Summary
Oestrogens play an important role in the development and progression of papillary thyroid carcinoma (PTC) through oestrogen receptor (ER)‐α and ‐β, which may exert different or even opposing actions in PTC. The roles of ERβ in ERα‐negative PTC are still not clear. This study investigated the expression dynamics of ERβ1 (wild‐type ERβ) and its clinical significance in female ERα‐negative PTC patients. ERβ1 expression was detected in thyroid tissues of 136 female patients diagnosed with PTC. The relationships between ERβ1 expression and clinicopathological/biological factors were also analysed in female ERα‐negative PTC patients. The total score for ERβ1 was significantly lower in female ERα‐negative PTC patients with LNM or ETE when compared to those without LNM or ETE (Z = −2.923, P = 0.003 and Z = −3.441, P = 0.001). Accordingly, the total score for ERβ1 was significantly higher in ERα‐negative PTC patients expressing E‐cadherin compared to patients negative for E‐cadherin expression (Z = −2.636, P = 0.008). The total score was lower in ERα‐negative PTC patients positive for VEGF expression compared to those negative for VEGF expression (Z = −1.914, P = 0.056). This preliminary study indicates that reduced expression of ERβ1 in female ERα‐negative PTC patients is associated with greater progression of the disease. This may provide insights into the underlying molecular mechanisms of ERβ1 and could help design targeted approaches for treating or even preventing this disease.
Keywords: immunohistochemistry, oestrogen receptor‐α, oestrogen receptor‐β, papillary thyroid cancer
Introduction
Thyroid cancer is the most common endocrine malignancy. The incidence of papillary thyroid cancer (PTC) in particular has increased worldwide over the last few decades (La Vecchia et al. 2015; Lim et al. 2017). According to recent cancer statistics in China, thyroid cancer is the eighth most common cancer in Chinese women, and its incidence is increasing. Thyroid cancer occurs three times more frequently in women than in men (Chen et al. 2016). This female predominance and the predilection of thyroid cancer to afflict postpubertal and premenopausal women suggest that oestrogen might play a role in the development and growth of thyroid cancer. However, no significant molecular or genetic factors explaining this gender disparity have been identified (Enewold et al. 2009; Nagataki & Nyström 2002).
Oestrogen binds to oestrogen receptors (ERs). Activated ERs bind DNA and regulate the expression of many different target genes. ERs are encoded by two distinct genes: ERα (ESR1) and ERβ (ESR2). Several splice variants of ERβ have been identified, which are either exon deletions or products of alternative splicing that code for proteins truncated at the C‐terminus that do not bind the oestrogen ligand. ERβ1 (wild‐type ERβ) is the only fully functional isoform that can bind oestrogen. ERα and ERβ act in distinct ways in several oestrogen target cells, including PTC tissues. Specifically, ERα exerts proliferative, antiapoptotic, autophagic and metastatic effects in PTC cells, whereas ERβ has differentiation‐inducing and pro‐apoptotic effects (Dong et al. 2013; Fan et al. 2015; Zeng et al. 2008). Both receptors have been described in both neoplastic and non‐neoplastic human thyroid tissues with extremely variable results (Ahn et al. 2015; Ceresini et al. 2006; Chen et al. 2015; Dong et al. 2012; Eldien et al. 2017; Fan et al. 2015; Huang et al. 2014; Magri et al. 2012; Sturniolo et al. 2016; Vaiman et al. 2010; Vannucchi et al. 2015). ERα is detectable in PTC tissues with positive percentages ranging from 9.9% to 66.5% (Ahn et al. 2015; Chen et al. 2015; Eldien et al. 2017; Fan et al. 2015; Huang et al. 2014; Magri et al. 2012; Sturniolo et al. 2016; Vannucchi et al. 2015). However, ERβ expression is more frequently found with positive percentages ranging from 44.4% to 97.8% (Ahn et al. 2015; Ceresini et al. 2006; Dong et al. 2012; Huang et al. 2014; Magri et al. 2012; Vaiman et al. 2010). It is important to note that there are two groups of ERβ‐positive PTC: those that co‐express both ERα and ERβ, and those expressing ERβ only. There are accumulating studies that investigated the level and frequency of expression of ERβ1 and its association with clinicopathological parameters, established markers of prognosis and clinical outcome in ERα‐negative breast carcinoma (Chantzi et al. 2013; Gruvberger‐Saal et al. 2007; Honma et al. 2008; Novelli et al. 2008; Poola et al. 2005; Reese et al. 2014; Skliris et al. 2006). However, previous studies focused on the expression and clinical significance of ERβ in PTC without regard for the expression of ERα. They explored the expression of ERβ protein and its association with important clinicopathological factors (e.g. tumour size), disease outcomes and biological markers (e.g. Ki‐67) by immunohistochemical (IHC) or quantitative analyses of the associations between ESR1 and ESR2 gene expression levels, the ESR1/ESR2 ratio and the clinicopathological characteristics of PTC patients using data from the Cancer Genome Atlas (TCGA)(Ahn et al. 2015; Huang et al. 2014; Yi et al. 2017). However, there are little data exploring PTCs that express ERβ alone. Thus, the roles of ERβ1 in ERα‐negative PTC remain unknown. In this study, we examined the expression dynamics and clinical significance of ERβ1 in female ERα‐negative PTC patients.
