risk is an easy way for clinicians to communicate future risk of atrial fibrillation to individuals. Estimating the lifetime risk of atrial fibrillation in various subgroups with one or multiple elevated or borderline-elevated risk factors might also help to design preventive strategies.11 16 In this community based cohort study, we hypothesized that the risk factor burden—categorized as optimal, borderline, or elevated—is associated with an increased lifetime risk of atrial fibrillation.
Methods
We included participants in the Framingham Heart Study who did not have atrial fibrillation at one or more index ages of 55, 65, and 75 years. The lifetime risk of atrial fibrillation was estimated from the index age to 95 years of age, with adjustment for the competing risk of death without atrial fibrillation. We considered smoking, alcohol consumption, body mass index, blood pressure, diabetes, and history of myocardial infarction or heart failure at an index age. We then defined the risk factor burdens as optimal (that is, all risk factors were optimal), elevated (at least one risk factor elevated), and borderline (otherwise), and compared the lifetime risk estimates according to those levels of risk factor burden.
Data source
We used longitudinal data from the Framingham Heart Study. In 1948, participants were enrolled in the original cohort (n=5209), and they were examined every two years with standardized Framingham Heart Study examinations comprising a collection of data (medical history, physical examination, and laboratory tests). In 1971, children of the original cohort and their spouses were enrolled in the offspring cohort (n=5124), and were examined every four to eight years.17 In 2002, adult children from the offspring cohort were enrolled in the third generation cohort (n=4095), and were examined every six to eight years.18
Study samples
Participants from the original cohort’s examination cycle 11 to 28 (from year 1968 to 2005), the offspring cohort’s examination cycle one to nine (from 1971 to 2014), and the third generation’s examination cycle one or two (from 2002 to 2014) could enter one or more of three age study samples, provided that their risk factors were available. The three study samples were at index age 55 years, index age 65 years, and index age 75 years.
Smoking, alcohol consumption, body mass index, blood pressure, and diabetes were recorded from Framingham Heart Study examinations, and history of cardiovascular disease was recorded from hospital records. A panel of three medical doctors (the Framingham Endpoint Review Committee) validated the Framingham Heart Study sequence of cardiovascular events, and the review was based on medical records and study participant history. Cardiovascular disease was considered to have developed if there was a definite manifestation of coronary heart disease (angina pectoris, coronary insufficiency, myocardial infarction), intermittent claudication, congestive heart failure, or stroke or transient ischemic attack in the absence of a previous manifestation of any of these diseases.
The Framingham Heart Study examination or hospital record closest to the age benchmark was used. At index age 55 years, we selected participants according to the following criteria:
Excluded participants who developed atrial fibrillation or died before age 55 years
Excluded participants who did not have a Framingham Heart Study examination, between ages 50 and 59 years (that is, <60 years)
Included individuals who had covariates measured between age 50 and 59 years (that is, <60 years)
Excluded participants with atrial fibrillation present before the recording of risk factors.
If risk factors were measured between age 50 and 55 years, the risk time started at 55 years. If risk factors were measured between age 55 and 59 years (that is, <60 years), the risk time started at the specific age when risk factors were measured. We used the same approaches to select participants for study samples with index ages 65 years (supplementary fig 1) and 75 years (supplementary fig 2).
Risk factor categories
Risk factors were selected on the basis of the CHARGE-AF risk score and literature.1 8 10 19 The risk factors comprised smoking, alcohol consumption, body mass index, blood pressure, diabetes mellitus (type 1 or 2), and history of myocardial infarction or heart failure. We defined optimal, borderline, and elevated categories for each risk factor (except for alcohol consumption and history of heart failure or myocardial infarction) according to a previous study,10 as described in table 1.
Table 1.
Definitions of risk factor categories
| Risk factor and category | Definition |
|---|---|
| Smoking | |
| Optimal | Never smoker |
| Borderline | Former smoker |
| Elevated | Current smoker |
| Alcohol | |
| Optimal | Men ≤14 units of alcohol a week; women ≤7 units of alcohol a week |
| Elevated | Men >14 units of alcohol a week; women >7 units of alcohol a week |
| Body mass index | |
| Optimal | <25 |
| Borderline | 25-29 |
| Elevated | ≥30 |
| Blood pressure* | |
| Optimal | Systolic blood pressure <120 mm Hg and diastolic blood pressure <80 mm Hg, and no treatment for hypertension |
| Borderline | Systolic blood pressure 120-139 mm Hg or diastolic blood pressure 80-89 mm Hg, and no treatment for hypertension |
| Elevated | Systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, and/or treatment for hypertension |
| Diabetes mellitus (type 1 or 2) | |
| Optimal | Fasting blood glucose <100 mg/dL (if fasting blood glucose was not available, non-fasting blood glucose <140 mg/dL was used) and no treatment for diabetes |
| Borderline | Fasting blood glucose 100-125 mg/dL (if fasting blood glucose was not available, non-fasting blood glucose 140-199 mg/dL was used) and no treatment for diabetes |
| Elevated | Fasting blood glucose ≥126 mg/dL (if fasting blood glucose was not available, non-fasting blood glucose ≥200 mg/dL was used) or treatment for diabetes |
| History of heart failure or myocardial infarction | |
| Optimal | No history of heart failure or myocardial infarction |
| Elevated | History of heart failure or myocardial infarction |
1 mg/dL=0.06 mmol/L.
Optimal, borderline, and elevated categories in table correspond to categories of normal, elevated and hypertension stage 1, and hypertension stage 2 from the American College of Cardiology/American Heart Association 2017 high blood pressure guidelines, respectively.
Presence of multiple borderline or elevated risk factors were evaluated according to three levels: all risk factors were optimal (optimal), presence of borderline risk factors and absence of any elevated risk factor (borderline), or presence of elevated risk factors (elevated).
Measurement of atrial fibrillation
Atrial fibrillation was diagnosed as either atrial flutter or fibrillation. We determined atrial fibrillation by electrocardiograms from Framingham Heart Study examinations, participants’ medical appointments, or hospital records. Framingham Heart Study personnel contacted hospitals and medical clinics to collect the participants’ medical records. Two cardiologists evaluated records for newly diagnosed atrial fibrillation.
