Abstract
Background
Many plant original foods have been shown beneficial effects in humans. In the previous work, we have developed a compound capsule which contains major constituents of walnut oil and grape seed extract.
Objective
To investigate the antioxidant effects of the Compound Walnut Oil Capsule (WOC) in aging model induced by D-gal.
Design
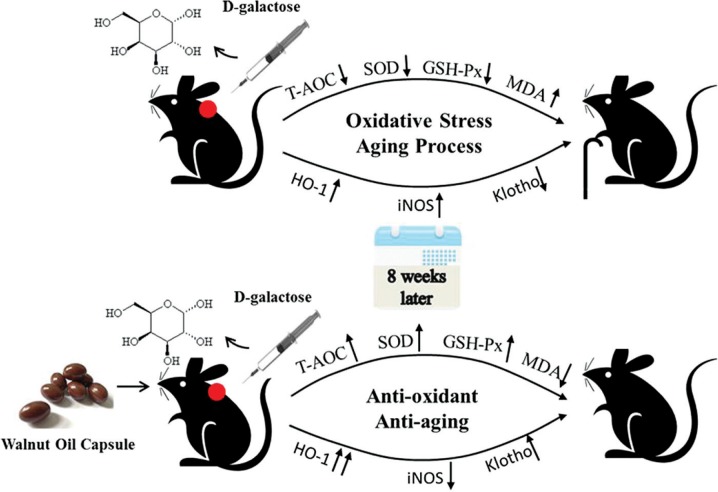
70 C57BL/6J mice were randomly divided into seven groups. Mice in normal group received daily subcutaneous injection of saline while the control group, WOC groups, Vitamin C (VC) group and pure walnut oil group received daily subcutaneous injection of D-galactose (D-gal) for 8 weeks. Total antioxidant capacity (T-AOC), super dismutase (SOD), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) in serum, liver and brain were determined. The expression of Heme Oxygenase (HO-1), iNOS and Klotho in liver and brain were obtained.
Results
WOC could improve the pathologic lesions caused by oxidative stress and significantly enhance the T-AOC, increase the activities of SOD, GSH-Px and decrease the contents of MDA in serum, liver and brain. Also, the WOC could obviously up-regulate the expression of HO-1 and Klotho and down-regulate the expression of iNOS.
Conclusion
WOC can be used as an anti-aging food for its effectively eliminating free radicals, enhancing the antioxidant capacity and alleviating the damages of oxidative stress.
Keywords: walnut oil capsule, oxidative stress, antioxidant, D-galactose, aging model
Aging is a complex natural process closely related to oxidative stress and free radicals, which has become a hot topic nowadays (1, 2). Accumulation of free radicals can affect the functions and abilities of human body such as lung, heart, and brain (3, 4). It is important to select appropriate scavengers to protect body from the damage of free radicals and improve the quality of life.
Natural drugs for their low toxicity and low side-effects are widely used in the field of medicine. It is reported that many plant original foods and medicines have potential antioxidant capacity in vitro and in vivo, such as Asparagus cochinchinensis (Lour.), (5) Trollius chinensis Bunge (6), and Cinnamomum verum (7). In the research, we have developed a compound capsule which contains major constituents of walnut oil and grape seed extract. Walnut oil is rich in linolenic acid and linoleic acid; previous studies showed that it has several health benefits including anti-inflammatory and antioxidant effects (8, 9). Many researches indicated that the main ingredients of grape seed extract are anthocyanin and procyanidin, which have strong anti-inflammatory and anti-lipid peroxidation effects (10–12).
Nowadays, the aging model induced by D-galactose (D-gal) is widely used in the field of oxidative stress and antioxidant research for its easy operation, cheapness, and short periods, which was first reported by Gong et al. in 1997 (13). Long-time injection of high dosage of D-gal can cause massive production of free radicals, neurotoxicity, tissue injury, and inflammation, followed by senescence (14, 15). The inbred C57BL/6J mice were widely used in the brain aging research for its lower growth rate and smaller individual difference (16). In our work, we used walnut oil capsule (WOC) to investigate its antioxidant effects in C57BL/6J mice aging model established by D-gal. Not only did we detect the activities of oxidative stress–related enzymes but also discussed the expression of some free radicals and aging-related enzymes at the molecular level. The histopathologic examination was also observed to see the effects of WOC on liver and brain. The research aimed to develop the WOC as an antioxidant health food for elderly people.