Materials and methods
Patients and tissue specimens
Thyroid tissue specimens were obtained from 136 female Chinese PTC patients. The mean age of the patients was 43.38 years old (range, 18–79 years). All of these patients were admitted to our hospital for a standard thyroidectomy between 2008 and 2013. Their diagnoses were confirmed by histopathological examination. None of these patients had a family history of thyroid cancer or external neck irradiation. Clinicopathological data, such as age at diagnosis, menopausal status, tumour size, lymph node metastasis (LNM) and the presence of extrathyroidal extension (ETE), were retrieved from the patients’ medical records. ETE was defined as gross extrathyroidal invasion of the subcutaneous soft tissues, larynx, trachea, oesophagus, recurrent laryngeal nerve and prevertebral fascia encasing the carotid artery or mediastinal vessels from a tumour of any size (Tuttle et al. 2016). The cancer stage was defined according to the 8th edition of the tumour, node and metastasis system classification by the American Joint Committee on Cancer (Tuttle et al. 2016). The study protocol was performed according to the Declaration of Helsinki and approved by the Medical Ethics Committee of China Medical University (AF‐S0P‐07‐1.0‐01). Written and verbal informed consent was obtained from all participants.
Immunohistochemistry (IHC)
IHC staining was performed to analyse the expression of ERα, ERβ1, Ki‐67, E‐cadherin and VEGF in PTC as previously described (Huang et al. 2014). Briefly, IHC was conducted on formalin‐fixed and paraffin‐embedded sections (4 μm thick) of surgical specimens from PTC patients using the Elivision™ plus two‐step system (Maxim Biotech Inc., Fuzhou, China). This method has proven superior to biotin‐based SP and ABC detection systems due to the presence of large amounts of endogenous biotin in the thyroid (Huang et al. 2014; Kanehira et al. 2008). The tissue sections were deparaffinized, rehydrated and subjected to microwave antigen retrieval in 10 mM citrate buffer (pH 6.0) for 20–25 min. Endogenous peroxidase was then blocked using 3% H2O2 for 10 min. After washing three times in phosphate‐buffered saline (PBS), the sections were incubated with a primary antibody against ERα (Clone 1D5, Dako; 1:200), ERβ1 (Clone PPG5/10, Serotec; 1:20), Ki‐67 (Clone SP6, Abcam; 1:100), E‐cadherin (Clone HECD‐1, Abcam; 1:100) or VEGF (Clone EP1176Y, Abcam; 1:100) in a humidified chamber at 4°C overnight. Staining was performed according to the manufacturer's instructions. Colour reactions were performed using 3,3′‐diaminobenzidine (DAB; Maxim Biotech Inc.). The sections were then counterstained with haematoxylin, washed, dehydrated into alcohol, cleared in xylene and mounted with coverslips. Appropriate positive and negative controls were run simultaneously with the patient specimens. Negative control staining was performed using normal rabbit or mouse IgG in place of a specific primary antibody.
Review and scoring of stained tissue sections
Immunostained tissue sections were reviewed, scored and interpreted using the Allred score as previously described (Huang et al. 2014). In brief, each section was carefully evaluated under the light microscope. A proportion staining score (PS) was assigned, which represented the estimated proportion of positive‐staining tumour cells as follows: 0, no staining; 1, <1/100; 2, 1/100 to 1/10; 3, 1/10 to 1/3; 4, 1/3 to 2/3; and 5, >2⁄3. An intensity score (IS) was also assigned to represent the average intensity of positive tumour cells in each section as follows: 0, no staining; 1, weak; 2, intermediate; and 3, strong. Finally, a total score (TS) was calculated from the sum of PS and IS (ranging from 0, 2–8). ‘Positive’ staining was defined as any score ≥3. A double‐blind analysis was performed by two independent investigators (Wang ZH and Sun W). If discrepancies occurred, a third investigator (Dong WW) evaluated the tissue sections, and the common result was used.
Statistical analysis
Descriptive statistics was used based upon the distribution of variables. The Mann–Whitney U test was used to compare quantitative variables between groups. The chi‐square test or Fisher's exact test was used to compare qualitative variables between groups. All statistical analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant if P < 0.05.