Participants in the three age samples (at ages 55, 65, and 75 years) were followed from the attained age free of atrial fibrillation to the earliest of the following dates: first diagnosed atrial fibrillation, last Framingham Heart Study visit or medical contact in which the participant was known as free of atrial fibrillation, age 95 years, death, or end of follow-up (31 December 2014).
Statistical analysis
We calculated the lifetime risks for the first incident atrial fibrillation from index ages 55, 65, and 75 years up to age 95 years. For example, the remaining lifetime risk for atrial fibrillation for 55 year old participants was the cumulative incidence of atrial fibrillation over 40 years. The standard Kaplan-Meier estimates do not take into account the competing risk of death (meaning that participants who die without atrial fibrillation no longer are at risk of atrial fibrillation) and overestimate absolute risks. Therefore, we used a modified Kaplan-Meier estimator with age as the time scale, accounting for the competing risk of death to compute the lifetime cumulative risk of atrial fibrillation and associated 95% confidence intervals.20
We computed lifetime risk in subgroups of participants according to their risk profile at a specified index age (optimal, borderline, and elevated), for each risk factor separately and for the combination of risk factors. Lifetime risk estimates in the elevated and borderline risk groups were compared with the lifetime risk estimated in the optimal risk group by a z ratio test (that is, the difference in lifetime risk between two groups divided by its standard error). We also tested for an interaction between sex and the risk categories in their effect on the subdistribution hazard of atrial fibrillation. The analyses were broken down further according to the number of borderline or elevated risk factors. Moreover, we fitted a multivariable Fine and Gray model, adjusted for competing risk of death to predict the lifetime risk of atrial fibrillation (details shown in the supplementary methods). We checked the assumptions of the model.21 The proportional hazards assumption was violated for body mass index, sex, smoking, and history of myocardial infarction or heart failure. Interaction terms were added between each covariate and the natural logarithmic transformation of time. We present the predicted lifetime risk of atrial fibrillation for 16 risk profiles, in men and women separately.
In secondary analyses, we estimated unadjusted cumulative risks, in which participants who died free of atrial fibrillation were censored, for each risk factor separately.22 The unadjusted cumulative risks were reported to assist in the interpretation of the results for smoking. We performed two sensitivity analyses. Firstly, to account for the exclusion of participants with missing data on risk factors, we compared the estimated lifetime risk of atrial fibrillation based on complete cases and multiple imputation (details shown in the supplementary methods). Secondly, we re-estimated the lifetime risk after discarding participants with a body mass index less than 18.5 from the optimal risk category for body mass index. The two sided level of significance was set at less than 5%. Data management and statistical analyses were performed by SAS (version 9.4 for Windows, SAS Institute) and R.23
Patient involvement
No participants from the Framingham Heart Study were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No participants were asked to advise on interpretation or writing up of results. We report research findings from the Framingham Heart Study on the study website and selected research results are disseminated to participants as part of mailed newsletters annually.
Results
Study samples at index ages 55, 65, and 75 years
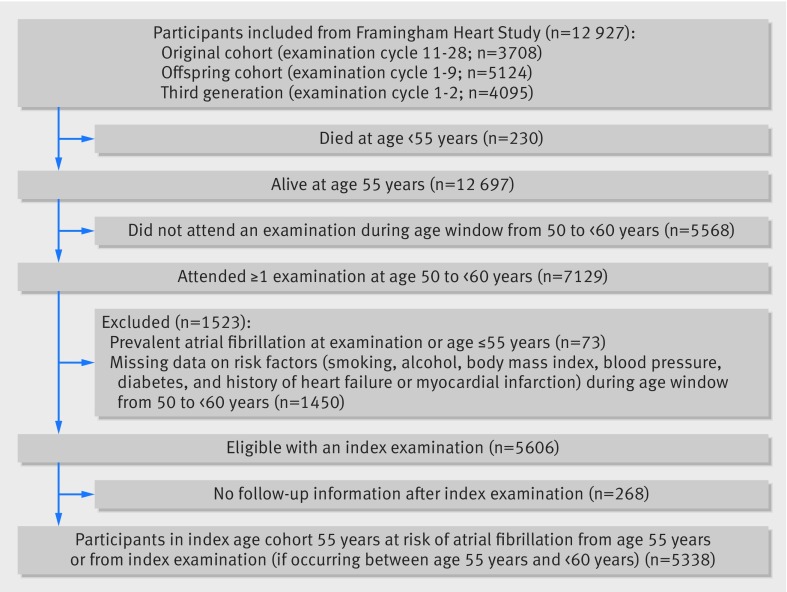
The selection of participants for the study samples with index ages 55, 65, and 75 years are shown in figure 1 and supplementary figures 1 and 2, respectively. Final numbers of participants in the three age study samples were as follows: index age 55 years had 5338 participants free of atrial fibrillation and 47% were men; index age 65 years had 4805 participants free of atrial fibrillation and 44% were men; and index age 75 years had 3199 participants free of atrial fibrillation and 40% were men. We report characteristics of the three study samples in table 2. The most common elevated risk factor among participants was elevated blood pressure (38% at age 55 years, 57% at 65 years, and 70% at 75 years). A gradual increase in the presence of elevated blood pressure, diabetes, and history of myocardial infarction or heart failure was observed as index age increased. As index age increased from 55 years to 75 years, the composition of individuals with smoking as an elevated risk factor changed from 21% to 9%. The characteristics of participants divided into optimal, borderline, and elevated smoking profiles are reported in supplementary table 1.
Fig 1.
Selection of study sample with index age 55 years
Table 2.