Materials and methods
Drugs and reagents
WOC was prepared by us according to designed prescription and the China patent (No:201410564056.x). Pure walnut oil was purchased from Valder Fields CO., Ltd (Yuxi, China), and Vitamin C (VC) from Solarbio Science & Technology CO., Ltd (Beijing, China). D-gal was purchased from Sigma-Aldrich (St Louis, MO, USA). 1,1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from TCI (Shanghai) Development Co., Ltd (Shanghai, China). Total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) and malondialdehyde (MDA) kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). BCA protein assay kit and SDS-PAGE kits were purchased from Multi Sciences Biotech CO., Ltd (Hangzhou, China). Anti-Heme Oxygenase-1 (HO-1) antibody and anti-Klotho antibody were purchased from Abcam (Shanghai, China). Anti-iNOS antibody and anti-β-actin antibody were purchased from cell signaling technology (Danvers, USA).
Measurement of radical scavenging in vitro
DPPH free radicals scavenging test has been effectively used to evaluate the free radical scavenging capacity of antioxidant drugs in vitro (17). Briefly, 20 μL of sample was quickly mixed with 180 μL 0.1 mmol/L DPPH and placed in the dark for 0 min, 10 min, 20 min, and 30 min. Then the absorbance at 517 nm was measured. Absolute alcohol was used as negative control. The DPPH scavenging rate was determined using K% = [1-(Ai–Aj)/Ao] × 100% (Ai, the absorbance of sample mixed with DPPH; Aj, the absorbance of sample mixed with absolute alcohol; Ao, the absorbance of negative control).
Treatment of animals
Seventy 3-month-old C57BL/6J male mice, weighing about 20 ± 2 g, were obtained from CAVENS Laboratory Animal Co., Ltd (Changzhou, China; certification No. SCXK (Su) 2016-0010). The mice were housed in an SPF environment at 25°C and 60% relative humidity under 12-/12-h dark/light cycle with free access to food and water. The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China. After a week of acclimation, the mice were randomly divided into seven groups (10 mice/group), including a normal group; an aging model group as control; WOC low, medium, and high groups; a VC group; and a pure walnut oil group as positive control. For the control group, WOC groups, positive control groups, all mice received daily subcutaneous injection of 1,000 mg/kg D-gal for 8 weeks. For mice in normal group, 0.2 mL physiological saline was injected for 8 weeks. From the second week, mice in the WOC, positive control groups received daily intragastric administration of WOC at doses of 6 mL/kg, 12 mL/kg, 18 mL/kg; VC at a dose of 200 mg/kg; pure walnut oil at a dose of 12 mL/kg, while the normal and control groups received intragastric administration of distilled water at a dose of 12 mL/kg.Twenty-four hours after final administration, the mice were anaesthetized by intraperitoneal injection of 10 mL/kg 5% chloral hydrate; 0.5 mL blood samples were extracted from peri-orbital sinus and the serum was collected after being centrifuged at 3,000 r/min for 10 min. Mice were sacrificed, followed by rapid isolation of liver and brain.
Histomorphological observation of liver and brain
Tissues were fixed in 10% formalin at room temperature for 48 h, then desiccated and embedded in paraffin. After that, tissues were sliced into 5 μm slices for hematoxylin-eosin (H&E) staining. Specimens were scanned and pathological changes were observed using Case Viewer 2.0 (3D HISTECH, Ltd, Budapest, Hungary).
Western blotting
Western blot analysis was performed as described previously (18). Briefly, tissues were lysed into homogenate using RIPA lysis buffer and PMSF, and total proteins were separated by SDS-PAGE and then transferred to PVDF membranes at 300 mA for 1 h. After being transferred, the membranes were placed in blocking buffer (5% non-fat milk in Tris-buffered saline containing Tween-20 [TBST]) for 1 h, and the blots were incubated with an appropriate primary antibody (anti-Klotho antibody [1:1,000], anti-HO-1 antibody [1:20,000], anti-iNOS antibody [1:1,000], anti-β-actin antibody [1:1,000]) at 4°C overnight, and then treated with secondary antibody (1:5,000) at room temperature for 1 h. Then the chemiluminescent indicator was applied to membranes and specific proteins were detected by fluorchem device.