Results
Expression of ERβ1 protein in female ERα‐negative PTC patients
In this study, we first examined ERα expression in 136 cases of PTC tissue specimens using IHC. ERα was detected in 48.5% (66/136) of all PTC patient samples. Next, we selected the 70 samples that were ERα‐negative for further study. Of these samples, 95.7% were positive for ERβ1. Nuclear expression only, both nuclear and cytoplasmic expression, and cytoplasmic expression only for ERβ1 protein were observed in 15.7%, 65.7% and 14.3% of samples respectively. The staining score frequencies of ERβ1 in 70 cases of PTC tissue specimens were Score 0 (three cases, 4.3%), Score 3 (two cases, 2.9%), Score 4 (three cases, 4.3%), Score 5 (14 cases, 20.0%), Score 6 (18 cases, 25.7%), Score 7 (24 cases, 34.3%) and Score 8 (six cases, 8.6%). Representative images are shown in Figure 1.
Figure 1.
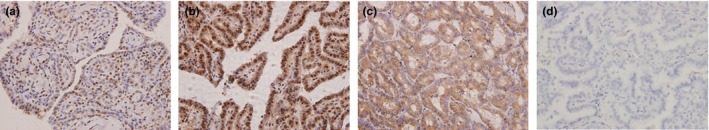
Immunohistochemical staining of ERβ1 in PTC lesions. Formalin‐fixed and paraffin‐embedded PTC tissue sections were stained with the Elivision™ plus two‐step system and specific antibodies against ERβ1. PTC tissues showed typical staining patterns of nuclear ERβ1 staining (a), nuclear and cytoplasmic ERβ1 staining (b),cytoplasmic ERβ1 staining (c) and negative control (d) (magnification ×400). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Relationships between ERβ1 expression and clinicopathological factors of female ERα‐negative PTC patients
In female ERα‐negative PTC patients, no significant difference was observed for the incidence of ERβ1 expression after examining the total scores and subcellular distributions of ERβ1 and comparing this data to ages at diagnosis (<55 years vs. ≥55 years), menopausal status (pre‐ vs. postmenopausal), tumour size (≤20 mm vs. >20 mm) or TNM stage (I/II vs. III/IV). The total score of ERβ1, but not the positive percentage or subcellular distribution, was significantly decreased in ERα‐negative female PTC patients with LNM or ETE compared to the corresponding patients without LNM or ETE (Z = −2.923, P = 0.003 and Z = −3.441, P = 0.001) (Table 1).
Table 1.
Associations between ERβ1 expression and clinicopathological factors in female ERα‐negative PTC tissue specimens
| Patient characteristics | Positive percentage | P‐value | Total scorea | P‐value | Subcellular localization | P‐value | ||
|---|---|---|---|---|---|---|---|---|
| Nu | Nu+Cyto | Cyto | ||||||
| Age at diagnosis (years) | ||||||||
| <55 (n = 47) | 46 (97.9) | 0.250 | 5.98 ± 1.50 | 0.841 | 9 (19.6) | 30 (65.2) | 7 (15.2) | 0.564 |
| ≥55 (n = 23) | 21 (91.3) | 5.70 ± 2.08 | 2 (9.5) | 16 (76.2) | 3 (14.3) | |||
| Menopausal status | ||||||||
| Pre (n = 33) | 32 (97.0) | 1.000 | 5.85 ± 1.52 | 0.580 | 6 (18.8) | 20 (62.5) | 6 (18.8) | 0.565 |
| Post (n = 37) | 35 (94.6) | 5.92 ± 1.86 | 5 (14.3) | 26 (74.3) | 4 (11.4) | |||
| Tumour size (cm) | ||||||||
| ≤20 mm (n = 45) | 44 (97.8) | 0.289 | 6.04 ± 1.46 | 0.410 | 10 (22.7) | 29 (65.9) | 5 (11.4) | 0.114 |
| >20 mm (n = 25) | 23 (92.0) | 5.60 ± 2.06 | 1 (4.3) | 17 (73.9) | 5 (21.7) | |||
| LNM | ||||||||
| − (n = 40) | 38 (95.0) | 1.000 | 6.25 ± 1.69 | 0.003 | 6 (15.8) | 28 (73.7) | 4 (10.5) | 0.476 |
| + (n = 30) | 29 (96.7) | 5.40 ± 1.61 | 5 (17.2) | 18 (62.1) | 6 (20.7) | |||
| ETE | ||||||||
| − (n = 47) | 45 (95.7) | 1.000 | 6.23 ± 1.64 | 0.001 | 9 (20.0) | 31 (68.9) | 5 (11.1) | 0.300 |
| + (n = 23) | 22 (95.7) | 5.17 ± 1.61 | 2 (9.1) | 15 (68.2) | 5 (22.7) | |||
| TNM stage | ||||||||
| I/II (n = 60) | 58 (96.7) | 0.375 | 5.97 ± 1.63 | 0.293 | 10 (17.2) | 39 (67.2) | 9 (15.5) | 0.816 |
| III/IV (n = 10) | 9 (90.0) | 5.40 ± 2.12 | 1 (11.1) | 7 (77.8) | 1 (11.1) | |||
PTC, papillary thyroid cancer; LNM, lymph node metastases; ETE, extrathyroidal extension; TNM stage, tumour node metastasis stage; n, number of patients. Nu, exclusively nuclear staining; Nu + Cyto, combined nuclear and cytoplasmic staining; Cyto, exclusively cytoplasmic staining.
