Abstract
Objective: To describe patient characteristics, treatment patterns, healthcare resource utilization (HRU), and costs among patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) receiving ceritinib in second or later line of therapy.
Methods: Adult patients with NSCLC receiving ceritinib were identified from two large US claims databases (2006–2015). Patient characteristics, comorbidity profile, treatment patterns prior to ceritinib, and ceritinib dosing patterns were described. All-cause, HRU, and costs incurred during the observation period after ceritinib initiation were reported per patient per six months.
Results: One hundred sixty-four patients were included (mean age 54.2 years, 57.3% female); the majority had metastatic disease (94.5%) and the average Charlson Comorbidity Index was 7.6. 150 (91.5%) patients received crizotinib prior to ceritinib – average crizotinib duration was 10.2 months and time between crizotinib discontinuation and ceritinib initiation was 2.1 months (median= 0; 25th–75th percentile= 0–0.8). Most patients (73.8%) initiated ceritinib on the recommended dose (750 mg) and maintained the dose until the end of the observation period (mean of 7.4 months) or ceritinib discontinuation; 61 (37.2%) patients discontinued ceritinib during the observation period. A total of 76 (46.3%) patients had at least one inpatient admission during the observation period after ceritinib initiation. Mean total healthcare cost per patient per six months was $111,468.
Conclusions: Patients with ALK-positive NSCLC receiving ceritinib had a high comorbidity burden and generally started ceritinib on the recommended dose quickly after crizotinib discontinuation. Medical costs accounted for nearly a half of the total healthcare costs.
Keywords: Non-small cell lung cancer, anaplastic lymphoma kinase, ceritinib
Introduction
Rearrangements in the anaplastic lymphoma kinase (ALK) gene are found in 3–7% of patients with non-small cell lung cancer (NSCLC) [1]. In 2011, the Food and Drug Administration (FDA) approved the first ALK inhibitor, crizotinib, for the treatment of ALK-positive NSCLC [2]. Although clinical trial data have demonstrated longer median progression-free survival (PFS) and higher response rates in patients with ALK-positive NSCLC treated with crizotinib compared to those treated with chemotherapy [3,4], resistance to crizotinib typically arises within one to two years of treatment initiation [5–8].
Until 2014, treatment options for patients who became resistant to or progressed on crizotinib were limited, and many of these patients did not receive further antineoplastic pharmacological therapy [9–11]. In response to patients’ resistance to crizotinib and the need for effective post-crizotinib treatment options, second-generation ALK inhibitors have been developed against secondary ALK mutations responsible for resistance to crizotinib [7]. One of these second-generation ALK inhibitors is ceritinib, approved in April 2014 in the United States (US) by the FDA for the treatment of patients with metastatic ALK-positive NSCLC [12]. The approval of ceritinib was based on an open-label, single-arm trial and clinical activity has been confirmed in subsequent phase II and III studies [13–16]. Remarkably, in enzymatic assays, ceritinib was found to be 20 times more potent than crizotinib against ALK [17].
Most of the published data on ceritinib efficacy and safety come from clinical trial data; real-world data are limited. The first real-world description of patient characteristics, treatment and dosing patterns, and early outcomes associated with the use of ceritinib following crizotinib therapy in US clinical practice was provided by a recent chart review study including 58 patients with locally advanced or metastatic ALK-positive NSCLC [18]. Results from this study showed that 69% of these crizotinib-experienced patients achieved complete or partial response to ceritinib, regardless of metastatic sites or initial dose received. Results from this study also suggested that administering ceritinib at a lower dose with food might lead to fewer gastrointestinal (GI) adverse events (AE) without apparent changes in the response rate. Data from the ongoing ASCEND-8 trial has confirmed that the administration of ceritinib 450 mg daily with food significantly reduces GI side effects with a pharmacokinetic profile similar to 750 mg taken in a fasted state [19,20]. In addition, ASCEND-8 demonstrated that administering ceritinib 450 mg with a low-fat diet led to less frequent dose reductions/interruptions, a better relative dose intensity than the 750 mg fasted dose, and comparable clinical efficacy with a median PFS of 17.6 (95% CI: 8.5-NE) months and a 15-month PFS rate of 66.4% (95% CI: 46.5–80.4) [20].
In this study, the objective was to analyse patient characteristics, treatment patterns, healthcare resource utilization (HRU), and healthcare costs among patients with ALK-positive NSCLC initiated on ceritinib in second or later line of therapy in US clinical practice.