Statistical analysis
All data were expressed as mean ± SD and analyzed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The significance of difference among groups was tested by one-way analysis of variance (ANOVA) test, and intergroup differences were compared using least-significant difference t-test.
Results
In vitro radical scavenging of WOC
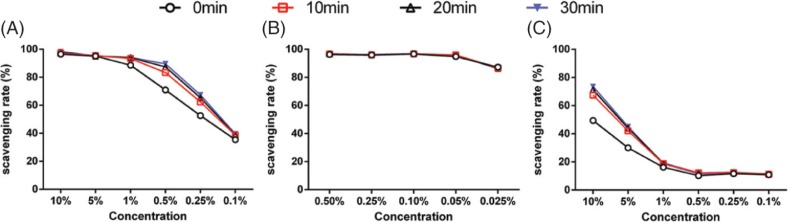
The scavenging rate of WOC and pure walnut showed concentration dependence and time dependence. The WOC results showed that when the concentration was higher than 1%, the changes of scavenging rate was not obvious with the decrease of concentration, while when the concentration was lower than 1%, the scavenging rate decreased obviously. On the contrary, when the concentration was above 1%, pure walnut oil showed that the scavenging rate decreased sharply with the decrease of concentration, but it flattened out below 1%. As regards the result of VC, there was no obvious concentration dependence and time independence. Instead, the scavenging rate remained almost unchanged with the decrease in concentration, and as time went on, the scavenging rate was almost constant, which indicated the scavenging rate of VC reached the maximum in a short time after reaction. In other words, the DPPH scavenging rate followed VC > WOC > pure walnut oil in vitro (Fig. 1).
Fig. 1.
DPPH scavenging rates of WOC, VC, and pure walnut oil at different time points. (A) WOC; (B) VC; (C) Pure walnut oil.
General condition of mice
Mice were weighed daily and the weight changes were recorded in order to observe the effects of different drugs on mice body weight. Result showed that there were no significant differences among groups in body weight both on the first day and the last day (Table 1). But the growth rate in the normal group was higher than that in other groups (p < 0.05 or p < 0.01). It indicated that not only D-gal but also WOC, VC, and pure walnut oil could affect body weight in mice.
Table 1.
The weight changes in mice
| Group | First day (g) | Last day (g) | Growth rate (%) |
|---|---|---|---|
| Normal | 25.21 ± 1.28 | 30.07 ± 1.35 | 19.80 ± 2.94 |
| Control | 25.53 ± 1.07 | 28.74 ± 1.81 | 12.51 ± 3.10** |
| WOC low | 25.72 ± 1.19 | 29.49 ± 1.67 | 14.68 ± 4.18* |
| WOC medium | 24.88 ± 1.61 | 28.43 ± 1.52 | 14.43 ± 4.68* |
| WOC high | 25.19 ± 1.04 | 28.39 ± 1.70 | 12.44 ± 3.47** |
| VC | 25.03 ± 1.76 | 28.57 ± 2.79 | 14.13 ± 7.81* |
| Pure walnut oil | 25.12 ± 1.21 | 28.35 ± 1.90 | 12.78 ± 2.13* |
The body weight change in C57BL/6J mice on the first and last day.
p < 0.05
p < 0.01 versus normal group (n = 10 in each group).
WOC, walnut oil capsule; VC, Vitamin C.