Total score was calculated from the sum of the proportion staining score and intensity score to reflect the expression level, which is given as the mean ± S.D.
Relationships between ERβ1 expression and biological markers in female ERα‐negative PTC patients
The expression of Ki‐67, E‐cadherin and VEGF in PTC lesions is examined as shown in Figure 2. As a cell proliferation‐associated antigen, Ki‐67 is expressed in the nuclei of PTC cells and has been found to be a marker for cell proliferation and a prognostic factor for both disease‐free survival (DFS) and cause‐specific survival (CSS) in PTC patients (Ito et al. 2014; Pan et al. 2017). However, there was no significant difference of Ki‐67 expression between patients with tumour size ≤2 cm and >2 cm (Z = −0.309, P = 0.757). As a transmembrane glycoprotein, E‐cadherin is involved in cell–cell adhesion and epithelial–mesenchymal transition (EMT). Loss of E‐cadherin expression at primary tumour sites has been associated with increased rates of metastasis and poor patient outcomes of various malignant tumours, including gastric carcinomas, breast carcinoma and non‐small cell lung carcinoma (Mitchell et al. 2016). E‐cadherin was detected at the cell membrane in PTC lesions, and loss of its expression was reported to be an independent prognostic factor for PTC (Erdem et al. 2011; von Wasielewski et al. 1997). We found E‐cadherin expression was significantly lower in patients with ETE or LNM than that in those patients without ETE or LNM (Z = −2.505, P = 0.012 and Z = −2.420, P = 0.016). VEGF is a well‐known pro‐angiogenic factor and may be an important biological marker that determines the angiogenic and lymphangiogenic potentials of PTC cells. VEGF negatively correlates with disease‐free cancer mortality and recurrence (Hsueh et al. 2011; Klein et al. 2001). Similarly, cytoplasmic localization of VEGF was observed in PTC tissues. We found patients with ETE, but not LNM, had significantly higher VEGF expression than those patients without these clinical features (Z = −3.235, P = 0.001). In our study, no difference was found in the incidence of ERβ1‐positive patients or the subcellular distribution of ERβ1 in ERα‐negative PTC patients positive for Ki‐67, E‐cadherin or VEGF expression compared to the corresponding patients who were negative for these factors. However, the total score for ERβ1 was significantly higher in ERα‐negative PTC patients expressing E‐cadherin compared to the corresponding patients negative for E‐cadherin (Z = −2.636, P = 0.008). Meanwhile, the score for ERβ1 was lower in ERα‐negative PTC patients positive for VEGF expression compared to the corresponding patients negative for VEGF expression (Z = −1.914, P = 0.056) (Table 2).
Figure 2.
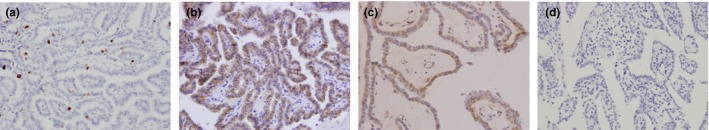
Immunohistochemical staining of Ki‐67, E‐cadherin and VEGF in PTC lesions. Formalin‐fixed and paraffin‐embedded PTC tissue sections were stained with the Elivision™ plus two‐step system and specific antibodies against Ki‐67, E‐cadherin and VEGF. PTC tissues showed typical staining patterns of Ki‐67 (a), E‐cadherin (b) and VEGF (c) and negative control (d) (magnification ×400). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Associations between ERβ1 expression and other biological markers in female ERα‐negative PTC tissue specimens
| Biological markers | Positive percentage | P‐value | aTotal score | P‐value | Subcellular localization | P‐value | ||
|---|---|---|---|---|---|---|---|---|
| Nu | Nu+Cyto | Cyto | ||||||
| Ki‐67 | ||||||||
| − (n = 8) | 7 (87.5) | 0.309 | 5.50 ± 2.67 | 0.939 | 3 (42.9) | 3 (42.9) | 1 (14.3) | 0.130 |
| + (n = 62) | 60 (96.8) | 5.94 ± 1.56 | 8 (13.3) | 43 (71.7) | 9 (15.0) | |||
| E‐cadherin | ||||||||
| − (n = 14) | 12 (85.7) | 0.100 | 4.71 ± 2.33 | 0.008 | 2 (16.7) | 6 (50.0) | 4 (33.3) | 0.131 |
| + (n = 56) | 55 (98.2) | 6.18 ± 1.38 | 9 (16.4) | 40 (72.7) | 6 (10.9) | |||
| VEGF | ||||||||
| − (n = 14) | 14 (100.0) | 1.000 | 6.64 ± 0.84 | 0.056 | 4 (28.6) | 7 (50.0) | 3 (21.4) | 0.224 |
| + (n = 56) | 53 (94.6) | 5.70 ± 1.81 | 7 (13.2) | 39 (73.6) | 7 (13.2) | |||
PTC, papillary thyroid cancer; Nu, exclusively nuclear staining; Nu + Cyto, combined nuclear and cytoplasmic staining; Cyto, exclusively cytoplasmic staining.