Methods
Data source
Data were obtained and pooled together from two of the largest US administrative commercial claims databases covering the period from 1 January 2006 to 31 December 2015. The data represent the medical and pharmacy claims of insured employees and their dependents, as well as Medicare-eligible retirees with employer-provided Medicare Supplemental plans. Data were fully de-identified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act; no institutional review board approval was required.
Inclusion criteria and study design
A retrospective, observational cohort design was used. Patients were included in the study if they were at least 18 years of age, had at least one diagnosis of lung cancer ([International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification] ICD-9 CM codes 162.2x-162.9x and ICD-10 CM codes C34.xx), had at least one prescription fill for ceritinib, and had continuous health plan enrolment for at least six months before (baseline period) and at least one month after the first prescription fill for ceritinib. Patients were excluded if they were enrolled in a clinical trial during their continuous health plan enrolment (ICD-9 CM code V70.7 and ICD-10 CM code Z006).
Description of patient characteristics
Patient characteristics assessed at the time of ceritinib initiation included age, gender, and type of health plan. Characteristics assessed during the baseline period included comorbidities from a comprehensive list of physical and mental conditions; only those with a prevalence of >5% were reported [21,22]. Furthermore, the Charlson Comorbidity Index (CCI) score [23] and location of metastases were measured based on diagnoses recorded during the pre-ceritinib period (i.e. from start of continuous healthcare plan enrolment to ceritinib initiation).
Treatment patterns
Descriptive information on cancer-directed therapies (chemotherapy, targeted therapy, radiotherapy, radiosurgery, and lung surgery) received during the pre-ceritinib period was summarized. The proportion of patients who received crizotinib prior to ceritinib, crizotinib treatment duration, and the number of months between crizotinib discontinuation (i.e. last day of supply) and ceritinib initiation were also reported. In addition, among patients who used crizotinib and did not immediately switch from crizotinib to ceritinib, the proportion of patients who used another cancer-directed therapy and the type of therapy received between crizotinib discontinuation and ceritinib initiation were summarized.
Ceritinib starting daily dose, average daily dose during the course of therapy, dose modification patterns (i.e. ceritinib daily dose changes over time, regardless of the number and duration of prescription fills), and time from ceritinib initiation to first dose reduction (i.e. decrease in the daily dose of at least 150 mg/day from initial dose [12]) were also described. The level of adherence to ceritinib was measured using the medication possession ratio (MPR), calculated as the number of days of medication supplied while on treatment divided by the duration of treatment, was also described. The number and duration of treatment interruptions (at least seven days) between ceritinib prescriptions, the proportion of patients who discontinued ceritinib (discontinuation date was defined as the last day of supply of the last prescription fill for ceritinib, regardless of treatment gaps) and the time from ceritinib initiation to discontinuation was also described. Among patients who discontinued ceritinib, the most commonly used therapies (antineoplastic, radiotherapy, radiosurgery, lung surgery, and other hospice care services) were also summarized.
Treatment patterns were summarized using means, medians, and standard deviations for continuous variables and frequencies and proportions for categorical variables. Dose modification and duration of therapy endpoints were assessed based on Kaplan–Meier analyses to account for censoring.
All-cause HRU and healthcare costs
All-cause HRU and healthcare costs were measured during the observation period after ceritinib initiation, that is, from ceritinib initiation until the end of continuous health plan enrolment or end of data availability, whichever occurred first. HRU included the number of inpatient (IP) admissions, number of IP days, days with durable medical equipment (DME) services, days with emergency care (EC) services, and number of outpatient (OP) visits.
Total healthcare costs included pharmacy and medical costs. Medical costs comprised IP, DME, EC, OP, and other medical costs. Healthcare costs were measured from a payers’ perspective and included the total amount reimbursed by the private payer and the amount covered by the coordination of benefits (COB), excluding deductibles and copayments. Costs were adjusted for inflation using the US Medical Care Consumer Price Index (CPI) from the Bureau of Labor Statistics from the US Department of Labor and were reported in 2015 US dollars (USD).
Healthcare resource utilization and healthcare costs were summarized using means, medians, and standard deviations and were reported per patient per 6 months of observation to account for different observation periods across patients.
