Abstract
We have previously determined that phospholipase D (PLD) is activated by abscisic acid (ABA), and this activation is required for the ABA response of the cereal aleurone cell. In this study, ABA-stimulated PLD activity was reconstituted in vitro in microsomal membranes prepared from aleurone protoplasts. The transient nature (20 min) and degree (1.5- to 2-fold) of activation in vitro were similar to that measured in vivo. Stimulation by ABA was only apparent in the membrane fraction and was associated with a fraction enriched in plasma membrane. These results suggest that an ABA receptor system and elements linking it to PLD activation are associated with the aleurone plasma membrane. The activation of PLD in vitro by ABA was dependent on the presence of GTP. Addition of GTPγS transiently stimulated PLD in an ABA-independent manner, whereas treatment with GDPβS or pertussis toxin blocked the PLD activation by ABA. Application of pertussis toxin to intact aleurone protoplasts inhibited the ability of ABA to activate PLD as well as antagonizing the ability of ABA to down-regulate gibberellic acid-stimulated α-amylase production. All of these data support the hypothesis that ABA stimulation of PLD activity occurs at the plasma membrane and is mediated by G-protein activity.
The cereal aleurone exhibits a well-defined suite of responses to the phytohormones gibberellic acid (GA3) and abscisic acid (ABA). GA3 increases the expression and secretion of hydrolytic enzymes (principally α-amylases), which break down the starchy endosperm providing resources for seed germination (Bethke et al., 1997; Ritchie and Gilroy, 1998a). ABA inhibits the responses to GA3 as well as causing the up-regulation of several ABA-responsive genes (for review, see Bethke et al., 1997).
The perception of GA3 and ABA in the cereal aleurone occurs at the plasma membrane (Hooley et al., 1991; Gilroy and Jones, 1994), although receptors have yet to be identified for either hormone. In addition, signal transduction events that lead from perception to cellular regulation remain poorly understood. There are however several candidate events that may be involved in early transduction of the ABA signal in the aleurone. These include changes in pH (van der Veen et al., 1992; Heimovaara-Dijkstra et al., 1994a), membrane potential (Heimovaara-Dijkstra et al., 1994b), mitogen-activated protein kinase-like activity (Knetsch et al., 1996), and the activity of the protein kinase, PKABA1 (Gomez-Cadenas et al., 1999). We have also demonstrated a role for phospholipase D (PLD) in the response of aleurone to ABA. Thus, ABA elicited a transient increase in PLD activity when applied to aleurone cells. Treatment of protoplasts with one of the products of PLD activity, phosphatidic acid (PtdOH), elicited the ABA response in the absence of ABA. Conversely inhibiting the production of PtdOH with a PLD antagonist (1-butanol) blocked the aleurone response to ABA (Ritchie and Gilroy, 1998b). We have also demonstrated a similar relationship between ABA and PLD in a very different ABA-responsive cell, the stomatal guard cell of broad bean (Jacob et al., 1999), and an involvement of PLD in ABA signal transduction regulating senescence has been suggested (Ryu and Wang, 1995; Fan et al., 1997). Changes in PLD expression and activity in response to dehydration stress have been shown to be correlated with drought tolerance of cowpea (Maarouf et al., 1999) and the resurrection plant (Frank et al., 2000). Whether these changes in response to water stress also reflect ABA signaling events remains to be determined.
The mechanism whereby ABA leads to activation of PLD remains unclear but GTP-binding proteins (G-proteins) represent one candidate. G-proteins are considered universal signal transduction elements and in the PLD-mediated deflagellation response of Chlamydomonas eugametos (Munnik et al., 1995, 1998), and in the regulation of PLD activities in carnation (Munnik et al., 1995; de Vrije and Munnik, 1997), G-protein activation of PLD has been proposed. In addition, in the stomatal guard cell, evidence has accumulated that heterotrimeric G-proteins are involved in regulating ion channels (Assmann, 1996), which are downstream effectors of ABA signaling in this cell. However, to date, evidence from aleurone cells more strongly supports G-protein involvement in transducing the GA3 rather than ABA signal. For example, application of the peptides mastoparan and Mas7 were able to mimic the GA3 response of the wild oat aleurone (Jones et al., 1998). These peptides are thought to imitate activated G-protein-coupled receptor motifs, suggesting that their action in the aleurone was through G-protein-mediated GA3 signal transduction. It has also been found that a dwarf mutant of rice, which is severely impaired in the GA3 response of the aleurone (Mitsunaga et al., 1994), has a lesion in a Gα gene (Fujisawa et al., 1999). Extracts of barley (Hordeum vulgare) aleurone protoplasts and embryos contain proteins that cross-react with antibodies to Gα and ras (a monomeric GTPase) and proteins that bind GTP (Wang et al., 1993; Visser et al., 1999). Recent evidence also tentatively suggests the involvement of G-protein activity in the response to ABA of the barley embryo (Visser et al., 1999).
We have therefore investigated further the link between PLD and ABA in the cereal aleurone by developing an in vitro assay in which ABA stimulates PLD activity. In this paper we report that ABA stimulation of PLD activity in the barley aleurone cell most likely takes place at the plasma membrane and requires a G-protein-type activity.
RESULTS
Stimulation of PLD Activity in Vitro by ABA
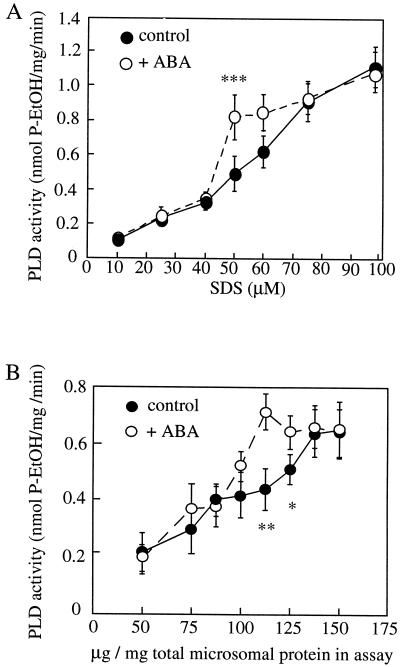
To investigate further the molecular interactions leading to the activation of PLD by ABA in the barley aleurone we reconstituted the PLD stimulation by ABA in a microsomal membrane fraction from protoplasts in vitro. ABA stimulation of PLD was detectable in these assays, but the stimulation was dependent on the SDS and protein concentration used in the assay (Fig. 1). Figure 1A shows that at 112.5 μg protein mL−1 and 50 to 60 μm SDS, ABA induced an approximately 1.5-fold stimulation of PLD activity in the aleurone cell extracts. This level of stimulation is similar to that induced by ABA in vivo (Ritchie and Gilroy, 1998b).