Characteristics of participants in three study samples. Data are number (%) of participants unless stated otherwise
| Characteristic and risk factor | Index age | ||
|---|---|---|---|
| 55 years (n=5338) | 65 years (n=4805) | 75 years (n=3199) | |
| Male | 2531 (47.4) | 2126 (44.3) | 1291 (40.4) |
| Age (years; mean (standard deviation)) | 55 (1) | 65 (1) | 75 (1) |
| Calendar year for Framingham Heart Study examination (median (interquartile range) | 1995 (1985-2005) | 1993 (1982-2005) | 1992 (1983-2001) |
| Length of follow-up (years; median (interquartile range)) | 14 (7-22) | 11 (6-18) | 8 (4-13) |
| Smoking | |||
| Optimal | 1935 (36.3) | 1570 (32.7) | 1233 (38.5) |
| Borderline | 2275 (42.6) | 2414 (50.2) | 1667 (52.1) |
| Elevated | 1128 (21.1) | 821 (17.1) | 299 (9.4) |
| Alcohol | |||
| Optimal | 4283 (80.2) | 3857 (80.3) | 2767 (86.5) |
| Elevated | 1055 (19.8) | 948 (19.7) | 432 (13.5) |
| Body mass index | |||
| Optimal | 1785 (33.4) | 1537 (32.0) | 1126 (35.2) |
| Borderline | 2136 (40.0) | 2008 (41.8) | 1364 (42.6) |
| Elevated | 1417 (26.6) | 1260 (26.2) | 709 (22.2) |
| Blood pressure | |||
| Optimal | 1539 (28.8) | 778 (16.2) | 272 (8.5) |
| Borderline | 1792 (33.6) | 1303 (27.1) | 676 (21.1) |
| Elevated | 2007 (37.6) | 2724 (56.7) | 2252 (70.4) |
| Diabetes | |||
| Optimal | 3805 (71.3) | 3137 (65.3) | 2249 (70.3) |
| Borderline | 1187 (22.2) | 1149 (23.9) | 584 (18.3) |
| Elevated | 346 (6.5) | 519 (10.8) | 366 (11.4) |
| History of heart failure or myocardial infarction | |||
| Optimal | 5069 (95.0) | 4236 (88.2) | 2623 (82.0) |
| Elevated | 269 (5.0) | 569 (11.8) | 576 (18.0) |
Single risk factor and lifetime risk of atrial fibrillation
The median follow-up time and number of participants with first developed atrial fibrillation were 14 years (interquartile range 7-22) and 816 for index age 55 years, respectively. Corresponding values were 11 (6-18) years and 1023 for index age 65 years, and eight (4-13) years and 776 for index age 75 years. The overall lifetime risk of atrial fibrillation was 37.0% (95% confidence interval 34.3% to 39.6%) for index age 55 years, 33.7% (31.9% to 35.5%) for index age 65 years, and 30.8% (28.9% to 32.7%) for index age 75 years. Table 3 reports the lifetime risk of atrial fibrillation associated with optimal, borderline, or elevated risk factors. The associated lifetime risk of atrial fibrillation was lowest if the risk factor profile was optimal. The lifetime risk of atrial fibrillation increased gradually as the risk factor profile changed from optimal to borderline and elevated at each index age. The opposite association was seen with a history of smoking; lifetime risk of atrial fibrillation was higher among participants with optimal (never) and borderline (former) smoking than among those with elevated smoking (current smoker at index examination).
Table 3.
Lifetime risk (%) of atrial fibrillation by individual risk factor, after adjustment for competing risk of death
| Risk factor and category | Index age 55 years | Index age 65 years | Index age 75 years | |||||
|---|---|---|---|---|---|---|---|---|
| No of atrial fibrillation cases/total | Lifetime risk (95% CI) | No of atrial fibrillation cases/total | Lifetime risk (95% CI) | No of atrial fibrillation cases/total | Lifetime risk (95% CI) | |||
| Smoking | ||||||||
| Optimal | 249/1935 | 38.1 (33.2 to 43.1) | 316/1570 | 33.7 (30.4 to 36.9) | 294/1233 | 30.2 (27.1 to 33.2) | ||
| Borderline | 343/2275 | 39.3 (34.9 to 43.6) | 525/2414 | 36.3 (33.6 to 39.1) | 410/1667 | 32.2 (29.4 to 35.0) | ||
| Elevated | 224/1128 | 32.1 (28.2 to 36.1) | 182/821 | 27.5 (24.0 to 31.0) | 72/299 | 26.0 (20.6 to 31.3) | ||
| Alcohol | ||||||||
| Optimal | 600/4283 | 35.1 (32.2 to 38.0) | 799/3857 | 33.2 (31.2 to 35.3) | 671/2767 | 30.8 (28.7 to 32.8) | ||
| Elevated | 216/1055 | 40.9 (36.1 to 45.7) | 224/948 | 35.6 (31.6 to 39.5) | 105/432 | 30.7 (25.4 to 36.0) | ||
| Body mass index | ||||||||
| Optimal | 236/1785 | 31.6 (27.5 to 35.7) | 314/1537 | 29.9 (27.0 to 32.8) | 255/1126 | 27.2 (24.2 to 30.2) | ||
| Borderline | 343/2136 | 38.2 (34.0 to 42.4) | 422/2008 | 33.3 (30.5 to 36.1) | 319/1364 | 30.1 (27.2 to 33.0) | ||
| Elevated | 237/1417 | 44.2 (38.5 to 50.0) | 287/1260 | 40.7 (36.7 to 44.8) | 202/709 | 38.7 (34.2 to 43.2) | ||
| Blood pressure | ||||||||
| Optimal | 152/1539 | 29.0 (24.2 to 33.9) | 118/778 | 27.5 (22.9 to 32.1) | 45/272 | 22.4 (16.4 to 28.5) | ||
| Borderline | 272/1792 | 36.5 (32.0 to 41.0) | 254/1303 | 30.2 (26.9 to 33.5) | 140/675 | 25.9 (22.1 to 29.8) | ||
| Elevated | 392/2007 | 41.1 (37.2 to 44.9) | 651/2724 | 37.1 (34.6 to 39.5) | 591/2252 | 33.1 (30.8 to 35.4) | ||
| Diabetes | ||||||||
| Optimal | 624/3805 | 36.5 (33.7 to 39.4) | 726/3137 | 32.8 (30.8 to 34.8) | 580/2249 | 30.1 (28.0 to 32.3) | ||
| Borderline | 128/1187 | 38.0 (24.6 to 51.5) | 171/1149 | 32.9 (27.0 to 38.7) | 110/584 | 31.7 (25.7 to 37.7) | ||
| Elevated | 64/346 | 34.8 (27.3 to 42.4) | 126/519 | 36.5 (30.7 to 42.2) | 86/366 | 32.3 (26.1 to 38.5) | ||
| History of heart failure or myocardial infarction | ||||||||
| Optimal | 747/5069 | 37.0 (34.3 to 39.8) | 864/4236 | 33.4 (31.4 to 35.3) | 607/2623 | 29.8 (27.7 to 31.9) | ||
| Elevated | 69/269 | 37.3 (29.1 to 45.4) | 159/569 | 35.9 (31.2 to 40.7) | 169/576 | 35.1 (30.4 to 39.8) | ||
For example, the lifetime risk of developing atrial fibrillation between age 55 and 95 years when blood pressure is optimal at age 55 years was 29.0%, after accounting for the competing risk of death.