Results
Patient characteristics
A total of 164 patients were included in the study. At the time of ceritinib initiation, the mean age was 54.2 years (14.6% were aged 65 years and older), and 57.3% of patients were female (Table 1). Most patients had metastatic disease (94.5%) and the most common metastatic sites were the respiratory system (62.8%), brain (59.1%), lymph nodes (50.0%), bone and bone marrow (47.0%), and liver (28.7%). The average CCI was 7.6 and the five most common comorbid conditions were hypertension (30.5%), fluid and electrolyte disorders (25.6%), chronic pulmonary disease (23.2%), cardiac arrhythmias (20.1%), and pulmonary circulation disorder (17.1%).
Table 1.
Patient characteristics.
| Ceritinib patients (N = 164) | |
|---|---|
| Age at ceritinib initiation (years), mean ± SD [median] | 54.2 ± 10.8 [55] |
| 65+ years old, N (%) | 24 (14.6%) |
| Female, N (%) | 94 (57.3%) |
| Type of coverage, N (%) | |
| Commercial only | 142 (86.6%) |
| Medicare supplemental | 22 (13.4%) |
| Patients with metastases, N (%) | 155 (94.5%) |
| Location of metastases, N (%) | |
| Respiratory system | 103 (62.8%) |
| Brain | 97 (59.1%) |
| Lymph nodes | 82 (50.0%) |
| Bone/bone marrow | 77 (47.0%) |
| Liver | 47 (28.7%) |
| Digestive system | 18 (11.0%) |
| Adrenal gland | 18 (11.0%) |
| Other digestive organs and spleen | 7 (4.3%) |
| Other | 8 (4.9%) |
| CCI, mean ± SD [median] | 7.6 ± 1.9 [7] |
| Baseline Comorbidities, with >5% prevalence, N (%) | |
| Hypertension | 50 (30.5%) |
| Fluid and electrolyte disorders | 42 (25.6%) |
| Chronic pulmonary disease | 38 (23.2%) |
| Cardiac arrhythmias | 33 (20.1%) |
| Pulmonary circulation disorder | 28 (17.1%) |
| Liver disease | 21 (12.8%) |
| Other neurological disorders | 20 (12.2%) |
| Congestive heart failure | 17 (10.4%) |
| Diabetes | 17 (10.4%) |
| Anxiety disorders | 19 (11.6%) |
| Valvular disease | 16 (9.8%) |
| Hypothyroidism | 16 (9.8%) |
| Sleep disorders | 14 (8.5%) |
| Depressive disorders | 13 (7.9%) |
| Weight loss | 13 (7.9%) |
| Deficiency anaemias | 12 (7.3%) |
| Renal failure | 10 (6.1%) |
| Trauma- and stressor-related disorders | 10 (6.1%) |
| Peripheral vascular disorder | 9 (5.5%) |
| Substance (alcohol and drug)-related disorders | 9 (5.5%) |
CCI: Charlson Comorbidity Index; N: number of patients; SD: standard deviation
Treatment patterns
The mean time from the first lung cancer diagnosis to ceritinib initiation was 19.0 months. A total of 160 (97.6%) patients received cancer-directed therapies in the pre- ceritinib period (Table 2): 101 (61.6%) patients had chemotherapy, 150 (91.5%) crizotinib, 105 (64.0%) radiotherapy, 45 (27.4%) radiosurgery, and 44 (26.8%) lung surgery. Among the 150 (91.5%) patients who received crizotinib in the pre-ceritinib period, the average crizotinib treatment duration was 10.2 months and the average time between crizotinib discontinuation and ceritinib initiation was 2.1 months (median = 0 month; 25th–75th percentile = 0–0.8 months) (Table 2).
Table 2.
Treatment patterns.