Figure 1.
The effect of varying SDS and protein concentration on PLD activity in microsomal membrane preparations in the presence or absence of ABA. PLD assays were carried out in the presence of various SDS (A) and protein (B) concentrations ± 10 μm ABA as described in “Materials and Methods.” In A the protein concentration used was 112.5 μg/mL per assay, in B the SDS concentration used was 55 μm. The results show the means ± se of three separate experiments for each graph. Asterisks show statistically different points (*, P < 0.05; **, P < 0.025; ***, P < 0.01, t test).
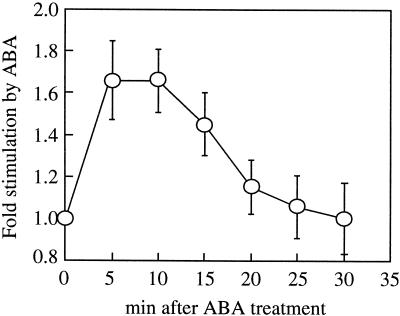
The PLD assays shown in Figure 1 were carried out after 10 min of ABA treatment but we have previously noted that the stimulation of PLD by ABA in vivo was transient, occurring 5 to 10 min after ABA application and subsiding by 20 to 25 min (Ritchie and Gilroy, 1998b). Thus once the conditions were established at which ABA-stimulated PLD activity in vitro could be detected, we conducted a time course of this activity. Figure 2 shows that the stimulation of PLD by ABA is apparent at the first time point we could assay after ABA addition (5 min), subsided after 20 min, and by 30 min no difference between PLD activity with or without ABA could be detected. Thus, the transient nature of the PLD activation in vitro parallels that reported in vivo, although we cannot determine if its onset may be faster.
Figure 2.
Time course of ABA stimulation of PLD activity in vitro. PLD assays were carried out using microsomal membrane preparations for various times ± 10 μm ABA. The stimulation by ABA was calculated by division of the PLD activity measured in assays run + ABA by that of the equivalent − ABA control. The results show the means ± se of three separate experiments.
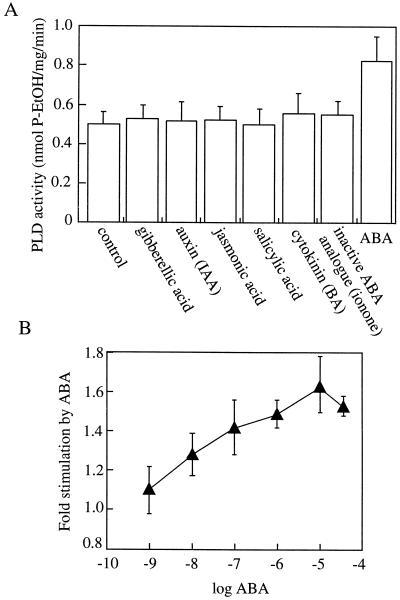
We next tested a range of other plant hormones to determine the specificity of PLD stimulation to ABA. In addition, ionone was used as a biologically inactive structural analog of ABA (van der Meulen et al., 1993). Figure 3A shows that under the assay conditions used, none of the other hormones tested had any stimulatory effect on PLD activity. Increasing ABA concentrations caused increasing PLD activation with the maximal effect at 10 μm ABA and with saturation of this effect at higher concentrations used. ABA concentrations as low as 10 nm caused a significant stimulation of PLD activation (Fig. 3B; t test, P < 0.05). This dose response parallels that of the responsiveness of intact aleurone cells to ABA (Ritchie et al., 1999).
Figure 3.
The stimulation of PLD by ABA is specific for this hormone and is dependent on the ABA concentration. PLD assays using microsomal membrane preparations were carried out in the presence of a range of plant hormones (all 10 μm) (A) and a range of ABA concentrations (B). The results show the means ± se of three separate experiments. BA, Benzyl adenine.
Stimulation of PLD by ABA Is G-Protein Mediated
We next asked the nature of the link between ABA perception and PLD activation. G-proteins are ubiquitous signaling intermediates in the membranes of eukaryotic cells. Therefore, we assayed the effect of molecules that modulate G-protein activity on PLD activation by ABA in the aleurone membrane fraction. The GTP analog GTPγS is hydrolyzed much more slowly than GTP, whereas GDPβS is a non-phosphorylatable form of GDP. These molecules render G-proteins in an active or inactive state, respectively (Ma and Weiss, 1995). Figure 4 shows that the inclusion of 10 μm GDPβS in PLD assays made the activity insensitive to ABA, whereas 10 μm GTPγS was able to significantly stimulate PLD in the absence of added ABA. To determine the specificity of these effects for GDP and GTP analogs, parallel assays were conducted in the presence of equivalent ADP and ATP analogs. Neither 10 μm ADPβS nor ATPγS showed any significant effect on PLD activity.
Figure 4.
The effect of non-hydrolyzable GTP analogs on ABA-stimulated PLD activity. PLD assays of microsomal membrane preparations were conducted using ±10 μm ABA in the presence of various nucleotides (10 μm). The results show the means ± se of three separate experiments. Asterisks show statistically different points (between control and + GTPγS; **, P < 0.025, t test).
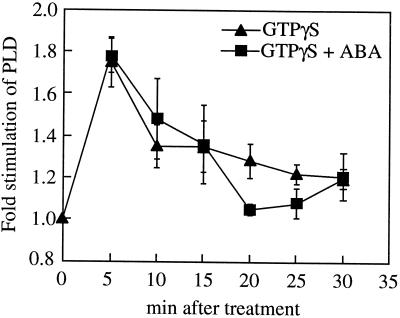
To test if GTPγS is also able to irreversibly activate PLD in our in vitro system, we conducted time courses of PLD activity in the presence of GTPγS and ABA. Figure 5 shows that, in the absence of ABA, GTPγS stimulated PLD activity with similar kinetics to ABA (Fig. 2), although the decline in stimulation by 10 min is more rapid than seen in ABA-treated samples, and its final extent may be lower (compare Figs. 2 and 5). Thus GTPγS was unable to prevent the inactivation of activated PLD normally seen in response to ABA.
Figure 5.