Total score was calculated from the sum of the proportion staining score and intensity score to reflect the expression level, which is given as the mean ± S.D.
Discussion
To our knowledge, this is the first study of the expression profiles of ERβ1 protein and its association with clinicopathological factors/biological markers in female ERα‐negative PTC patients. Our results revealed that the majority of PTC tissues were positive for ERβ1 expression, and these tissues predominantly displayed nuclear and cytoplasmic localization for ERβ1. PTC patients with highly aggressive clinicopathological features (e.g. LNM or ETE) had lower expression of ERβ1 protein than the corresponding patients without those features. Accordingly, the expression of ERβ1 positively correlated with E‐cadherin expression and negatively correlated with VEGF expression. These findings indicated that reduced expression of ERβ1 was associated with tumour invasion and metastasis in female ERα‐negative PTC patients. The constitutive expression of ERβ1 may still play a tumour‐suppressive role independently even in cases where ERα expression is lost in PTC.
The associations of ERβ1 expression with tumour progression and disease outcomes have been explored in some oestrogen‐related tumours, especially breast cancer. Some studies showed that ERβ1 protein expression correlated with less aggressive behaviour and good disease‐free and overall survival in breast cancer (Rosin et al. 2014; Sugiura et al. 2007). However, ERβ1 was not considered as a prognostic or predictive biomarker for breast cancer using extensive survival analyses in four large cohorts of human breast cancer patient (Wimberly et al. 2014). This discrepancy might be because they did not take the subcellular localization and the status of ERα into consideration. Thus, further studies showed nuclear ERβ1 was associated with low histological grade and an independent marker of lower rates of recurrence in patients with HER2‐positive and triple‐negative tumours treated with tamoxifen monotherapy (Chantzi et al. 2013; Honma et al. 2008). In this study, we found total score, but not positive percentage or subcellular localization, of ERβ1 was associated with clinicopathological parameter. That is, reduced expression of ERβ1 was associated with LNM and ETE in female ERα‐negative PTC patients, suggesting the antitumour activity of ERβ1 in this type of PTC. Moreover, we found that there was no difference of ERβ1 expression and its associations with clinicopathological factors/biological markers between ERα‐positive and ERα‐negative tumours in PTC (data not shown). Thus, a novel line of ERβ1 targeted drugs could be designed to treat ERα‐negative tumours similar to ERα blockers for ERα‐positive tumours.
Recently, some studies have focused on IHC markers and evaluated the expression of Galectin‐3, Ki‐67, E‐cadherin and VEGF in PTC (Erdem et al. 2011; Hsueh et al. 2011; Ito et al. 2014; Tang et al. 2016). These proteins have been considered useful markers reflecting the biological behaviour and prognosis for PTC. In this study, we analysed the associations between ERβ1 expression and the expression of Ki‐67, E‐cadherin and VEGF. There is increasing evidence suggesting that there is an association between Ki‐67 expression and outcomes of PTC (Ito et al. 2014; Matsuse et al. 2017; Pan et al. 2017). In this study, we did not find an association between ERβ1 and Ki‐67, which was consistent with our previous findings (Huang et al. 2014). The involvement of ERβ1 in proliferation in ERα‐negative breast cancer is also unclear. Skliris et al. reported that ERβ1 positively correlates with Ki‐67 in ERα‐negative breast cancer, suggesting that ERβ1 expression in ERα‐negative breast cancer is associated with a high proliferative index (Skliris et al. 2006). However, in another constitutive ERβ overexpression model, little or no effect of ERβ on proliferation occurs in MDA‐MB‐231 cells, an ERα‐negative breast cancer cell line (Rousseau et al. 2004). It is believed that reduced expression of E‐cadherin causes a loss of cell adhesion, leading to excessive proliferation, cancer progression and increased metastatic potential. Reduced expression of E‐cadherin in PTC correlates with capsule invasion, LNM, multiple foci, a tumour diameter of >10 mm and poor prognosis (Ceyran et al. 2015; Scheumman et al. 1995). In our study, reduced expression of E‐cadherin also correlates with ETE and LNM. ERβ1 expression significantly positively correlates with E‐cadherin expression. These findings indicated that ERβ may have suppressive effects on the ETE and LNM of PTC, and E‐cadherin may be involved in this process. Our previous study also suggested that DPN inhibits the migration and invasion of human PTC cell line BCPAP, which is modulated by E‐cadherin. This