| Ceritinib patients (N = 164) | |
|---|---|
| Patients who used crizotinib before ceritinib initiation, N (%) | 150 (91.5%) |
| Crizotinib treatment duration (months), mean ± SD [median] | 10.2 ± 8.1 [8] |
| Months between crizotinib interruption and ceritinib initiation, mean ± SD [median] | 2.1 ± 5.0 [0] |
| Patients who used another cancer-directed therapy between crizotinib and ceritinib initiation, N (%) | 39 (23.8%) |
| Chemotherapy | 29 (17.7%) |
| Radiotherapy | 16 (9.8%) |
| Lung surgery | 3 (1.8%) |
| Targeted therapy | 7 (4.3%) |
| Radiosurgery | 3 (1.8%) |
| Cancer-directed therapy prior to ceritinib initiation, N (%) | 160 (97.6%) |
| Chemotherapy | 101 (61.6%) |
| Targeted therapy | 150 (91.5%) |
| Radiotherapy | 105 (64.0%) |
| Radiosurgery | 45 (27.4%) |
| Lung surgery | 44 (26.8%) |
| MPR while on ceritinib, mean ± SD [median] | 93.9 ± 16.6 [98] |
| MPR <80%, N (%) | 30 (18.3%) |
| 80% ≤ MPR <90%, N (%) | 22 (13.4%) |
| 90% ≤ MPR, N (%) | 112 (68.3%) |
| Patients with ceritinib treatment gaps, N (%) | 85 (51.8%) |
| Number of gaps, mean ± SD [median] | 1.2 ± 1.8 [1] |
| Gap duration (each gap, days), mean ± SD [median] | 19.5 ± 15.5 [15] |
| Patients who discontinued ceritinib, N (%) | 61 (37.2%) |
| Patients who remained untreated with antineoplastic therapy, N (%) | 37 (60.7%) |
| Among patients who remained untreated: | |
| Follow-up after ceritinib (months), mean ± SD [median] | 3.7 ± 3.4 [2] |
| Other treatment received, N (%) | |
| Radiotherapy | 7 (11.5%) |
| Radiosurgery | 1 (1.6%) |
| Lung surgery | 0 (0.0%) |
| Medical service in hospice | 10 (16.4%) |
| Patients who received antineoplastic therapy after ceritinib discontinuation, N (%) | 24 (39.3%) |
| Chemotherapy | 23 (37.7%) |
| Targeted therapy | 7 (11.5%) |
MPR: medication possession ratio; SD: standard deviation
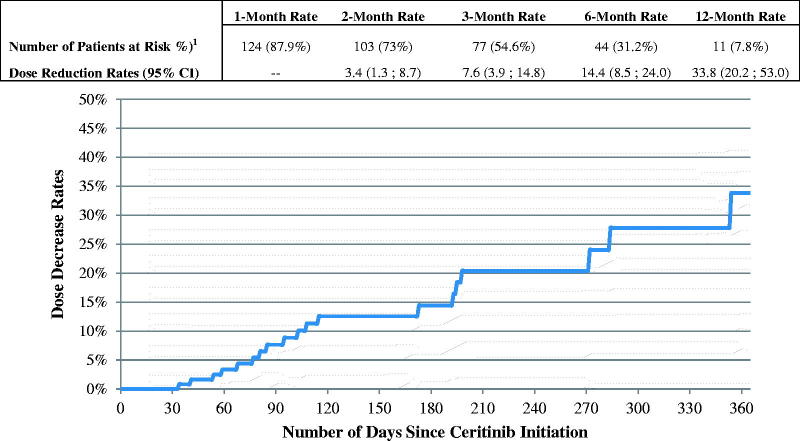
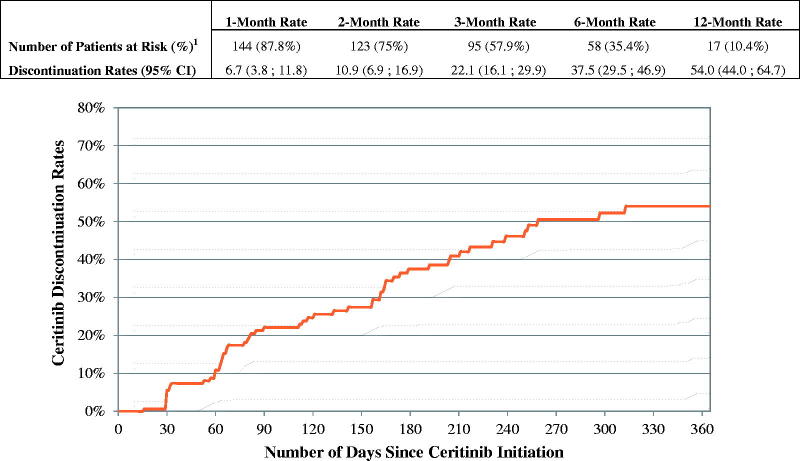
The mean ceritinib starting daily dose was 717.1 mg and the mean daily dose while on ceritinib was 695.3 mg (Table 3). The majority of patients (73.8%) started ceritinib on 750 mg and maintained that dose until the end of the observation period or until ceritinib discontinuation (Table 3). Among patients who initiated ceritinib on the 750 mg dose, 14.4% reduced their ceritinib dose within six months of initiating the 750 mg (Figure 1). The mean MPR while on ceritinib was 93.9% (Table 2). A total of 51.8% of patients had at least one treatment interruption while on ceritinib: the mean number of gaps was 1.2 per patient and the mean gap duration was 19.5 days. Over a mean observation period of 7.4 months after ceritinib initiation, 61 (37.2%) patients discontinued ceritinib. The 3 and 6-month discontinuation rates were 22.1% and 37.5%, respectively (Figure 2). Among the 61 patients who discontinued ceritinib, 24 received an antineoplastic therapy after ceritinib discontinuation; 23 received chemotherapy and 7 a targeted therapy (Table 2). Among the 37 (60.7%) patients who were not treated with an antineoplastic therapy after ceritinib discontinuation, 7 received radiotherapy, 1 patient received radiosurgery and 10 patients received hospice care services (Table 2).