Time course of GTPγS stimulation of PLD activity in vitro. PLD assays of microsomal membrane preparations were carried out for various times ±10 μm GTPγS and ±10 μm ABA. The stimulation by GTPγS was calculated by division of the activity in assays run + GTPγS ± ABA by those from assays −GTPγS and −ABA. The results show the means ± se of three separate experiments.
Treatment with ABA and GTPγS showed similar transient PLD activation to GTPγS or ABA alone (Fig. 5). This is in contrast to the increased stimulation of ABA plus GTPγS over GTPγS alone seen in Figure 4. The reason for this variability in PLD activation by GTPγS relative to the maximal stimulation seen with ABA is unknown but again may reflect the action of other regulators of PLD activity in the membrane preparation. However, importantly, in all the assays we have conducted, GTPγS elicited a significant (P < 0.05, t test) activation of PLD in the range seen to be elicited by ABA.
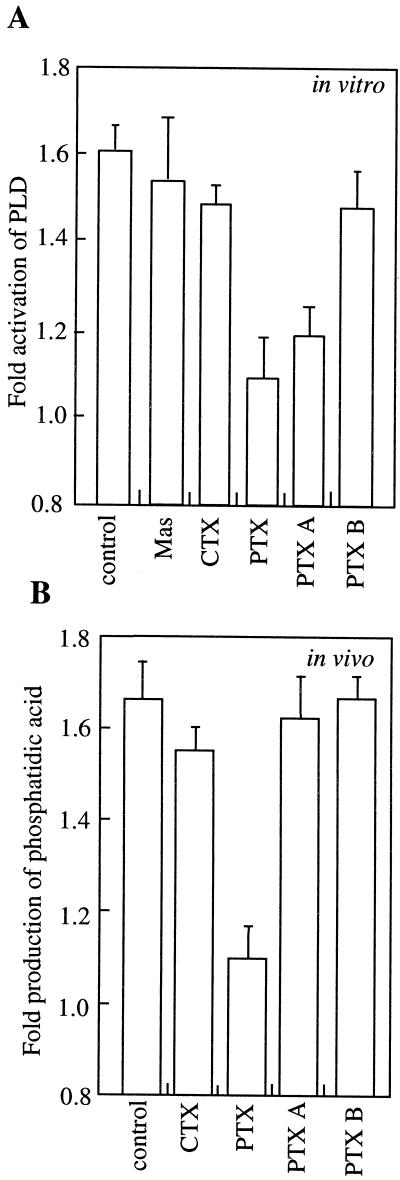
Pertussis toxin (PTX), cholera toxin (CTX), and mastoparan are also all used to perturb G-protein function (Ma and Weiss, 1995). PTX is able to inhibit some types of G-protein, whereas CTX activates others. Mastoporan elicits the activation of G-proteins by mimicking the conformation of activated G-protein coupled receptors. As shown in Figure 6A, PTX inhibited the ABA stimulation of PLD activity, whereas CTX and mastoparan had no significant effects (P > 0.05, t test). To determine the specificity of the effect of PTX, we also analyzed the activity of the two individual proteins that make the whole toxin molecule. The PTX A subunit has the enzymatic, ADP-ribosylation activity that alters G-protein function, whereas the B oligomer subunits enable entry of the A subunit into cells (Locht and Antoine, 1995). In the in vitro assay, we found that the A subunit alone inhibited the ABA stimulation of PLD activity, whereas the B subunit alone had no effect on the stimulation of PLD by ABA. PTX denatured by boiling was also ineffective against ABA stimulation of PLD (data not shown). In addition, neither the A subunit nor the full PTX holoenzyme inhibited basal PLD activity (control 0.51 ± 0.05 nmol P ethanol mg−1 min−1 compared with 0.49 ± 0.07 for PTX treated and 0.5 ± 0.05 for PTX-A treated). Their action was limited to antagonizing the ABA-activated component of PLD activity. Taken together, these data indicate that the effect of the PTX is unlikely to be due to a non-specific effect of the proteins involved since only the A subunit was able to inhibit the stimulation of PLD by ABA and only the ABA-regulated component of PLD activity was affected.
Figure 6.
The effect of G-protein agonists and antagonists on ABA-stimulated PLD activity and in vivo effect of PTX and CTX on ABA-stimulated PtdOH levels. A, In vitro PLD assays of microsomal membrane preparations were conducted ±10 μm ABA in the presence of a range of G-protein-related peptides (1 or 0.5 μg/mL for protomer A or oligomer B of PTX) and P-ethanol measured. B, Aleurone protoplasts were loaded with fluorescently labeled phosphatidylcholine and, after application of ABA (10 μm) and toxins (1 or 0.5 μg/mL for protomer A or oligomer B of PTX) for 15 min, lipids were extracted and fluorescent PtOH produced in vivo was quantified. The results show the means ± se of three separate experiments. For A, basal in vitro PLD activity was 0.5 ± 0.05 nmol P-ethanol mg−1 min−1. For B, basal in vivo fluorescent PtdOH production was 1.3 ± 0.15 arbitrary fluorescent units. The data are expressed as the ratio of P-ethanol (A) or PtdOH (B) produced with the treatment in the presence versus absence of ABA. None of the treatments (Mas, CTX, PTX, PTX-A, or PTX-B) significantly affected either PLD activity (P-ethanol produced, A) or production of fluorescent-PtdOH (B) in non-ABA-treated samples (P < 0.05, t test). Mas, Mastoparan; control, activation in presence of ABA without additional treatment.
As PTX was able to block ABA stimulation of PLD activity in vitro, we determined if it could affect ABA signaling and PLD activity in vivo. We monitored in vivo PLD activity using aleurone protoplasts loaded with fluorescent phospholipid and following the production of the fluorescently labeled product of PLD activity. After treatment with ABA the levels of fluorescent PtdOH extracted from aleurone protoplasts were elevated above those of controls indicating PLD activation. Figure 6B shows that PTX treatment abolished this effect, whereas CTX was much less effective at inhibiting ABA-induced PtdOH production. Treatment of protoplasts with either PTX A protomer or B oligomer alone did not have any effect on the response to ABA, consistent with a requirement of both parts for entry into the cells (B oligomer) and subsequent inhibition by the catalytic activity (A protomer).