The lifetime risks according to single risk factors stratified by sex are in supplementary tables 2-4 for index ages 55, 65, and 75 years, respectively. The lifetime risk of atrial fibrillation was lower in women than in men.
Multiple risk factors
Supplementary table 5 shows that among participants with index age 55 years, 247 (4.6%) had an optimal risk profile, 1415 (26.5%) had a borderline risk profile, and 3676 (68.9%) had an elevated risk profile. The proportion of participants with optimal risk factors changed from 4.6% to 2.1% and 1.0%, when index age increased from 55 to 65 and 75 years, respectively. Accordingly, the proportion with one or more borderline risk factors fell as age increased. The proportion of participants with at least three elevated risk factors increased gradually from 11.1% to 15.8% and 14.0% as index age rose from 55 to 65 and 75 years, respectively.
Multiple elevated or borderline risk factors and lifetime risk of atrial fibrillation
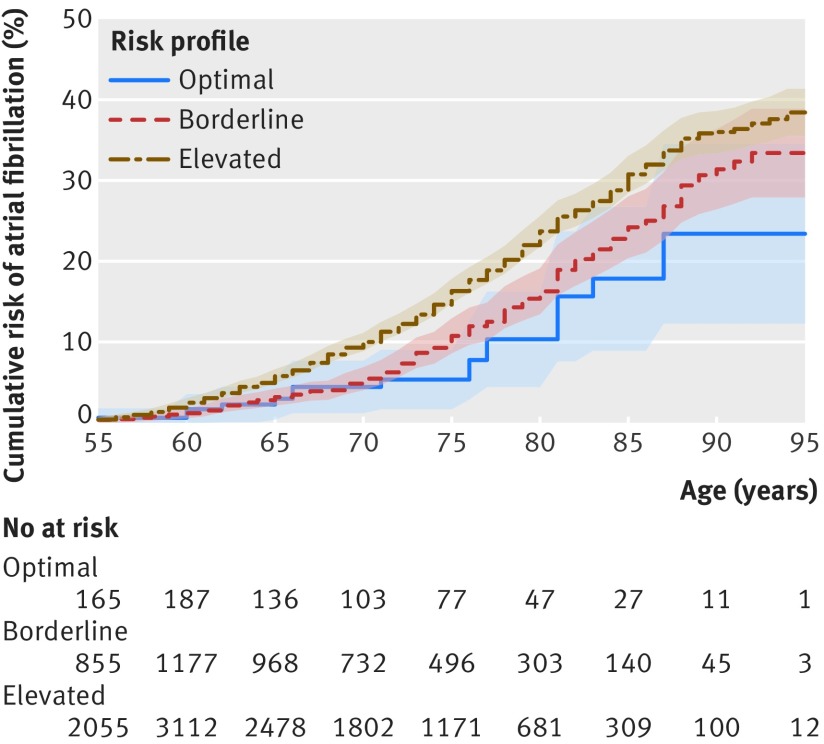
Figure 2 and supplementary figures 3 and 4 show the lifetime risk of atrial fibrillation for index ages 55, 65, and 75 years, respectively, with optimal, borderline, or elevated risk factor profiles. The lifetime risk of atrial fibrillation was lowest among those with optimal risk factors, whereas it was significantly higher in the group with elevated risk factors for index age 55 and 65 years (table 4). The lifetime risk of atrial fibrillation in each risk factor profile was higher among men than among women, with a quantitative interaction between sex and risk categories because the increase in lifetime risk from optimal to borderline risk profiles was larger in men than women.
Fig 2.
Cumulative risk (%) for atrial fibrillation according to risk factor burden (optimal, borderline, or elevated) at index age 55 years. Shading=95% confidence intervals. Participants entered the study sample between ages 55 and <60 years; therefore, the number at risk increased from age 55 years to <60 years
Table 4.
Lifetime risk (%) of atrial fibrillation in men and women according to risk factor burden, after adjustment for competing risk of death
| Study sample (index age) and risk category | All | Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of atrial fibrillation cases/total | Lifetime risk (95% CI) | P* | P† | No of atrial fibrillation cases/total | Lifetime risk (95% CI) | P* | P† | No of atrial fibrillation cases/total | Lifetime risk (95% CI) | P* | P† | |||
| 55 years | ||||||||||||||
| Optimal | 18/247 | 23.4 (12.8 to 34.5) | <0.001 | — | 7/55 | 29.8 (9.7 to 49.9) | 0.01 | — | 11/192 | 20.5 (7.3 to 33.7) | 0.006 | — | ||
| Borderline | 163/1415 | 33.4 (27.9 to 38.9) | 0.11 | 94/617 | 39.7 (31.7 to 47.7) | 0.37 | 69/798 | 28.0 (20.6 to 35.4) | 0.33 | |||||
| Elevated | 635/3676 | 38.4 (35.5 to 41.4) | 0.009 | 388/1859 | 43.3 (38.4 to 46.2) | 0.20 | 247/1817 | 34.6 (30.3 to 39.0) | 0.05 | |||||
| 65 years | ||||||||||||||
| Optimal | 9/100 | 18.1 (6.7 to 29.4) | <0.001 | — | 4/21 | 28.8 (3.0 to 54.6) | <0.001 | — | 5/79 | 14.9 (2.2 to 27.6) | <0.001 | — | ||
| Borderline | 136/921 | 26.1 (22.0 to 30.1) | 0.19 | 67/359 | 32.1 (25.3 to 38.9) | 0.81 | 69/562 | 22.1 (17.2 to 27.0) | 0.30 | |||||
| Elevated | 878/3784 | 35.8 (33.8 to 37.9) | 0.003 | 464/1746 | 39.7 (36.6 to 42.7) | 0.41 | 414/2038 | 32.7 (30.0 to 35.4) | 0.007 | |||||
| 75 years | ||||||||||||||
| Optimal | 4/33 | 15.4 (1.3 to 29.5) | <0.001 | — | 1/5 | 25.0 (0.0 to 67.4) | 0.09 | — | 3/28 | 13.8 (0.0 to 28.3) | 0.003 | — | ||
| Borderline | 87/492 | 23.6 (19.1 to 28.2) | 0.28 | 42/191 | 28.3 (20.6 to 36.1) | 0.85 | 45/301 | 20.6 (15.0 to 26.1) | 0.38 | |||||
| Elevated | 685/2674 | 32.2 (30.0 to 34.3) | 0.02 | 315/1095 | 36.4 (33.0 to 39.9) | 0.51 | 370/1579 | 29.3 (26.7 to 32.0) | 0.03 | |||||
Overall test comparing subhazard distributions across risk categories by Fine-Gray method. P<0.001 for interaction between sex and risk categories for index ages 55, 65, and 75 years.