Table 3.
Ceritinib dose modification patterns.
| Ceritinib patients (N = 164) | |
|---|---|
| Starting dose, mean ± SD [median] – min – max (mg/day) | 717.1 ± 95.5 [750] – 300 – 750 |
| Dose, mean ± SD [median] – min – max (mg/day) | 695.3 ± 107.1 [750] – 300 – 750 |
| Dose modification patterns (mg/day), N (%) | |
| 750 | 121 (73.8%) |
| 750 → 600 | 10 (6.1%) |
| 600 | 9 (5.5%) |
| 750 → 450 | 8 (4.9%) |
| 450 | 6 (3.7%) |
| 750 → 600 → 750 | 3 (1.8%) |
| 300 | 3 (1.8%) |
| 750 → 600 → 450 | 1 (0.6%) |
| 300 → 450 → 600 | 1 (0.6%) |
| 450 → 750 → 450 → 750 | 1 (0.6%) |
| 600 → 450 | 1 (0.6%) |
SD: standard deviation
Figure 1.
Time from ceritinib initiation to first dose reduction – among patients who initiated ceritinib on 750 mg dose. 1Number of patients at risk of having a dose reduction after elapsed time (still observed). Patients were observed from the ceritinib initiation until the first dose reduction, ceritinib treatment discontinuation, or end of continuous eligibility, whichever occurred first. CI: confidence interval.
Figure 2.
Time to ceritinib discontinuation. 1Number of patients at risk of discontinuing ceritinib therapy after elapsed time (still observed). Patients were observed from ceritinib initiation until ceritinib discontinuation or end of continuous eligibility, whichever occurred first. If a patient had less than 30 days of continuous eligibility after ceritinib discontinuation, the patient was censored at the last day of supply of the last prescription fill for ceritinib. CI: confidence interval.
All-cause HRU and healthcare costs
A total of 76 (46.3%) patients had at least one IP admission during the observation period after ceritinib initiation. Patients had a mean of 23.4 days with OP services and 0.8 days with EC services per patient per six months (Table 4). The mean total healthcare cost per patient per six months was $111,468. Medical costs accounted for 44.3% of the total healthcare costs and were mainly driven by OP costs (mainly office visit costs) and IP costs (Table 5).
Table 4.
Description of healthcare resource utilization during the observation period after ceritinib initiation.
| Healthcare resource utilization, per patient per six months | Ceritinib patients (N = 164) |
|---|---|
| IP admissions, N (%) | 1.1 ± 1.9 [0.0] |
| Patients with ≥1 IP admission | 76 (46.3%) |
| IP days, mean ± SD [median] | 10.3 ± 26.1 [0.0] |
| Days with DME services, mean ± SD [median] | 1.2 ± 3.4 [0.0] |
| Days with EC services, mean ± SD [median] | 0.8 ± 1.8 [0.0] |
| Days with OP services, mean ± SD [median] | 23.4 ± 14.3 [21.2] |
| Home care services | 3.4 ± 11.3 [0.0] |
| Skilled nursing facility services | 0.7 ± 3.0 [0.0] |
| Office visits | 18.3 ± 10.8 [16.3] |
| Ambulatory surgical centre visits | 0.1 ± 0.6 [0.0] |
| Other OP services | 0.9 ± 2.3 [0.0] |
| Days with drug administration-related claims | 3.7 ± 5.5 [1.7] |
| Days with laboratory tests | 6.6 ± 5.5 [6.1] |
DME: durable medical equipment; EC: emergency care; IP: inpatient; OP: outpatient; SD: standard deviation.
Table 5.