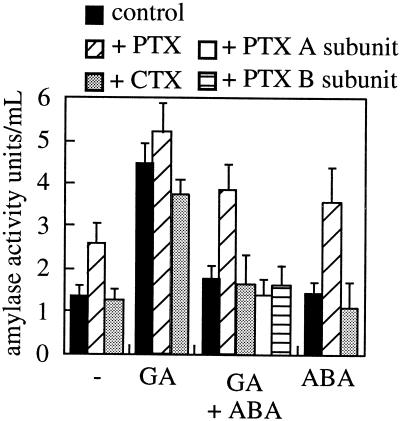
One further measure of ABA activity in the aleurone is its ability to inhibit GA3-induced α-amylase secretion. We therefore tested the effects of the toxins on this aspect of the ABA response of these cells. Figure 7 indicates that protoplasts treated with GA showed the expected elevated levels of amylase secretion that was inhibited by ABA. However, PTX blocked this ABA effect, maintaining levels of secretion in GA3+ABA-treated cells. As noted by other researchers (Jones et al., 1998), PTX did not inhibit the GA3 response, again arguing against PTX having non-specific toxic effects on the aleurone cell. When PTX was applied to ABA-only-treated samples or samples not treated with any hormone, there was a stimulation of α-amylase production, an activity normally associated with GA3 action. The same effect on secreted amylase activity was also seen when aleurone layers rather than protoplasts were treated with PTX (data not shown). When the individual subunits of PTX were applied instead of the whole holoenzyme there was no effect of the toxin on α-amylase secretion (Fig. 7), consistent with the lack of effect of the individual toxin subunits on ABA activation of PtdOH production in intact cells (Fig. 6B).
Figure 7.
The effect of PTX and CTX on α-amylase activity. Aleurone protoplasts were treated with combinations of GA (1 μm), ABA (10 μm), PTX, and CTX (1 or 0.5 μg/mL for individual subunits of PTX). After 48 h, secreted α-amylase activity was assayed. The results show the means ± se of three separate experiments.
ABA Stimulation of PLD Activity Occurs at the Plasma Membrane
To determine if the ABA stimulation of PLD activity occurred at the plasma membrane, we processed microsomal fractions from aleurone layers through three rounds of phase partitioning to generate an upper phase fraction enriched in plasma membrane and a lower phase enriched in other cellular membranes and depleted in plasma membrane. Aleurone layers were used as starting material rather than protoplasts because of the limitation in the amount of membrane material that could be generated using protoplasts. However, some experiments using protoplast-derived plasma membrane were performed, and the results were identical to those reported below for layers.
We had already established that the levels of protein and SDS in the assay are critical factors needing optimization to reconstitute ABA activation of PLD in microsomal membranes in vitro (Fig. 1). As the protein and lipid composition of phase partitioned membranes would likely differ from the microsomal preparation used previously, we repeated the protein and SDS optimization experiments outlined in Figure 1 for the fractions for the phase partition experiments. These experiments revealed that the optimal SDS level of 50 to 60 μm was identical to our previous experiments but that the optimal protein level for ABA-induced stimulation of PLD activity was 162.5 μg/mL. Therefore, we used this protein concentration in the phase partitioning PLD experiments reported below.
We next conducted membrane marker assays on the fractions generated by the phase partitioning to estimate the enrichment of plasma membrane in the final upper phase. Table I shows that in the upper phase the vanadate-sensitive ATPase, a marker used for plasma membranes (Briskin et al., 1987), comprised approximately 50% of the total ATPase activity. In comparison, in the lower phase the vanadate-sensitive activity was only 5% of total. Whereas, the markers for other membrane fractions (cytochrome C reductase, for the endoplasmic reticulum; ATPase sensitive to azide [for mitochondria] and molybdate [for the vacuole]; latent IDPase, for the Golgi apparatus) were enriched in the lower phase and reduced in the upper phase. From these marker enzyme assays, we estimate that the upper phase is enriched to approximately 80% plasma membrane.
Table I.
Marker enzyme assays using fractions prepared by two-phase partitioning of aleurone membranes
| Enzyme Activity | Total Microsomal Membranes | Lower Phase (Fold Enrichment) | Upper Phase (Fold Enrichment) |
|---|---|---|---|
| NADPH-cytochrome C reductase (Endoplasmic reticulum) | 0.284 ± 0.08 | 0.411 ± 0.11 (1.5) | 0.091 ± 0.019 (0.32) |
| Latent IDPase (Golgi) | 0.0158 ± 0.002 | 0.0194 ± 0.004 (1.23) | None detected |
| ATPase | |||
| No inhibitors | 0.069 ± 0.007 | 0.089 ± 0.004 | 0.147 ± 0.017 |
| + Nitrate | 0.055 ± 0.01 | 0.078 ± 0.005 | 0.153 ± 0.05 |
| + Nitrate, azide, and molybdate | 0.037 ± 0.01 | 0.033 ± 0.009 | 0.139 ± 0.05 |
| + Nitrate, azide, and molybdate + vanadate | 0.028 ± 0.005 | 0.029 ± 0.008 | 0.06 ± 0.02 |
| ATPase activity that is sensitive to: | |||
| Nitrate (tonoplast) | 0.014 | 0.011 (0.79) | None detected |
| Azide (mitochondria) + molybdate (acid phosphatase) | 0.018 | 0.045 (2.5) | 0.014 (0.78) |
| Vanadate (plasma membrane) | 0.009 | 0.004 (0.44) | 0.079 (8.78) |
Total membrane preparations before phase partitioning; the first lower phase and the third upper phase from partitioning were used. The data show the means ± se for three independent experiments, samples assayed in duplicate, except for latent IDPase activity and nitrate-inhibited ATPase activity, for which data from two independent experiments is shown. Specific activities are shown expressed as μmol mg−1 min−1 and -fold enrichment in activities are shown in parentheses. Average total protein in each fraction was: total, 16.4 mg; lower phase, 8.2 mg; and upper phase, 1.1 mg.
Figure 8 shows that when PLD activity was assessed in the upper and lower phases derived from the partitioning of aleurone membranes, activity was stimulated more than 3-fold by ABA in the upper phase fraction. Although the lower phase membranes had a much higher basal level of PLD activity, there was no significant stimulation by ABA (P > 0.05, t test). No stimulation was detected in the soluble fraction. We included 10 μm GTP in the assays shown in Figure 8A, and as shown in Figure 8B there was no ABA stimulation in the upper phase fraction in the absence of added GTP. ATP could not substitute for this GTP requirement. In the absence of ABA, both GTP and ATP caused an approximately 40% decrease in the basal amount of PLD activity. Also, in the presence of both ATP and GTP, ATP suppressed the level of PLD activity stimulated by ABA by approximately 30%.
Figure 8.