Test comparing lifetime risk in borderline and elevated risk groups with optimal risk group by z ratio test (that is, difference in lifetime risk between two groups divided by its standard error).
Table 5 and supplementary tables 6 and 7 show the lifetime risk of atrial fibrillation among participants with multiple borderline or elevated risk factors, and the lifetime risk when separated into men only and women only, respectively. If all risk factors were optimal, the lifetime risk of atrial fibrillation ranged from 15.4% to 23.4% across the three study samples. By contrast, if at least one risk factor was elevated, the lifetime risk of atrial fibrillation was at least 37.8%.
Table 5.
Lifetime risk (%) of atrial fibrillation by risk factor profile and number of elevated/borderline risk factors, after adjustment for competing risk of death
| Risk factor profile and number of elevated/borderline risk factors | Index age | |||||||
|---|---|---|---|---|---|---|---|---|
| 55 years | 65 years | 75 years | ||||||
| No of atrial fibrillation cases/ total | Lifetime risk (95% CI) | No of atrial fibrillation cases/ total | Lifetime risk (95% CI) | No of atrial fibrillation cases/ total | Lifetime risk (95% CI) | |||
| Optimal | ||||||||
| 0/0 | 18/247 | 23.4 (12.8 to 34.5) | 9/100 | 18.1 (6.7 to 29.4) | 4/33 | 15.4 (1.3 to 29.5) | ||
| Borderline | ||||||||
| 0/1 | 58/520 | 31.9 (24.5 to 40.3) | 44/279 | 24.9 (18.2 to 31.7) | 18/138 | 18.0 (10.2 to 25.8) | ||
| 0/2 | 68/549 | 38.6 (28.8 to 48.5) | 65/377 | 31.2 (24.6 to 37.9) | 40/214 | 23.8 (17.2 to 30.4) | ||
| 0/≥3 | 37/346 | 27.3 (18.4 to 36.2) | 27/265 | 19.7 (12.4 to 26.9) | 29/140 | 28.9 (19.3 to 38.6) | ||
| Elevated | ||||||||
| 1/any | 266/1863 | 37.8 (33.1 to 42.4) | 373/1671 | 35.7 (32.6 to 38.8) | 296/1253 | 29.2 (26.3 to 32.2) | ||
| 2/any | 225/1220 | 38.8 (34.0 to 43.5) | 313/1355 | 36.0 (32.6 to 39.4) | 268/972 | 34.9 (31.3 to 38.5) | ||
| ≥3/any | 144/539 | 39.1 (33.6 to 44.6) | 192/758 | 35.9 (31.6 to 40.2) | 121/449 | 34.4 (29.0 to 39.8) | ||
Predicted lifetime risk of atrial fibrillation
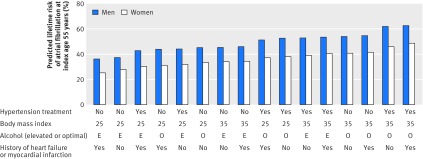
Supplementary table 8 presents the multivariable prediction model of the lifetime risk of atrial fibrillation. The predicted lifetime risk of atrial fibrillation is shown in figure 3 for 16 different risk profiles in men and women, separately. We found a higher predicted lifetime risk of atrial fibrillation in men than in women. Moreover, the presence of hypertension treatment, body mass index of 35, elevated alcohol consumption, and history of myocardial infarction or heart failure at index age 55 years was associated with the greatest predicted lifetime risk of atrial fibrillation in both men and women. Supplementary figure 5 shows the assessment of the calibration and discrimination of the prediction model.
Fig 3.
Predicted lifetime risk (%) of atrial fibrillation at index age 55 years across 16 risk profiles, in men and women. Profiles were defined according to treatment for hypertension (yes or no), body mass index (25 or 35), alcohol (elevated (E) or optimal (O)), and history of myocardial infarction or heart failure (yes or no). For each profile, participants with an average blood pressure level (mean systolic blood pressure 128 mm Hg and 124 mm Hg and mean diastolic blood pressure 80 mm Hg and 76 mm Hg for men and women, respectively), who never smoked, and who had no diabetes were considered
Secondary analysis
Supplementary table 9 reports the lifetime risk of atrial fibrillation based on a model unadjusted for competing risk of death. For all five risk factors, the lifetime risk of atrial fibrillation increased gradually from optimal to borderline and to elevated, including for smoking and diabetes. Similar patterns were seen in all three study samples at index ages 55, 65, and 75 years.
Sensitivity analysis
Firstly, we imputed missing data and estimated the lifetime risks of atrial fibrillation by multiple risk factors (supplementary table 10). Based on the imputed data, we found similar lifetime risks to our main results (table 4). Secondly, we removed participants with a body mass index of less than 18.5 (n=34, 44, and 44 for index age 55, 65, and 75 years, respectively) from the optimal body mass index category (supplementary table 11). The lifetime risks of atrial fibrillation remained consistent with our results in table 3.