Description of healthcare costs during the observation period after ceritinib initiation.
| Healthcare costs, per patient per six months | Ceritinib patients (N = 164) |
|---|---|
| Duration observation periods after ceritinib initiation (months), mean ± SD [median] | 5.7 ± 4.6 [4] |
| Total healthcare costs, mean ± SD [median] | 111,468 ± 63,100 [98,947] |
| Disease-related total medical costs2 | 37,107 ± 42,950 [19,665] |
| Medical costs | 49,338 ± 58,529 [30,971] |
| IP costs | 22,182 ± 47,548 [0] |
| DME costs | 120 ± 328 [0] |
| EC costs | 1,744 ± 4,753 [0] |
| OP costs | 25,294 ± 27,716 [16,045] |
| Home care costs | 1,622 ± 6,197 [0] |
| Skilled nursing facility costs | 330 ± 2,288 [0] |
| Office visit costs | 23,151 ± 26,473 [13,353] |
| Ambulatory surgical centre costs | 75 ± 804 [0] |
| Other OP costs | 115 ± 738 [0] |
| Laboratory test costs | 1,224 ± 3,123 [362] |
| Medical drug administration costs – any medical settings | 6,845 ± 14,567 [166] |
| Total pharmacy costs | 62,130 ± 28,765 [64,101] |
DME: durable medical equipment; EC: emergency care; IP: inpatient; OP: outpatient.
Discussion
Using data from two large administrative commercial claims databases, this study described patient characteristics, treatment patterns, and HRU and costs among patients with ALK-positive NSCLC receiving ceritinib in US clinical practice. Study results showed that patients with ALK-positive NSCLC who initiated ceritinib generally had a high comorbidity burden and extensive metastatic involvement. The large majority of patients were previously treated with crizotinib. While ceritinib was generally initiated shortly after crizotinib discontinuation (2.1 months), the initiation of ceritinib was delayed for about one fourth of the patients as they received other non-ALK inhibiting treatments between crizotinib discontinuation and ceritinib initiation. Most patients initiated ceritinib on the recommended dose (750 mg) and maintained that dose until the end of the observation period or ceritinib discontinuation. By the end of the observation period, 62.8% of the patients were still on ceritinib.
The rate of ceritinib dose modification was found to be relatively low. This finding is in line with the results from a recent chart review study conducted among patients with locally advanced or metastatic ALK-positive NSCLC who initiated ceritinib following crizotinib therapy [18]. Among patients who initiated ceritinib on the recommended 750 mg dose, 17.0% (7/41) of patients had a dose reduction following a GI AE over a median observation period of 3.9 months. The dose reduction rates in both the above chart review study [18] and the current study (14.4% at 6 months) are lower than those reported in the ASCEND-1 trial, which reported dose reduction due to adverse reaction in 59% of patients who initiated ceritinib on the recommended dose, with a median time to dose reduction of seven weeks [14]. Comparisons between the current study and clinical trials should, however, be made with caution given the fundamental differences in patients’ management in a protocol versus non-protocol setting, which may influence treatment patterns and outcomes. For example, in the chart review study mentioned above [18], authors reported that, although the label recommends ceritinib be administered on an empty stomach, in real-world practice, different types of administration instructions were given to patients for the proactive management of GI AEs. In fact, about 50.0% of patients were advised to fast two hours before and after taking ceritinib, as recommended, 17.2% were advised to take ceritinib with food, and 32.8% were not given any food-related instructions. Although the above study [18] was not specifically designed to assess the impact of ceritinib administration on the risk of GI AEs under fasting versus non-fasting conditions, its results suggest that, administering ceritinib at a lower dose with food may lead to fewer GI AEs and a lower ceritinib dose was not associated with apparent changes in response rates. These findings are also consistent with the results from the phase I ASCEND-8 clinical trial, which compared outcomes in patients with ALK-positive metastatic NSCLC receiving ceritinib 450 mg or 600 mg with a low-fat meal versus 750 mg in fasted state [24]. Results from this study suggest that ceritinib 450 mg taken with a low-fat meal may lead to a lower frequency and severity of GI-related AEs than the approved 750 mg fasted dose [24]. As demonstrated in ASCEND-8, the lower dose of ceritinib 450 mg yields similar efficacy, better safety and tolerability, and fewer discontinuations than the 750 mg dose [20].