ABA stimulation of PLD activity is localized to a plasma membrane-enriched fraction and is GTP dependent. A, Microsomal membrane preparations were processed through phase partitioning and the resulting upper and lower phases, plus the initial microsomal and soluble fractions were assayed for PLD activity (in the presence of 10 μm GTP) ± 10 μm ABA. B, The upper phase was assayed for PLD activity in the absence or presence of 10 μm ATP and/or GTP. The results show the means ± se of three separate experiments.
The requirement for GTP to detect ABA stimulation of PLD activity shown in Figure 8 is in contrast to experiments using freshly prepared microsomal membranes (Figs. 1–3) in which stimulation occurred without the need to add exogenous GTP. We hypothesized that this difference might be due to the presence of endogenous nucleotides in the microsomal preparation that have been removed or hydrolyzed during phase partitioning. Such support of G-protein activity by carry over of nucleotides in membrane fractions has been noted by other researchers (Graeser and Neubig, 1992). To test this possibility, we attempted to remove any nucleotides by washing our microsomal preparations twice by pelleting and resuspension in fresh buffer. When these final pellets were used PLD activity was no longer stimulated by ABA (−ABA: 0.26 ± 0.04 nmol P-etOH−1 mg−1 min−1 and +ABA: 0.27 ± 0.03). With inclusion of 10 μm GTP this basal PLD activity was unchanged but ABA now stimulated PLD 1.4 ± 0.2-fold (results from three independent experiments). The addition of ATP rather than GTP could not support stimulation of PLD by ABA. These data support the hypothesis that GTP hydrolysis is required for PLD stimulation by ABA but that sufficient GTP is present in fresh microsomal preparations for there to be no requirement for exogenous GTP.
DISCUSSION
We have previously demonstrated that ABA stimulates PLD activity in the barley aleurone and that this stimulation is involved in transduction of the ABA signal (Ritchie and Gilroy, 1998b). In this work we have developed an in vitro assay in which ABA, but not the other plant hormones tested, can also stimulate PLD in membrane preparations from barley aleurone. To detect any stimulation of PLD activity by ABA in this in vitro assay, the concentrations of protein and detergent had to be optimized. The critical ratio of detergent:protein (50 μm:112.5 μg/mL) is in the range in which the membrane components are likely to be essentially intact (Hjelmeland, 1990). Hence these conditions are likely to allow for interactions between membrane-associated proteins essentially as they would in vivo.
The in vitro stimulation of PLD by ABA shared important characteristics with the activation seen in intact cells. The 1.5- to 1.8-fold stimulation of PLD activity by ABA is similar in vivo (Ritchie and Gilroy, 1998b) and in vitro (e.g. Fig. 8). Similarly, in both the in vitro and in vivo assays the stimulation of PLD by ABA was transient in nature, subsiding after 15 to 20 min.
Scherer and Andre (1993) used an in vitro assay to complement in vivo work implicating PLA2 activity in auxin signaling. Their in vitro assay allowed them to establish a role of GTP, ATP, and auxin-binding protein in the interaction. In a similar manner the use of the in vitro assay described in this work has allowed us to investigate further the nature of the interaction between ABA and PLD. We found that using a plasma membrane-enriched fraction from aleurone the stimulation of PLD by ABA was greater (on average 3-fold) than the 1.5-fold using a total microsomal preparation (Fig. 8). This result suggests that the ABA receptor, signal transduction system, and ABA-regulated PLD activity may all be tightly associated with the plasma membrane. Current evidence suggests multiple ABA perception sites within the aleurone cell depending on the downstream event (Ritchie and Gilroy, 1998a) with one site of ABA perception at the plasma membrane (Gilroy and Jones, 1994). Our results support the model of ABA perception at the plasma membrane. In addition, as the activation system survived three rounds of membrane purification by phase partitioning, it seems unlikely that the PLD activation reflects recruitment of cytosolic factors or enzyme to the membrane as has been proposed for other PLD-activating systems (Wang, 1999).
The effects we have documented of G-protein-modulating agents on the in vitro and in vivo activation of PLD suggests G-proteins may be one membrane-associated factor acting between PLD and the ABA receptor system. In the in vitro assays, both PTX and GDPβS inhibited the PLD stimulation elicited by ABA (Figs. 4 and 6). In addition, GTP was required for PLD activation, and GTPγS was able to mimic the effect of ABA on PLD activity (Figs. 4 and 5). This is characteristic of some G-protein-mediated signaling systems and is thought to be due to low-level activity of the GTPase in the absence of agonist receptor activation (McFadzean and Brown, 1992). In other cells GTPγS is not able to mimic G-protein activation in the absence of the agonist, however if added concomitant with the agonist, the downstream G-protein-stimulated events are prolonged (McFadzean and Brown, 1992). This latter possibility did not occur in our studies suggesting that deactivation of the G-protein, and consequently downstream events, is independent of GTP turnover. Figure 8 indicates ATP-suppressed PLD activation, suggesting changes in the activity of an ATP-dependent factor, such as a protein kinase/phosphatase in the membrane fraction may be part of the PLD regulatory system.
In agreement with these in vitro data, PTX inhibited ABA-related events in vivo. This inhibition was evident when assayed either as a reduction in ABA-stimulated PLD activity (Fig. 6) or an inhibition of ABA antagonism of GA3-induced events (Fig. 7). It is interesting, however, that PTX treatment not only inhibited ABA-related events but also resulted in elevated levels of α-amylase activity in the absence of added GA3. There is significant genetic evidence supporting the hypothesis originally proposed by Brian (1957) that GA3 acts by relieving the action of a repressor systems (for review, see Harberd et al., 1998). In the case of the aleurone in particular it is plausible that a repressor system could be set up by ABA and other developmental cues during seed maturation, and this repressor system is finally “de-activated” by GA3 during seed germination. Our results with PTX suggest that a G-protein-mediated, perhaps ABA-related, process is operating in the aleurone to suppress the GA3 response.
The identity of the putative ABA-related G-protein-regulating PLD remains to be determined. Several genes encoding proteins homologous to α- and β-subunits of heterotrimeric G-proteins have been cloned from a variety of plants including oat aleurone (for review, see Hooley, 1999). In plants, a range of highly homologous Gα-subunits have been cloned, as well as a novel 99-kD Gα termed “extra-large GTP-binding protein.” This protein contains an additional domain with homology to a bacterial proteins involved in energy transfer across the plasma membrane and also to zinc-finger proteins (Lee and Assmann, 1999). This raises the exciting possibility that there may be other novel plant-specific G-proteins with similar heterologous combinations of domains and presumably properties. G-proteins have been proposed to act in many different types of signaling processes (for review, see Assmann, 1996; Bischoff et al., 1999; Hooley, 1999), suggesting a diverse family of G-proteins may regulate a wide range of cellular responses in plants.