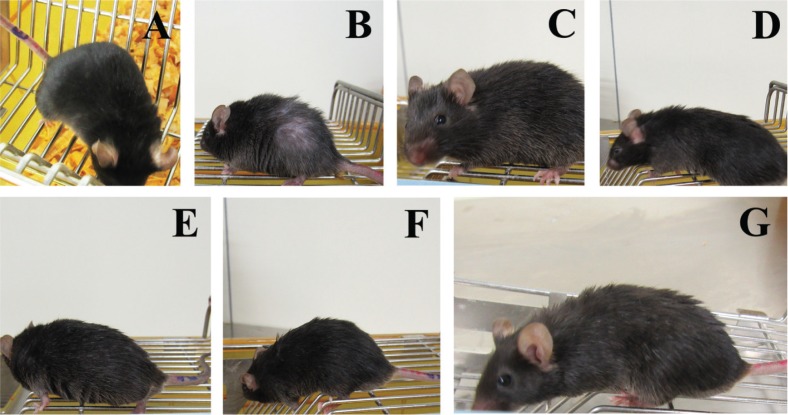
There were also differences in appearance among different groups (Fig. 2). Mice in the normal group had smooth hair and active spirit (A), but in the control group, mice were curled up and had coarse hair, severe hair loss, and poor spirit (B). Mice treated with WOC, VC, and pure walnut oil exhibited improvements in their hair color and spirit (C D E F G).
Fig. 2.
The appearance changes in different groups. (A) Normal group; (B) Control group; (C) WOC low group; (D) WOC medium group; (E) WOC high group; (F) VC group; (G) Pure walnut oil.
The effects of WOC on histological changes in liver and brain
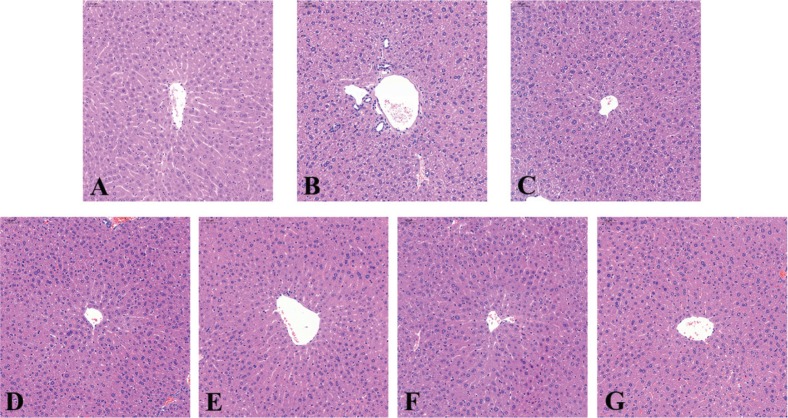
The pathological changes in liver were shown in Fig. 3. In the normal group, the shape of cell nucleus was large and round. Hepatic cords were neatly arranged and there were no cell necrosis and degeneration. There were spotty necrosis, hydropic degeneration, vacuolar degeneration and lymphocytic infiltration in the control group. And the hepatic cords were disarranged. Mice treated with WOC, VC, and pure walnut oil had exhibited improvements in these situations. And as the dose of WOC increased, the improvements got better.
Fig. 3.
Pathological changes in liver. (A) Normal group; (B) Control group; (C) WOC low group; (D) WOC medium group; (E) WOC high group; (F) VC group; (G) Pure walnut oil.
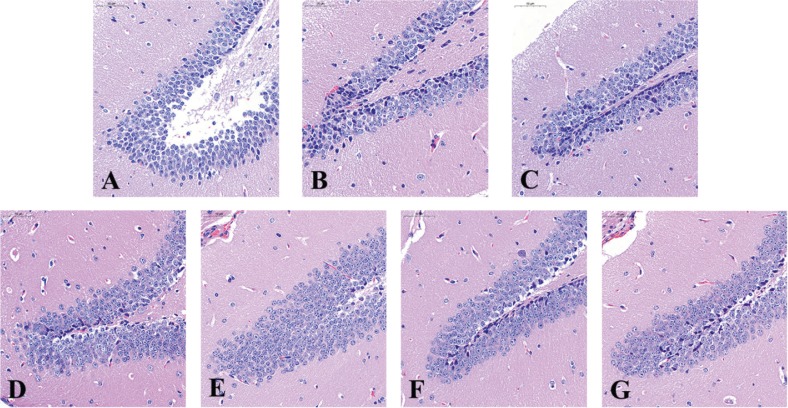
The pathological changes in hippocampus dentate gyrus were observed (Fig. 4). Compared with the normal group, the granular cells in the control group were smaller, fewer, and disarranged. Furthermore, cells with hyperchromatism, changes of nuclear shape, and karyopyknosis were also observed, which indicated cell aging (19, 20). Groups treated with WOC, VC, and pure walnut oil showed improvements in the cells number and arrangement.