Discussion
Principal findings
We used data from three generations of participants in the Framingham Heart Study to estimate the lifetime risk of atrial fibrillation in various subgroups according to the burden of modifiable risk factors. Firstly, among participants aged 55 years or older, the overall lifetime risk of atrial fibrillation was 37.0%. Secondly, the lifetime risk of atrial fibrillation was higher in men than in women. Thirdly, among participants aged 55 years or older and with an optimal risk factor profile, the lifetime risk of atrial fibrillation was about one in five. Finally, having at least one elevated risk factor was associated with a lifetime risk of atrial fibrillation of at least one in three. Our study shows that the risk factor burden and having multiple morbidities have a crucial role in the lifetime risk of atrial fibrillation.
Comparison with other studies
In 2004, Lloyd-Jones and colleagues reported that men and women aged 40 years or older had a lifetime risk of atrial fibrillation of one in four.12 The study was based on the Framingham Heart Study, with the last follow-up in 1999. Our study included more participants in the third generation cohort and had an additional 15 years of follow-up. In our study, the overall lifetime risk of atrial fibrillation ranged from 31% to 37%. The higher lifetime risk found in our study could be explained by several temporal changes, such as advancing age of the population and increased surveillance for atrial fibrillation.
Our study is also an extension to the 2004 study,12 because our current work elaborates on the lifetime risk of atrial fibrillation with multiple risk factors. With reference to an optimal risk factor profile, we found that only an elevated risk factor profile significantly increased the lifetime risk of atrial fibrillation, whereas a borderline risk factor profile was not associated with a significantly increased lifetime risk. Moreover, we found that individuals with all optimal risk factors had a lifetime risk of atrial fibrillation of about one in five, whereas individuals with multimorbidity (defined as at least two elevated risk factors) had a lifetime risk greater than one in three.
Despite the remarkable increase in lifetime risk among individuals with elevated versus optimal risk factor burdens, those with an optimal risk factor profile still had about a 20% risk of developing atrial fibrillation in our study. A meta-analysis of 18 cohorts showed that among individuals aged 55 years and with an optimal risk factor profile, the lifetime risk of atherosclerotic cardiovascular disease was 14.6%.24 Our data suggest that the lifetime risk of atrial fibrillation is higher than the lifetime risk of atherosclerotic cardiovascular disease if a person has an optimal risk factor profile at age 55 years. However, future studies comparing lifetime risk of different diseases and in different racial and ethnic groups are warranted.
We found that both the lifetime risk and predicted lifetime risk of atrial fibrillation were higher in men than in women, which accords with the results from a European cohort study.25 In the European study, the authors further explored the association between sex differences in risk of atrial fibrillation and presence of risk factors. They found that women had a more favorable risk profile of atrial fibrillation than men—that is, women smoked less, consumed less alcohol, had a lower body mass index and blood pressure, and had lower levels of diabetes.25
The Rotterdam Study, a population based prospective study in the Netherlands with 6808 participants, also investigated the lifetime risk of atrial fibrillation; the results were similar to the 2004 study by Lloyd-Jones and colleagues.12 13 Although comparable results were found in a Swedish study,15 researchers in a Chinese study estimated the lifetime risk of atrial fibrillation to be one in five at age 55 years.14 Other studies have reported that the incidence of atrial fibrillation is lower among Asians than European ancestry populations,26 and the Multi-Ethnic Study of Atherosclerosis from the USA reported an incidence of 3.9 in Asians versus 11.2 in white people per 1000 person years.27 These four earlier studies did not take into account the influence of the risk factor burden on lifetime risk; but in our study, we report that the risk factor burden, comprising modifiable risk factors, has a crucial role on the lifetime risk of atrial fibrillation. In another study, our group has shown that the lifetime risk of atrial fibrillation also depends on genetic predisposition.28
Based on 14 598 participants from the Atherosclerosis Risk in Communities (ARIC) Study, Huxley and colleagues examined the importance of individuals being exposed to optimal, borderline, or elevated risk factors and the associated risk of incident atrial fibrillation over a mean period of 17.1 years.10 The risk factor categories were similar to our present study. Huxley and colleagues found that a profile of optimal, borderline, or elevated risk factors was associated with an incidence of atrial fibrillation of 2.19, 3.68, and 6.59 per 1000 person years, respectively. The results from our study also showed a gradual increase in risk of atrial fibrillation when having an optimal, borderline, or elevated risk factor profile.
Among the modifiable risk factors included in our study, we found that obesity was the most prominent risk factor influencing the lifetime risk of atrial fibrillation. Another study from the Framingham Heart Study reported the temporal changes in risk factors of atrial fibrillation over 50 years and found that obesity has been a significant risk factor of atrial fibrillation in the past five decades.1 The same study showed that body mass index was associated with an increasing trend in the population attributable fraction of incident atrial fibrillation. In the period from 1998 to 2007, the population attributable fraction was −2.1 for systolic blood pressure, 19.5 for hypertension treatment, 16.9 for body mass index, no substantial contribution for smoking, 5.9 for diabetes, 1.4 for heart failure, and 3.6 for myocardial infarction.1 The study by Huxley and colleagues confirmed that obesity and elevated blood pressure had the highest population attributable fractions of developing atrial fibrillation (12.7 and 21.6, respectively).10 In our study, diabetes was not associated with the lifetime risk of atrial fibrillation, and history of myocardial infarction or heart failure had a decreasing role on the lifetime risk of atrial fibrillation with greater index age.