Gα has been immunologically localized to the plasma membrane, the site of the aleurone ABA-activated PLD, and also to the endoplasmic reticulum in a variety of tissues and species (for review, see Hooley, 1999). However, antisense-RGA1 plants (a rice Gα) have a dwarf phenotype, suggesting alterations in a GA3-response pathway (Fujisawa et al., 1999). In addition, a previously characterized dwarf mutant of rice has mutations in the Gα-subunit. This mutant has impaired response of its aleurone to GA3 (Mitsunaga et al., 1994). Jones et al. (1998) have shown Gα and Gβ expression in oat aleurone and provided evidence for G-protein action in the GA3 signaling system in this plant. Homann and Tester (1997) also provided evidence for a G-protein activity maintaining secretory vesicle fusion with the plasma membrane in the aleurone cell. Together these observations provide strong support for the involvement of G-proteins in GA3 signaling and the cellular machinery of secretion of the cereal aleurone. In contrast in our study, G-protein activity appears to be involved in ABA action. These results highlight the potential for multiple G-proteins and G-protein-regulated events in the cell. Results from studies on intact cells attempting to probe G-protein action must be interpreted in light of these possible multiple effects. Thus, for example, Jones et al. (1998) noted no effect of PTX on the GA3 response system of the aleurone, but our studies indicate it is likely affecting ABA responsiveness of these same cells. This complexity highlights the advantage of analyzing such in vivo responses in parallel with a defined in vitro system such as the reconstituted PLD/ABA activation system where the potential for multiple signaling events is reduced.
In summary, our results demonstrate that the kinetics of PLD activity stimulated by ABA in vitro is similar to that seen in vivo in aleurone cells. This stimulation requires membrane components and is likely to be localized at the plasma membrane. We have also shown that the stimulation of PLD by ABA most likely involves the activity of a G-protein and that this activity appears essential for ABA action in the aleurone cell. Recent data indicate ABA-regulated PLD activity in guard cells. There is also a wealth of data indicating G-protein involvement in guard cell signaling as well as a plasma membrane site for ABA action in these cells. Thus, it is tempting to speculate that a G-protein-activated PLD may be a conserved feature of ABA signaling in diverse plant cells. Defining the molecular identity of this G-protein signaling system is the challenge for future research.
MATERIALS AND METHODS
Plant Material and Chemicals
For protoplast preparation barley (Hordeum vulgare cv Himalaya; Department of Agronomy, Washington State University, Pullman) grains were de-embryonated, cut into quarters, and prepared for protoplast isolation as described by Hillmer et al. (1993). Freshly isolated protoplasts were used for microsomal protein extraction and PLD assays. For in vivo treatment of protoplasts with PTX (Sigma, St. Louis; PTX A protomer and B oligomer, Calbiochem-Novabiochem, La Jolla, CA) and CTX (Sigma), protoplasts were incubated in 10 mm CaCl2 and combinations of 1 μm GA3, and 10 μm ABA, PTX, or CTX as indicated. After 48 h of treatment secreted amylase activity was assayed as in Bush et al. (1986).
The fluorescent lipid, 1-acyl-2[-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino dodecanoyl]-sn-glycerol-3-phosphocholine (NBD-PC) (R1, 16:0 [62%, w/v], 18:0 [29%, w/v], 18:1 [5.5%, w/v]; R2, 12:0-N-NBD), was from Avanti Polar Lipids Inc. (Alabaster, AL) and was stored at −80°C. Prior to use the NBD-PC was dried under a stream of N2 and emulsified in protoplast incubation medium (for in vivo loading) or water (for in vitro assays) by sonication.
Measurement of in Vivo PtdOH Changes
Freshly released protoplasts (1.7 mL per sample of 0.5 × 106 protoplasts) were incubated with 25 μg/mL NBD-PC. Protoplasts were left to incubate for 2 h in the presence of 10 mm CaCl2. Then 10 μm ABA, 0.5 μg/μL PTX, or 0.5 μg/μL CTX was added as indicated for 10 min, after which the protoplasts were settled under 1g for 5 min. Five hundred microliters of settled protoplasts was processed for measurement of NBD-labeled PtdOH levels as described by Ritchie and Gilroy (1998b).
PLD Extraction and Assays
Freshly released protoplasts were settled for 5 min at 1g, all further procedures were carried out at 4°C or on ice unless otherwise stated. Four hundred microliters of media containing the settled protoplasts was added to 800 μL of extraction buffer (50 mm Tris [tris(hydroxymethyl)aminomethane]-acetate, 5 mm EDTA, 5 mm EGTA, pH 8.8, 1 mm dithiothreitol, and 10 μg/μL each of leupeptin, pepstatin, and aprotinin) and homogenized in a glass/Teflon homogenizer. The extract was centrifuged at 2,000g for 10 min, and the resulting supernatant was centrifuged at 100,000g for 45 min (TL-100 Ultracentrifuge, Beckman, Palo Alto, CA). The resulting microsomal pellet was resuspended in extraction buffer and the protein concentration determined by the method of Bradford (1976) using bovine serum albumin as a standard and a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Unless otherwise stated, the protein concentration was adjusted to 0.45 μg/μL using extraction buffer.
PLD assays were conducted as described by Ritchie and Gilroy (1998b) with the following modifications: a final volume of 40 μL contained 10 μL of microsomal protein, 50 mm MES (2-[N-morpholino]ethanesulfonic acid)/NaOH, pH 6.5, 25 mm CaCl2, 5 mm MgCl2, 55 μm SDS (unless otherwise indicated), 1.5% (v/v) ethanol, 150 μm NBD-PC. Ten micromolars ABA was added as indicated from a stock in ethanol. The final ethanol concentration was 0.1% (v/v), which was also added to the controls. The reaction was initiated by the addition of protein sample and incubated at room temperature for 10 min (or increments of 5 min for experiments described in Figs. 2 and 5) with shaking at 100 rpm. The reaction was stopped by the addition of 150 μL of chloroform:methanol (1:2 v/v). Chloroform (40 μL) and 2 m KCl (40 μL) was added, and the samples were processed for measurement of the NBD-phosphatidylethanol (NBD-P-ethanol) produced using thin layer chromatography as described by Ritchie and Gilroy (1998b). Since a non-saturating amount of ethanol was added into the PLD assays, some NBD-PtdOH was produced in addition to NBD-P-ethanol (approximately 30%). Levels of NBD-PtdOH varied in the same manner as NBD-P-ethanol (e.g. they increased in sample treated with ABA), however the levels were lower, and we chose to measure NBD-P-ethanol only.