molecular mechanism has also been demonstrated in breast cancer. ERβ1 inhibits the migration and invasion of breast cancer cells through upregulation of E‐cadherin in an Id1‐dependent manner (Zhou et al. 2015). Downregulation of ERβ increases the expression levels of the epithelial marker E‐cadherin and cell junctions, followed by a reduction in various cell behaviours, such as proliferation, migration, spreading capacity, invasion and adhesion to collagen I (Piperigkou et al. 2016). Members of the VEGF family are key stimulators of both angiogenesis and lymphangiogenesis, which are fundamental processes for tumour progression. VEGF, also known as VEGF‐A, is a critical regulator of tumour angiogenesis, and it induces proliferation, migration, invasion and endothelial cell survival. VEGF expression is upregulated in PTC, and it correlates with the pathological parameters and metastatic status of PTC (Erdem et al. 2011; Salajegheh et al. 2013; Tian et al. 2008). Therefore, regulation of VEGF is a way to modulate tumour progression in PTC. In our study, increased expression of VEGF correlates with ETE, but not LNM. ERβ1 expression negatively correlates with VEGF expression. These findings indicate that ERβ may inhibit tumour invasion of PTC, and VEGF may be involved in this process. Our previous study suggested that both the incidence and total score for VEGF expression are significantly decreased in female PTC patients of reproductive age with exclusively nuclear ERβ1 expression compared with those with extranuclear localization of ERβ1. This suggests that oestrogen may suppress VEGF expression through transcriptional effects mediated by ERβ1 when it localizes to the nuclei of PTC cells. This molecular mechanism has also been demonstrated in some other malignant tumours. ERβ attenuates the hypoxic induction of VEGF mRNA by directly inhibiting the binding of HIF‐1α to the VEGF promoter in breast cancer (Lim et al. 2011). Treatment of DPN reduces VEGF expression, and cotreatment with the ERβ‐specific antagonist PHTPP abrogates this effect in PC3 prostate cancer cells (Park & Lee 2014).
This study has characterized the expression and clinical significance of ERβ1 in female ERα‐negative PTC patients. The association of ERβ1 expression with LNM, ETE, E‐cadherin and VEGF expression suggests that ERβ1 may exert inhibitory effects on tumour invasion and metastasis in PTC patients, and E‐cadherin and VEGF may be involved in this process. This has clinical implications for selective targeting of ERβ1 for therapeutic and preventative strategies against ERα‐negative PTC. However, we recognize that the limitations of this study relate to the relatively small number of cases we examined and missing information regarding patient outcomes. Thus, the potential clinical significance of ERβ1 in female ERα‐negative PTC patients is worth considering, but these results should be confirmed in a larger number of patients under long‐term follow‐up.
Conflict of interests
The authors declare no conflict of interests.
Funding
This work was supported by National Natural Science Foundation of China [grant numbers 81402208, 81502319] and Liaoning Province PhD Start‐up Fund [grant numbers 20141042, 201501008].
Acknowledgements
We would like to thank the native English‐speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
References
- Ahn H.Y., Kim M.S., Kim M.J. et al (2015) Loss of ERβ expression in papillary thyroid carcinoma is associated with recurrence in young female. Clin. Endocrinol. 82, 300–306. [DOI] [PubMed] [Google Scholar]
- Ceresini G., Morganti S., Graiani V. et al (2006) Estrogen receptor (ER)‐β, but not ER‐α, is present in thyroid vessels: immunohistochemical evaluations in multinodular goiter and papillary thyroid carcinoma. Thyroid 16, 1215–1220. [DOI] [PubMed] [Google Scholar]
- Ceyran A.B., Şenol S., Şimşek B.Ç., Sağıroğlu J. & Aydın A. (2015) Role of cd56 and e‐cadherin expression in the differential diagnosis of papillary thyroid carcinoma and suspected follicular‐patterned lesions of the thyroid: the prognostic importance of e‐cadherin. Int. J. Clin. Exp. Pathol. 8, 3670. [PMC free article] [PubMed] [Google Scholar]
- Chantzi Ν.Ι., Tiniakos D.G., Palaiologou M. et al (2013) Estrogen receptor beta 2 is associated with poor prognosis in estrogen receptor alpha‐negative breast carcinoma. J. Cancer Res. Clin. Oncol. 139, 1489–1498. [DOI] [PubMed] [Google Scholar]
- Chen D., Qi W., Zhang P., Guan H. & Wang L. (2015) Expression of the estrogen receptor α, progesterone receptor and epidermal growth factor receptor in papillary thyroid carcinoma tissues. Oncol. Lett. 10, 317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zheng R., Baade P.D. et al (2016) Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132. [DOI] [PubMed] [Google Scholar]