Results from the current study showed that 46.3% of patients had at least one IP admission during the observation period after ceritinib initiation (mean time 7.4 months) and that patients incurred average total healthcare costs of $111,468 per patient per six months. Medical costs represented 44.3% of the total healthcare costs and were mainly driven by OP costs (mostly office visits costs) and IP costs. Although studies on the economic outcomes associated with the real-world use of ceritinib are lacking, the healthcare costs observed in the present study are lower than those reported in a previous study assessing healthcare costs in patients with ALK-positive NSCLC treated with antineoplastic therapies following crizotinib in a pre-second-generation ALK inhibitor era [25]. In that study, the authors reported average monthly total healthcare costs of $22,160 ($141,629 per patient per 6 months after conversion to 2015 USD), versus the $111,468 per patient per 6 months found in the current study. Particularly, the average monthly IP costs of $6,419 ($41,054 per patient per six months after conversion to 2015 USD) reported in the previous study are almost twice as high as the cost reported in the current study ($21,182 per patient per six months). These results seem to suggest that, following crizotinib failure, the healthcare costs may be lower for patients who switch to ceritinib versus other (non-ALK inhibitor) antineoplastic therapy, especially in terms of IP costs, which are likely driven by progression-related events. These results are not surprising as in the phase III ASCEND-5 study, conducted among crizotinib-pretreated patients with ALK-positive NSCLC who received ceritinib versus chemotherapy, ceritinib was associated with a significantly improved median PFS (5.4 vs. 1.6 months) and an increased overall response rate compared to chemotherapy (39.1% vs. 6.9%) [26]. By limiting disease progression, ceritinib may thus contribute to lowering HRU and medical service costs. However, further studies are warranted to directly compare the economic burden of crizotinib-pretreated ALK-positive NSCLC patients treated with ceritinib versus other antineoplastic therapy and versus other recently approved second-generation ALK-inhibitors.
This study is subject to some limitations. First, there is no specific diagnosis code available to identify patients with ALK-positive NSCLC. Therefore, a prescription fill for ceritinib in addition to a diagnosis of lung cancer was used as a proxy to identify patients with ALK-positive NSCLC. Second, claims databases only report diagnostic and procedural codes recorded for reimbursement purposes and do not include information on reasons underlying treatment changes (e.g. dose reduction). Third, patients included in this study were selected from two large administrative commercial claims databases; thus, the sample of patients was limited to privately insured employees and their dependents diagnosed. Accordingly, this study may have limited generalizability to patients covered only by other insurance programs such as Medicare and Medicaid. Lastly, claims data are subject to coding errors and/or data omission.
Despite the limitations listed above, this study provides a meaningful understanding of the characteristics and early outcomes of ALK-positive NSCLC patients who receive ceritinib in clinical practice and contributes to informing the treatment landscape and management of this patient population. As physicians become more familiar with ceritinib and more clinical and safety data are made available, treatment patterns and outcomes are likely to change over time. Further studies with a larger sample size and longer data follow-up are warranted to assess longer-term treatment patterns and outcomes in ALK-positive NSCLC patients who receive ceritinib in clinical practice.
Conclusions
This claims data study showed that, upon initiation of ceritinib, patients with ALK-positive NSCLC had a high comorbidity burden and metastatic involvement. The majority of patients had received crizotinib prior to ceritinib, which was generally initiated shortly after crizotinib discontinuation. Most patients initiated ceritinib on the recommended dose and maintained it until the end of the observation period or ceritinib discontinuation. By the end of the observation period, 62.8% of patients were still on ceritinib. Medical costs accounted for nearly a half of the total healthcare costs.
Funding Statement
Financial support for this study was provided by Novartis Pharmaceuticals Corporation.
Acknowledgements
We would like to thank Cinzia Metallo, PhD, an employee of Analysis Group, Inc., for medical writing assistance.
Preliminary data were presented at the AMCP Annual Meeting 2017, on 27–30 March 2017 in Denver, Colorado.