Fig. 4.
Pathological changes in brain (H&E staining, ×500). (A) Normal group; (B) Control group; (C) WOC low group; (D) WOC medium group; (E) WOC high group; (F) VC group; (G) Pure walnut oil.
The effects of WOC on T-AOC, SOD, GSH-Px, and MDA
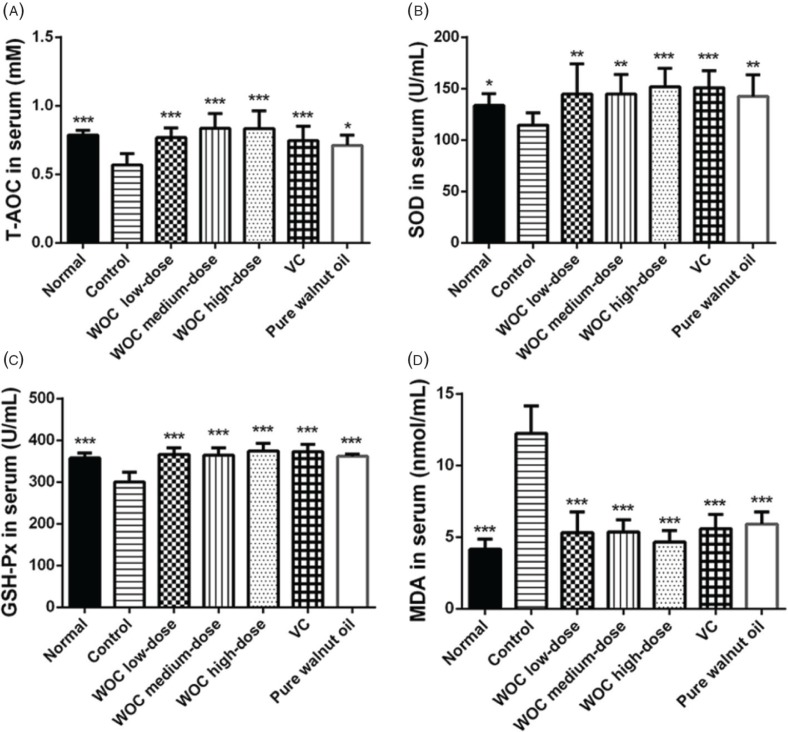
In the result of serum (Fig. 5), decreased levels of T-AOC, SOD, and GSH-Px, and increased contents of MDA were found in the control group compared with the normal group (p < 0.05 or p < 0.001). Mice treated with WOC, VC, and pure walnut oil showed improvements in T-AOC, SOD, GSH-Px, and MDA compared with the control group (p < 0.05 or p < 0.01 or p < 0.001), and there were significant differences between WOC high group and pure walnut oil group (p < 0.05) in MDA result. But there were no significant differences found among WOC, VC, and pure walnut oil groups in other results.
Fig. 5.
Biochemical indicators in serum. (A) Effects of WOC on T-AOC; (B) Effects of WOC on the activities of SOD; (C) Effects of WOC on the activities of GSH-Px; (D) Effects of WOC on the contents of MDA. *p < 0.05, **p < 0.01,*p < 0.001 versus the control group.