Phase Partitioning and Membrane Marker Assays
Aleurone layers (1,000–2,000) were ground in liquid N2 and homogenized in a pestle and mortar in 100 mL of extraction buffer (as for protoplasts above, plus 0.1% [w/v] PVP). The extract was filtered through two layers of cheesecloth and centrifuged as described above for protoplasts to obtain a microsomal membrane fraction. The microsomal pellets were resuspended in 50 mm Tris-acetate, 0.5 mm EDTA, and 0.5 mm EGTA (resuspension buffer) and added to a phase partitioning mix of 36 g final weight as previously described (Larsson et al., 1987; Robbins et al., 1999). The phase mixture was inverted gently more than 20 times, incubated on ice for 10 min, and centrifuged at 1,000g for 5 min. The upper phase was removed and added to fresh lower phase, the mixing and centrifugation were repeated, and the second upper phase processed through a further third round of partitioning. The final upper phase was diluted >2-fold, and the first lower phase >10-fold with resuspension buffer. These diluted phases were centrifuged for 60 min at 90,000g (L5–50E Ultracentrifuge, Beckman), and the resulting membrane pellets were resuspended in the same buffer to give a protein concentration of 2 to 4 mg/mL. These fractions were further diluted to 0.65 mg/mL for use in PLD assays or used undiluted for marker enzyme assay.
Antimycin insensitive cytochrome C reductase activity was assayed using 10 μL of sample in a total volume of 600 μL containing 10 mm KH2PO4, pH 7.3, 25 μm cytochrome C, 250 μm NADH, 12.5 mm KCN, and 1 μm antimycin as described by Briskin et al. (1987). Vanadate-sensitive and -insensitive ATPase activities were assayed using 10 μL of sample in a volume of 120 μL of assay mix containing 330 mm Suc, 50 mm MES-KOH, pH 6.5, 3 mm ATP, 0.02% (v/v) Triton X-100, 4 mm MgSO4, 50 mm KNO3, with or without 1 mm NaN3 and 0.1 mm Na6 Mo7O24, or 0.1 mm NaVO4. Latent IDPase activity was assayed as described by Briskin et al. (1987), using 10 μL of sample in a 200-μL reaction volume. Latent activity was released by the addition of 0.05% (v/v) Triton X-100 (Ray et al., 1969) or after storage of samples for 5 d at 4°C. The degree of latency was similar using either method. In both ATPase and IDPase assays, inorganic phosphate release was assayed using the method of LeBel et al. (1978).
ACKNOWLEDGMENTS
The authors would like to thank Dr. Xi-Qing Wang and Scott MacCleery for critical reading of the manuscript.
Footnotes
This work was supported by a grant from the U.S. Department of Agriculture (to S.G.).
LITERATURE CITED
- Assmann S. Guard cell G protein. Trends Plant Sci. 1996;1:73–74. [Google Scholar]
- Bethke PC, Schuurink R, Jones RL. Hormonal signaling in cereal aleurone. J Exp Bot. 1997;48:1337–1356. [Google Scholar]
- Bischoff F, Molendijk A, Rajendrakumar CS, Palme K. GTP-binding proteins in plants. Cell Mol Life Sci. 1999;55:233–256. doi: 10.1007/s000180050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of micrograms of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brian PW. The effects of some microbial metabolic products on plant growth. Symp Soc Exp Biol. 1957;11:166–182. [PubMed] [Google Scholar]
- Briskin DP, Leonard RT, Hodges TK. Isolation of the plasma membrane: membrane markers and general principles. Methods Enzymol. 1987;148:542–558. [Google Scholar]
- Bush DS, Cornejo MJ, Huang CM, Jones RL. Ca2+-stimulated secretion of α-amylase during development of barley aleurone protoplasts. Plant Physiol. 1986;82:566–574. doi: 10.1104/pp.82.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrije T, Munnik T. Activation of phospholipase D by calmodulin antagonists and mastoparan in carnation petals. J Exp Bot. 1997;48:1631–1637. [Google Scholar]
- Fan L, Zheng S, Wang X. Antisense suppression of phospholipase Dα retards abscisic acid- and ethylene-promoted senescence of post-harvest arabidopsis leaves. Plant Cell. 1997;9:2183–2196. doi: 10.1105/tpc.9.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W, Munnik T, Kerkmann K, Salamini F, Bartels D. Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell. 2000;12:111–124. doi: 10.1105/tpc.12.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Asahi T, Iwasaki Y. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Natl Acad Sci USA. 1999;96:7575–7580. doi: 10.1073/pnas.96.13.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Jones RL. Perception of gibberellin and abscisic acid at the external face of the plasma membrane of barley (Hordeum vulgare L.) aleurone protoplasts. Plant Physiol. 1994;104:1185–1192. doi: 10.1104/pp.104.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho T-HD, Walker-Simmons MK. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic-acid-suppressed gene expression in barley aleurone layers. Proc Natl Acad Sci USA. 1999;96:1767–1772. doi: 10.1073/pnas.96.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeser D, Neubig RK. Methods for the study of G-protein interactions. In: Milligan G, editor. Signal Transduction, A Practical Approach. Oxford: IRL Press; 1992. pp. 1–30. [Google Scholar]
- Harberd NP, King KE, Carol P, Cowling RJ, Peng J, Richards DE. Gibberellin: inhibitor of an inhibitor of? BioEssays. 1998;20:1001–1008. doi: 10.1002/(SICI)1521-1878(199812)20:12<1001::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Heimovaara-Dijkstra S, Heistek JC, Wang M. Counteractive effects of ABA and GA3 on extracellular and intracellular pH and malate in barley aleurone. Plant Physiol. 1994a;106:359–365. doi: 10.1104/pp.106.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovaara-Dijkstra S, Vanduijn B, Libbenga KR, Heidekamp F, Wang M. Abscisic acid-induced membrane potential changes in barley aleurone protoplasts: a possible relevance for the regulation of Rab gene expression. Plant Cell Physiol. 1994b;35:743–750. [Google Scholar]
- Hillmer S, Gilroy S, Jones RL. Visualization of secretion from single barley aleurone protoplasts. Plant Physiol. 1993;102:279–286. doi: 10.1104/pp.102.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland LM. Solubilization of native membrane proteins. Methods Enzymol. 1990;182:253–264. doi: 10.1016/0076-6879(90)82021-s. [DOI] [PubMed] [Google Scholar]