Declaration of interest
AAD, AM, and KWC are employees of Novartis Pharmaceuticals Corporation and may own stock or stock options. AG is an employee of Analysis Group, Inc., which has received consultancy fees from Novartis for this work. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Sullivan I, Planchard D.. ALK inhibitors in non-small cell lung cancer: the latest evidence and developments. Ther Adv Med Oncol. 2016;8:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazandjian D, Blumenthal GM, Chen HY, et al. . FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 2014;19:e5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, et al. . Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 4.Solomon BJ, Mok T, Kim D-W, et al. . First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 5.Chun SG, Choe KS, Iyengar P, et al. . Isolated central nervous system progression on crizotinib: an achilles heel of non-small cell lung cancer with EML4-ALK translocation? Cancer Biol Ther. 2012;13:1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki T, Koivunen J, Ogino A, et al. . A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Savooji J, Liu D.. Second- and third-generation ALK inhibitors for non-small cell lung cancer. J Hematol Oncol. 2016;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Solomon B. [Internet]. Anaplastic lymphoma kinase (ALK) fusion oncogene positive non-small cell lung cancer. 2016; [cited 2016 Sept]. Available from: http://www.uptodate.com/contents/anaplastic-lymphoma-kinase-alk-fusion-oncogene-positive-non-small-cell-lung-cancer [Google Scholar]
- 9.Peters S.Emerging options after progression during crizotinib therapy. J Clin Oncol. 2016;34:643–645. [DOI] [PubMed] [Google Scholar]
- 10.Kayaniyil S, Hurry M, Wilson J, et al.. Treatment patterns and survival in patients with ALK-positive non-small-cell lung cancer: a Canadian retrospective study. Curr Oncol. 2016;23:e589–ee97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad MM, Shaw AT. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12:429–439. [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration (FDA) [Internet]. Highlights of prescribing information. Zykadia® (ceritinib). 2017. [cited 2017 Aug]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205755s012lbl.pdf [Google Scholar]
- 13.Khozin S, Blumenthal GM, Zhang L, et al. . FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21:2436–2439. [DOI] [PubMed] [Google Scholar]
- 14.Raedler LA.Zykadia (Ceritinib) approved for patients with crizotinib-resistant ALK-positive non–small-cell lung cancer. Am Health Drug Benefits. 2015;8(Spec Feature):163–166. [PMC free article] [PubMed] [Google Scholar]
- 15.Crino L, Ahn MJ, De Marinis F, et al. . Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016;34:2866–2873. [DOI] [PubMed] [Google Scholar]
- 16.Soria JC, Tan DSW, Chiari R, et al. . First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 17.Friboulet L, Li N, Katayama R, et al. . The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendaly E, Dalal AA, Culver K, et al. . Treatment patterns and early outcomes of ALK-positive non-small cell lung cancer patients receiving ceritinib: a chart review study. Adv Ther. 2017;34:1145–1156. [DOI] [PubMed] [Google Scholar]
- 19.Cho BC, Kim DW, Bearz A, et al. . ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with Anaplastic Lymphoma Kinase (ALK)-Rearranged Metastatic Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol. 2017;12:1357–1367. [DOI] [PubMed] [Google Scholar]
- 20.Cho B, Obermannova R, Bearz A, et al. Efficacy and updated safety of ceritinib (450 mg or 600 mg) with low-fat meal vs 750 mg fasted in ALK + metastatic NSCLC. International Association for the Study of Lung Cancer (IASLC) 18th World Conference on Lung Cancer, October 2017, Yokohama, Japan. 2017. [cited 2017 Nov]. [Internet]; Available from: https://s3.amazonaws.com/iaslc/pdf/WCLC2017_Abstract_Book_Web.pdf [Google Scholar]
- 21.Agency for Healthcare Research and Quality [Internet]. HCUP Methods Series. Comorbidity Software Documentation. 2004. [cited 2016 Sept]. Available from: https://www.hcup-us.ahrq.gov/reports/methods/ComorbiditySoftwareDocumentationFinal.pdf [Google Scholar]
- 22.American Psychiatric Association [Internet]. Diagnostic and Statistical Manual of Mental Disorders (DSM–5). 2016. [cited 2016 Sept]. Available from: https://www.psychiatry.org/psychiatrists/practice/dsm [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 24.Dziadziuszko R, Kim D-W, Bearz A, etet al. P3.02a-036 Phase 1 Study of Ceritinib 450 mg or 600 mg Taken with a Low-Fat Meal versus 750 mg in Fasted State in ALK + Metastatic NSCLC. J Thoracic Oncol. 2017;12:S1184. [DOI] [PubMed] [Google Scholar]
- 25.Guerin A, Sasane M, Wakelee H, et al. . Treatment, overall survival, and costs in patients with ALK-positive non-small-cell lung cancer after crizotinib monotherapy. Curr Med Res Opin. 2015;31:1587–1597. [DOI] [PubMed] [Google Scholar]
- 26.European Society for Medical Oncology (ESMO) [Internet]. Ceritinib improves progression-free survival in phase 3 trial of ALK rearranged lung cancer. 2016. [cited 2016 Sept]. Available from: https://www.eurekalert.org/pub_releases/2016-10/esfm-cip100816.php [Google Scholar]