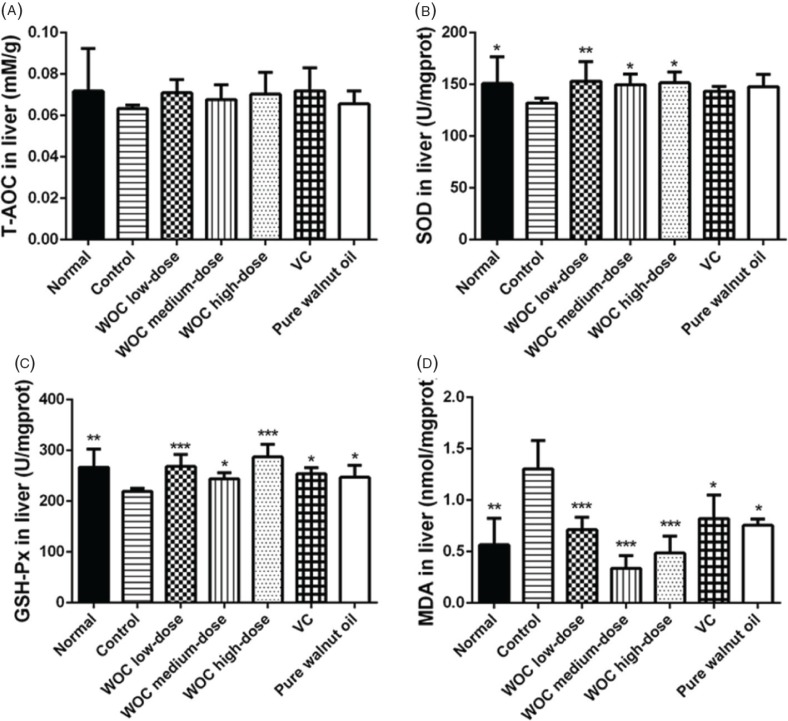
As shown in the result of liver (Fig. 6), there were no significant differences among groups in T-AOC. In WOC groups, the SOD and GSH-Px were significantly higher than the control group and the MDA lower than that in the control group (p < 0.05 or p < 0.01 or p < 0.001). There were also significant differences between the control group and the positive control groups in the results of GSH-Px and MDA (p < 0.05) but not in the SOD result. Moreover, the improvement levels of a high dose of WOC was higher than VC and pure walnut oil in GSH-Px (p < 0.05), also the MDA level in WOC medium group was lower than positive control groups (p < 0.05 or p < 0.01).
Fig. 6.
Biochemical indicators in liver. (A) Effects of WOC on T-AOC; (B) Effects of WOC on the activities of SOD; (C) Effects of WOC on the activities of GSH-Px; (D) Effects of WOC on the contents of MDA. *p < 0.05, **p < 0.01,*p < 0.001 versus the control group.
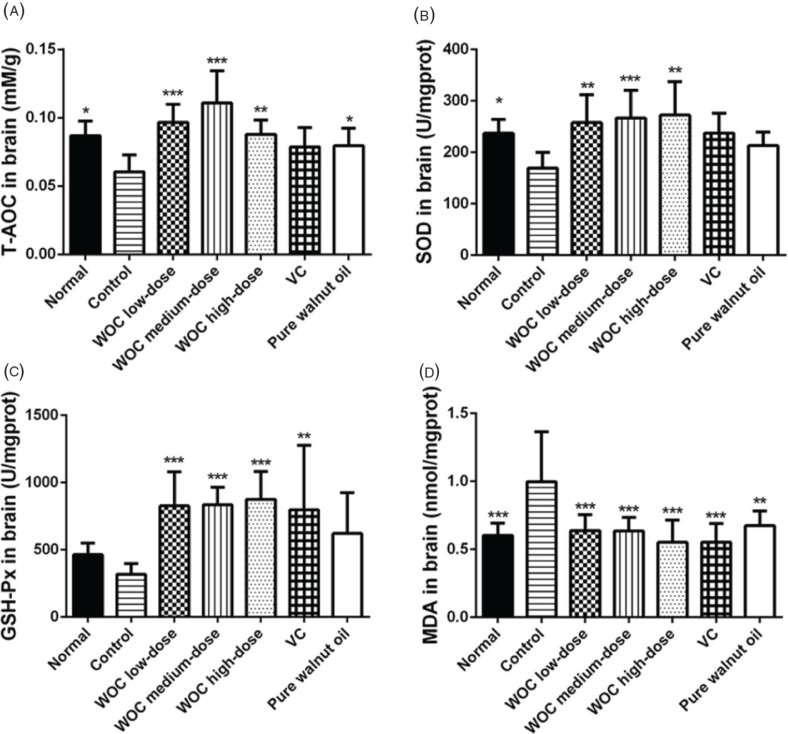
For the result of brain (Fig. 7), the T-AOC levels and the activities of SOD and GSH-Px in WOC groups were significantly higher than that in the control group. The contents of MDA decreased significantly in WOC groups compared with the control group (p < 0.01 or p < 0.001). Moreover, the T-AOC levels in the WOC medium group were significantly higher than that in the positive control groups (p < 0.001).