For smoking as a risk factor, we observed a pattern in which the lifetime risk of atrial fibrillation increased from optimal to borderline risk burdens, but attenuated from borderline to elevated risk burdens. This attenuation in lifetime risk could have several explanations. Firstly, in the models that were unadjusted for competing risk of death, the lifetime risk of atrial fibrillation was highest among participants with an elevated smoking profile, suggesting that they had a higher risk of death than those with an optimal or borderline smoking profile. A plausible explanation is that current smokers were more likely to die before they developed atrial fibrillation. Atrial fibrillation is also an age related disease, with events occurring at advanced ages, but most smokers die before they reach such advanced ages.1 29 Similarly, Mamun and colleagues concluded in an observational study that current smokers had a shorter life expectancy than never smokers; therefore, current smokers had a diminished or the same lifetime risk of cardiovascular disease as never smokers.29
Secondly, based on the models adjusted for competing risk of death, the results for smoking could have been influenced by survivorship bias. One hypothesis is that participants who were current smokers at index age 75 years and survived the following 10 years without developing atrial fibrillation could have had a higher chance of survival and being free of atrial fibrillation than with those who were never smokers or former smokers at index age 75 years, because of a low predisposition to cardiovascular disease.30 31 Lastly, among older people, smokers are more likely to quit smoking than never smokers are to initiate smoking; smoking cessation during follow-up could have neutralized the anticipated high lifetime risk of atrial fibrillation among those with an elevated smoking profile at index age. The awareness of smoking related diseases has increased, supportive actions in smoking cessation have progressed, and changes in social policy might all have had a temporal effect and enhanced smoking cessation during follow-up in our study.32
In the USA, median inpatient expenses related to atrial fibrillation per Medicare beneficiary have increased substantially, from US$2932 (£2068; €2373) in 1999 to $4719 in 2013.33 Furthermore, many patients with atrial fibrillation may undergo catheter ablation procedures, rates of hospital admissions related to atrial fibrillation have increased by 1% per year, stroke risk in patients with atrial fibrillation have increased by a factor of five, and patients with atrial fibrillation are at a higher risk of developing other cardiovascular diseases.19 33 34 The prevention of atrial fibrillation is essential both from the societal health and economic perspective, as well as for individual patients burdened with atrial fibrillation. Our study indicates that in practice, primary prevention of atrial fibrillation could begin with the management of modifiable borderline and elevated risk factors, together with reductions in multimorbidity. Early intervention and control of modifiable risk factors could reduce the number of individuals developing atrial fibrillation. Therefore, there is the potential for the burden of atrial fibrillation to be reduced in addition to the costs associated with atrial fibrillation.
Strengths and limitations of study
The first major limitation in our study was related to the risk factor exposures. The risk factors examined in our study were the most established risk factors for atrial fibrillation. However, our study did not include other potential risk factors for atrial fibrillation, such as family history of atrial fibrillation due to risk of misclassification bias or heart murmur, social deprivation, ethnicity, or genetic risk profiles owing to missing data.1 8 28 Our study was also not performed with time dependent updates of the risk factor profiles during follow-up.35 In the borderline and elevated risk categories for smoking, we did not take into account the number of pack years and the time since a participant had discontinued smoking in the borderline group. Moreover, the definition of optimal, borderline, and elevated blood pressure varies between different countries and has changed with recent guidelines, which could affect the generalizability.36 Furthermore, some participants had multiple overlapping risk factors, but in the current analyses we were not able to take into account the diverse weights of multiple risk factors on lifetime risk of atrial fibrillation. We also used univariate lifetime risk models, and had limited statistical power in some of our analyses, in particular at advanced index age in the participant group with optimal risk factor profiles. The limited statistical power might have weakened the clinical implications of our results.
The second major limitation was linked to outcome. We were not able to distinguish between atrial fibrillation subtypes in our study. Moreover, some individuals could have had undiagnosed atrial fibrillation, resulting in an underestimated lifetime risk of atrial fibrillation. In the Framingham Heart Study, we cannot rule out surveillance bias. Finally, our observational study design limits the ability to establish causal pathways, and only associations between risk factor profiles and lifetime risk of atrial fibrillation can be concluded from our study. The Framingham Heart Study is comprised mostly of individuals of European ancestry, which limits the generalizability to populations from other races or ethnicities. Our model to predict the lifetime risk of atrial fibrillation was based on only one cohort, with moderate discriminative ability, and the prediction model will need validation in other cohorts.
What is already known on this topic
The lifetime risk of atrial fibrillation has been estimated to be about one in four at age 40 years or above
Short term clinical risk factors for atrial fibrillation are well established, but how the risk factor burden affects the lifetime risk of atrial fibrillation is not known
What this study adds
The overall lifetime risk of atrial fibrillation was 37.0% among individuals aged 55 years or older; lifetime risk was higher among men than women
Individuals with an optimal risk factor profile at 55 years had a lifetime risk of atrial fibrillation of about one in five, whereas those with elevated risk factors had a lifetime risk of at least one in three
Preventive efforts to reduce the disease burden should target modifiable borderline and elevated risk factors plus consider multimorbidity
Acknowledgments
We thank the participants and staff of the Framingham Heart Study for their valuable contributions.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: LS, EJB, and LT developed the hypothesis and study design. LS, BW, SRP, and LT did the statistical analysis. LS wrote the first and successive drafts of the manuscript. All authors contributed to study concept and design, analysis and interpretation of data, and drafting or critical revision of the manuscript for important intellectual content or additionally to data acquisition. PTE, KLL, and EJB obtained funding for the study. BW, SRP, and LT had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. EJB is the guarantor. EJB and LT are joint contributing last authors.
Funding: This work was supported by the Boston University School of Medicine, and the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract NIH/NHLBI N01-HC25195l; HHSN268201500001I; 2R01HL092577; 1R01HL128914; 1P50HL120163). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of this manuscript. Additional support for this project from the Velux Foundation (LS); National Institutes of Health (NIH) grants K23HL114724 and Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105 (SAL); NIH grant K24HL105780, Established Investigator Award from the American Heart Association (13EIA14220013) and the Foundation Leducq (14CVD01) (PTE); NIH grants R01HL126911, R01HL135219, R01HL136660, UH3 TR000921-04, and National Science Foundation grant NSF-12-512 (DDM); postdoctoral fellowship from the American Heart Association (2017D000729) (LCW); grants 2R01HL092577, 1R01HL128914, and 1P50HL120163 (EJB).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: main support from the Boston University School of Medicine, and the National Heart, Lung, and Blood Institute’s Framingham Heart Study for the submitted work; SAL reports personal fees from St Jude Medical/Abbott and Quest Diagnostics, and grants from Boehringer Ingelheim, Biotronik, and Bayer HealthCare outside the submitted work; PTE reports grants from National Institutes of Health (NIH; K24HL105780), Established Investigator Award from the American Heart Association (13EIA14220013), Foundation Leducq (14CVD01), and Bayer HealthCare outside the submitted work; DDM receives sponsored research support from Bristol Myers Squibb, Pfizer, Biotronik, and Philips Healthcare, has consulted for Bristol Myers Squibb, FlexCon, Samsung, Philips, and Pfizer, has equity in Mobile Sense Technologies; and received grants from the NIH (R01HL126911, R01HL135219, R01HL136660, UH3 TR000921-04) and National Science Foundation (NSF-12-512) outside the submitted work; LF reports personal fees from Pfizer outside the submitted work; EJB reports funding from 1R01HL128914, 2R01 HL092577, 1P50HL120163, and the Robert Wood Johnson Foundation.