- Homann U, Tester M. Ca2+-independent and Ca2+-GTP-binding protein-controlled exocytosis in a plant cell. Proc Natl Acad Sci USA. 1997;94:6565–6570. doi: 10.1073/pnas.94.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley R. A role for G proteins in plant hormone signaling? Plant Physiol Biochem. 1999;37:393–402. [Google Scholar]
- Hooley R, Beale MH, Smith SJ. Gibberellin perception at the plasma membrane of Avena fatua aleurone protoplasts. Planta. 1991;183:274–280. doi: 10.1007/BF00197799. [DOI] [PubMed] [Google Scholar]
- Jacob T, Ritchie S, Assmann SM, Gilroy S. ABA signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HD, Smith SJ, Desikan R, Plakidou-Dymock S, Lovegrove A, Hooley R. Heterotrimeric G proteins are implicated in gibberellin induction of α-amylase gene expression in wild oat aleurone. Plant Cell. 1998;10:245–253. doi: 10.1105/tpc.10.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knetsch MLW, Wang M, Snaar-Jagalska BE, Heimovaara-Dijkstra S. Abscisic acid induces mitogen-activated protein kinase activation in barley aleurone protoplasts. Plant Cell. 1996;8:1061–1067. doi: 10.1105/tpc.8.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C, Widell S, Kjellbom P. Preparation of high-purity plasma membranes. Methods Enzymol. 1987;148:558–568. [Google Scholar]
- LeBel D, Poirier GG, Beaudoin AR. A convenient method for the ATPase assay. Anal Biochem. 1978;85:86–89. doi: 10.1016/0003-2697(78)90277-4. [DOI] [PubMed] [Google Scholar]
- Lee Y-RJ, Assmann SM. Arabidopsis thaliana “extra-large GTP-binding protein” (AtXLG1): a new class of G-protein. Plant Mol Biol. 1999;40:55–64. doi: 10.1023/a:1026483823176. [DOI] [PubMed] [Google Scholar]
- Locht C, Antoine R. A proposed mechanism of ADP-ribosylation catalyzed by the pertussis toxin S1 subunit. Biochimie. 1995;77:333–340. doi: 10.1016/0300-9084(96)88143-0. [DOI] [PubMed] [Google Scholar]
- Ma H, Weiss CA. In vitro analysis of G-protein functions. Methods Cell Biol. 1995;49:471–485. doi: 10.1016/s0091-679x(08)61474-0. [DOI] [PubMed] [Google Scholar]
- Maarouf HE, Zuily-Fodil Y, Gareil M, d'Arcy-Lameta A, Pham-Thi AT. Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp.: differing in drought tolerance. Plant Mol Biol. 1999;39:1257–1265. doi: 10.1023/a:1006165919928. [DOI] [PubMed] [Google Scholar]
- McFadzean I, Brown DA. Electrophysiology applied to G-protein function. In: Milligan G, editor. Signal Transduction, A Practical Approach. Oxford: IRL Press; 1992. pp. 167–179. [Google Scholar]
- Mitsunaga S, Tashiro T, Yamaguchi J. Identification and characterization of gibberellin-insensitive mutants selected from among dwarf mutants of rice. Theor Appl Genet. 1994;87:705–712. doi: 10.1007/BF00222896. [DOI] [PubMed] [Google Scholar]
- Munnik T, Arisz SA, de Vrije T, Musgrave A. G protein activation stimulates phospholipase D signaling in plants. Plant Cell. 1995;7:2197–2210. doi: 10.1105/tpc.7.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Himbergen JAJ, ter Riet B, Braun FJ, Irvine RF, van den Ende H, Musgrave A. Detailed analysis of the turnover of polyphosphoinositides and phosphatidic acid upon activation of phospholipase C and D in Chlamydomonas cells treated with non-permeabilizing concentrations of mastoparan. Planta. 1998;207:133–145. [Google Scholar]
- Ray PM, Shininger TL, Ray MM. Isolation of B-glucan synthetase particles from plant cells and identification with Golgi membranes. Proc Natl Acad Sci USA. 1969;64:605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S. Gibberellins: regulating genes and germination. New Phytol. 1998a;140:363–383. doi: 10.1111/j.1469-8137.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S. Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1998b;95:2697–2702. doi: 10.1073/pnas.95.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S, McCubbin A, Ambrose G, Kao T-H, Gilroy S. The sensitivity of barley aleurone tissue to gibberellin is heterogenous and may be spatially determined. Plant Physiol. 1999;10:361–370. doi: 10.1104/pp.120.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins KM, Bhuvarahamurthy N, Pliska-Matyshak G, Murthy PPN. The isolation and characterization of right-side-out plasma membrane vesicles from barley aleurone cells. Lipids. 1999;34:75–82. doi: 10.1007/s11745-999-340-5. [DOI] [PubMed] [Google Scholar]
- Ryu SB, Wang X. Expression of phospholipase D during castor bean senescence. Plant Physiol. 1995;108:713–719. doi: 10.1104/pp.108.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer GFE, Andre B. Stimulation of phospholipase A2 by auxin in microsomes from suspension-cultured soybean cells is receptor-mediated and influenced by nucleotides. Planta. 1993;191:515–523. [Google Scholar]
- van der Meulen RM, Heidekamp F, Jarstorff B, Horgan R, Wang M. Effects of abscisic acid analogues on abscisic acid-induced gene expression in barley aleurone protoplasts: relationship between structure and function of the abscisic acid molecule. J Plant Growth Reg. 1993;12:13–19. [Google Scholar]
- van der Veen R, Heimovaara-Dijkstra S, Wang M. Cytosolic alkalinization mediated by abscisic acid is necessary, but not sufficient, for abscisic acid-induced gene expression in barley aleurone protoplasts. Plant Physiol. 1992;100:699–705. doi: 10.1104/pp.100.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser K, Kijne JW, Wang M. GTP-binding activity of membrane proteins in isolated barley embryo is enhanced by abscisic acid. Plant Sci. 1999;148:139–145. [Google Scholar]
- Wang X. The role of phospholipase D in signaling cascades. Plant Physiol. 1999;120:645–651. doi: 10.1104/pp.120.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Sedee NJA, Heidekamp F, Snaar-Jagalska BE. Detection of GTP-binding proteins in barley aleurone protoplasts. FEBS Lett. 1993;329:245–248. doi: 10.1016/0014-5793(93)80230-r. [DOI] [PubMed] [Google Scholar]