- Dong W., Li J., Huang Y., Zhang H., Shan Z., Teng W. (2012) Differential expression patterns of estrogen receptor (ER)‐β splice variants between papillary thyroid cancer and nodular thyroid goiter. Med. Sci. Monit. 18, BR351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Zhang H., Li J. et al (2013) Estrogen induces metastatic potential of papillary thyroid cancer cells through estrogen receptor α and β. Int J. Endocrinol. 2013, 941568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldien M.M.S., Abdou A.G., Rageh T., Abdelrazek E. & Elkholy E. (2017) Immunohistochemical expression of ER‐α and PR in papillary thyroid carcinoma. Ecancermedicalscience 11, 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enewold L., Zhu K., Ron E. et al (2009) Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980‐2005. Cancer Epidemiol. Biomark. Prev. 18, 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem H., Gündogdu C. & Şipal S. (2011) Correlation of E‐cadherin, VEGF, COX‐2 expression to prognostic parameters in papillary thyroid carcinoma. Exp. Mol. Pathol. 90, 312–317. [DOI] [PubMed] [Google Scholar]
- Fan D., Liu S.Y., van Hasselt C.A. et al (2015) Estrogen receptor alpha induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J. Clin. Endocrinol. Metab. 100, E561–E571. [DOI] [PubMed] [Google Scholar]
- Gruvberger‐Saal S.K., Bendahl P.‐O., Saal L.H. et al (2007) Estrogen receptor β expression is associated with tamoxifen response in ERα‐negative breast carcinoma. Clin. Cancer Res. 13, 1987–1994. [DOI] [PubMed] [Google Scholar]
- Honma N., Horii R., Iwase T. et al (2008) Clinical importance of estrogen receptor‐β evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J. Clin. Oncol. 26, 3727–3734. [DOI] [PubMed] [Google Scholar]
- Hsueh C., Lin J.D., Wu I. et al (2011) Vascular endothelial growth factors and angiopoietins in presentations and prognosis of papillary thyroid carcinoma. J. Surg. Oncol. 103, 395–399. [DOI] [PubMed] [Google Scholar]
- Huang Y., Dong W., Li J., Zhang H., Shan Z. & Teng W. (2014) Differential expression patterns and clinical significance of estrogen receptor‐α and β in papillary thyroid carcinoma. BMC Cancer 14, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Miyauchi A., Kobayashi K. & Miya A. (2014) Prognosis and growth activity depend on patient age in clinical and subclinical papillary thyroid carcinoma. Endocr. J. 61, 205–213. [DOI] [PubMed] [Google Scholar]
- Kanehira K., Hu J., Pier T., Sebree L. & Huang W. (2008) High endogenous avidin binding activity: an inexpensive and readily available marker for the differential diagnosis of kidney neoplasms. Int. J. Clin. Exp. Pathol. 1, 435–439. [PMC free article] [PubMed] [Google Scholar]
- Klein M., Vignaud J.‐M., Hennequin V. et al (2001) Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J. Clin. Endocr. Metab. 86, 656–658. [DOI] [PubMed] [Google Scholar]
- La Vecchia C., Malvezzi M., Bosetti C. et al (2015) Thyroid cancer mortality and incidence: a global overview. Int. J. Cancer 136, 2187–2195. [DOI] [PubMed] [Google Scholar]
- Lim W., Park Y., Cho J. et al (2011) Estrogen receptor beta inhibits transcriptional activity of hypoxia inducible factor‐1 through the downregulation of arylhydrocarbon receptor nuclear translocator. Breast Cancer Res. 13, R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H., Devesa S.S., Sosa J.A., Check D. & Kitahara C.M. (2017) Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974‐2013. JAMA 317, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri F., Capelli V., Rotondi M. et al (2012) Expression of estrogen and androgen receptors in differentiated thyroid cancer: an additional criterion to assess the patient's risk. Endocr. Relat. Cancer 19, 463–471. [DOI] [PubMed] [Google Scholar]
- Matsuse M., Yabuta T., Saenko V. et al (2017) TERT promoter mutations and Ki‐67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factors. Sci. Rep. 7, 41752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B., Dhingra J.K. & Mahalingam M. (2016) BRAF and epithelial‐mesenchymal transition: lessons from papillary thyroid carcinoma and primary cutaneous melanoma. Adv. Anat. Pathol. 23, 244–271. [DOI] [PubMed] [Google Scholar]
- Nagataki S. & Nyström E. (2002) Epidemiology and primary prevention of thyroid cancer. Thyroid 12, 889–896. [DOI] [PubMed] [Google Scholar]
- Novelli F., Milella M., Melucci E. et al (2008) A divergent role for estrogen receptor‐beta in node‐positive and node‐negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 10, R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D.‐H., Luo Y.‐H., Chen G., Yang H., Chen J.‐Q. & He Y. (2017) The diagnostic and prognostic values of Ki‐67/MiB‐1 expression in thyroid cancer: a meta‐analysis with 6,051 cases. Onco. Targets Ther. 10, 3261–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. & Lee Y. (2014) Overexpression of ERβ is sufficient to inhibit hypoxia‐inducible factor‐1 transactivation. Biochem. Biophys. Res. Comm. 450, 261–266. [DOI] [PubMed] [Google Scholar]