Ethical approval: Boston University Medical Center’s institutional review board approved all study protocols. Participants signed informed consent.
Data sharing: Participant level data are available at the database of Genotypes and Phenotypes (https://www.ncbi.nlm.nih.gov/gap/) and BioLINCC (https://biolincc.nhlbi.nih.gov/home/).
The lead authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154-62. 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaupel JW. Biodemography of human ageing. Nature 2010;464:536-42. 10.1038/nature08984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest 2012;142:1489-98. 10.1378/chest.11-2888 [DOI] [PubMed] [Google Scholar]
- 4. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25. 10.1161/CIRCULATIONAHA.105.595140 [DOI] [PubMed] [Google Scholar]
- 5. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142-7. 10.1016/j.amjcard.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 6. Piccini JP, Hammill BG, Sinner MF, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J 2014;35:250-6. 10.1093/eurheartj/eht483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamberlain AM, Alonso A, Gersh BJ, et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. Am Heart J 2017;185:74-84. 10.1016/j.ahj.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2:e000102. 10.1161/JAHA.112.000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet 2009;373:739-45. 10.1016/S0140-6736(09)60443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501-8. 10.1161/CIRCULATIONAHA.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 12. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042-6. 10.1161/01.CIR.0000140263.20897.42 [DOI] [PubMed] [Google Scholar]
- 13. Heeringa J, van der Kuip DAM, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949-53. 10.1093/eurheartj/ehi825 [DOI] [PubMed] [Google Scholar]
- 14. Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GYH. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest 2015;147:109-19. 10.1378/chest.14-0321 [DOI] [PubMed] [Google Scholar]
- 15. Mandalenakis Z, Von Koch L, Eriksson H, et al. The risk of atrial fibrillation in the general male population: a lifetime follow-up of 50-year-old men. Europace 2015;17:1018-22. 10.1093/europace/euv036 [DOI] [PubMed] [Google Scholar]
- 16. Godino JG, van Sluijs EMF, Marteau TM, Sutton S, Sharp SJ, Griffin SJ. Lifestyle advice combined with personalized estimates of genetic or phenotypic risk of type 2 diabetes, and objectively measured physical activity: a randomized controlled trial. PLoS Med 2016;13:e1002185. 10.1371/journal.pmed.1002185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975;4:518-25. 10.1016/0091-7435(75)90037-7 [DOI] [PubMed] [Google Scholar]
- 18. Splansky GL, Corey D, Yang Q, et al. The third generation cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328-35. 10.1093/aje/kwm021 [DOI] [PubMed] [Google Scholar]
- 19. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501-17. 10.1161/CIRCRESAHA.117.309732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med 2000;19:1495-522. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Scheike TH, Zhang M-J. Checking Fine and Gray subdistribution hazards model with cumulative sums of residuals. Lifetime Data Anal 2015;21:197-217. 10.1007/s10985-014-9313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet 2002;359:1686-9. 10.1016/S0140-6736(02)08594-X [DOI] [PubMed] [Google Scholar]
- 23.R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.https://www.R-project.org/
- 24. Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321-9. 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magnussen C, Niiranen TJ, Ojeda FM, et al. BiomarCaRE Consortium Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (biomarker for cardiovascular risk assessment in Europe). Circulation 2017;136:1588-97. 10.1161/CIRCULATIONAHA.117.028981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 2013;128:2470-7. 10.1161/CIRCULATIONAHA.113.002449 [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez CJ, Soliman EZ, Alonso A, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol 2015;25:71-6, 76.e1. 10.1016/j.annepidem.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weng L-C, Preis SR, Hulme OL, et al. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation 2018;137:1027-38. 10.1161/CIRCULATIONAHA.117.031431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mamun AA, Peeters A, Barendregt J, Willekens F, Nusselder W, Bonneux L, NEDCOM, The Netherlands Epidermiology and Demography Compression of Morbidity Research Group Smoking decreases the duration of life lived with and without cardiovascular disease: a life course analysis of the Framingham Heart Study. Eur Heart J 2004;25:409-15. 10.1016/j.ehj.2003.12.015 [DOI] [PubMed] [Google Scholar]
- 30. Tucker NR, Ellinor PT. Emerging directions in the genetics of atrial fibrillation. Circ Res 2014;114:1469-82. 10.1161/CIRCRESAHA.114.302225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newman AB, Murabito JM. The epidemiology of longevity and exceptional survival. Epidemiol Rev 2013;35:181-97. 10.1093/epirev/mxs013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yi Z, Mayorga ME, Hassmiller Lich K, Pearson JL. Changes in cigarette smoking initiation, cessation, and relapse among U.S. adults: a comparison of two longitudinal samples. Tob Induc Dis 2017;15:17. 10.1186/s12971-017-0121-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for Medicare beneficiaries, 1999-2013. Circulation 2017;135:1227-39. 10.1161/CIRCULATIONAHA.116.022388 [DOI] [PubMed] [Google Scholar]
- 34. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016;354:i4482. 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 35. Rahman F, Yin X, Larson MG, et al. Trajectories of risk factors and risk of new-onset atrial fibrillation in the Framingham Heart Study. Hypertension 2016;68:597-605. 10.1161/HYPERTENSIONAHA.116.07683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2017;S0735-1097(17)41518-X. 29146533 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material