- Piperigkou Z., Bouris P., Onisto M. et al (2016) Estrogen receptor beta modulates breast cancer cells functional properties, signaling and expression of matrix molecules. Matrix Biol. 56, 4–23. [DOI] [PubMed] [Google Scholar]
- Poola I., Fuqua S.A., De Witty R.L., Abraham J., Marshallack J.J. & Liu A. (2005) Estrogen receptor α–negative breast cancer tissues express significant levels of estrogen‐independent transcription factors, ERβ1 and ERβ5: potential molecular targets for chemoprevention. Clin. Cancer Res. 11, 7579–7585. [DOI] [PubMed] [Google Scholar]
- Reese J.M., Suman V.J., Subramaniam M. et al (2014) ERβ1: characterization, prognosis, and evaluation of treatment strategies in ERα‐positive and‐negative breast cancer. BMC Cancer 14, 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin G., De Boniface J., Karthik G., Frisell J., Bergh J. & Hartman J. (2014) Oestrogen receptors β1 and βcx have divergent roles in breast cancer survival and lymph node metastasis. Br. J. Cancer 111, 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau C., Nichol J.N., Pettersson F., Couture M.‐C. & Miller W.H. (2004) ERβ sensitizes breast cancer cells to retinoic acid: evidence of transcriptional crosstalk. Mol. Cancer Res. 2, 523–531. [PubMed] [Google Scholar]
- Salajegheh A., Pakneshan S., Rahman A. et al (2013) Co‐regulatory potential of vascular endothelial growth factor–A and vascular endothelial growth factor–C in thyroid carcinoma. Hum. Pathol. 44, 2204–2212. [DOI] [PubMed] [Google Scholar]
- Scheumman G., Hoang‐Vu C., Cetin Y. et al (1995) Clinical significance of E‐cadherin as a prognostic marker in thyroid carcinomas. J. Clin. Endocr. Metab. 80, 2168–2172. [DOI] [PubMed] [Google Scholar]
- Skliris G., Leygue E., Curtis‐Snell L., Watson P. & Murphy L. (2006) Expression of oestrogen receptor‐β in oestrogen receptor‐α negative human breast tumours. Br. J. Cancer 95, 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturniolo G., Zafon C., Moleti M., Castellví J., Vermiglio F. & Mesa J. (2016) Immunohistochemical expression of estrogen receptor‐α and progesterone receptor in patients with papillary thyroid cancer. Eur. Thyroid J. 5, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H., Toyama T., Hara Y. et al (2007) Expression of estrogen receptor β wild‐type and its variant ER β cx/β 2 is correlated with better prognosis in breast cancer. Jpn. J. Clin. Oncol. 37, 820–828. [DOI] [PubMed] [Google Scholar]
- Tang W., Huang C., Tang C., Xu J. & Wang H. (2016) Galectin‐3 may serve as a potential marker for diagnosis and prognosis in papillary thyroid carcinoma: a meta‐analysis. Onco. Targets Ther. 9, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Cong M., Zhou W., Zhu J. & Liu Q. (2008) Relationship between protein expression of VEGF‐C, MMP‐2 and lymph node metastasis in papillary thyroid cancer. J. Int. Med. Res. 36, 699–703. [DOI] [PubMed] [Google Scholar]
- Tuttle R., Morris L., Haugen B. et al (2016) Thyroid: differentiated and anaplastic carcinoma. AJCC cancer staging manual. New York, NY: Springer; 1024. [Google Scholar]
- Vaiman M., Olevson Y., Habler L., Kessler A., Zehavi S. & Sandbank J. (2010) Diagnostic value of estrogen receptors in thyroid lesions. Med. Sci. Monit. 16, BR203–BR207. [PubMed] [Google Scholar]
- Vannucchi G., De Leo S., Perrino M. et al (2015) Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. Eur. J. Endocrinol. 173, 29–36. [DOI] [PubMed] [Google Scholar]
- von Wasielewski R., Rhein A., Werner M. et al (1997) Immunohistochemical detection of E‐cadherin in differentiated thyroid carcinomas correlates with clinical outcome. Can. Res. 57, 2501–2507. [PubMed] [Google Scholar]
- Wimberly H., Han G., Pinnaduwage D. et al (2014) ERβ splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Res. Treat. 146, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.W., Kim S.‐J., Kim J.K. et al (2017) Upregulation of the ESR1 gene and ESR Ratio (ESR1/ESR2) is associated with a worse prognosis in papillary thyroid carcinoma: the impact of the estrogen receptor α/β expression on clinical outcomes in papillary thyroid carcinoma patients. Ann. Surg. Oncol. 24, 1–9. [DOI] [PubMed] [Google Scholar]
- Zeng Q., Chen G., Vlantis A., Tse G. & Van Hasselt C. (2008) The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. J. Pathol. 214, 425–433. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Ming J., Xu Y., Zhang Y. & Jiang J. (2015) ERβ1 inhibits the migration and invasion of breast cancer cells through upregulation of E‐cadherin in a Id1‐dependent manner. Biochem. Biophys. Res. Comm. 457, 141–147. [DOI] [PubMed] [Google Scholar]