Fig. 7.
Biochemical indicators in brain. (A) Effects of WOC on T-AOC; (B) Effects of WOC on the activities of SOD; (C) Effects of WOC on the activities of GSH-Px; (D) Effects of WOC on the contents of MDA. *p < 0.05, **p < 0.01,*p < 0.001 versus the control group.
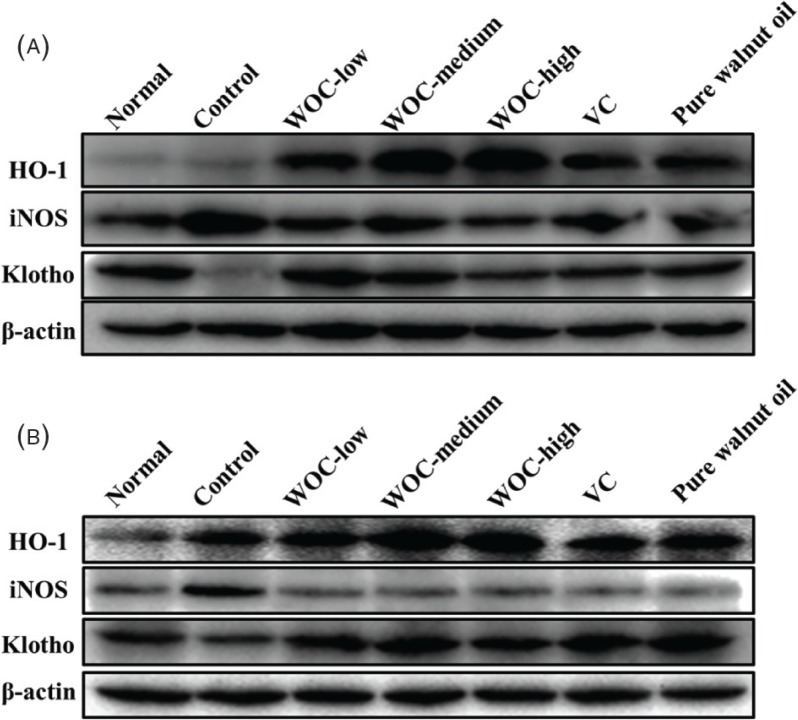
Expression of HO-1, iNOS, and Klotho in liver and brain
In the results of western blotting (Fig. 8), the expression of HO-1 in D-gal-induced aging mice was significantly increased compared with the normal group. Mice treated with WOC, VC, and pure walnut oil all exhibited the higher upregulation of HO-1 than the control group and the enhancements of WOC were higher than VC and pure walnut oil. iNOS found high expression in the control group. WOC, VC, and pure walnut oil could obviously downregulate the expression of iNOS both in liver and brain. Mice in the control group showed extremely low expression of Klotho, but WOC, VC, and pure walnut oil differently upregulated the expression of Klotho in liver and brain.
Fig. 8.
Western blotting assays of HO-1, iNOS, and Klotho. (A) The expression of HO-1, iNOS, and Klotho in liver; (B) The expression of HO-1, iNOS, and Klotho in brain.
Discussion
Aging is an inevitable process which can lead to oxidative stress and affect the function of kidney, brain, liver, and so on (21–24). In recent years, discovery of many antioxidant ingredients has made it possible to delay senescence, and finding effective anti-aging foods or drugs has become a hot topic. Many plants and plant extracts are rich in bioactive constituents and have been widely used in pharmaceutical application and research; thus, they are potential in the screening of antioxidant drugs. In this study, we investigated the compound capsule of walnut oil and grape seed extract to verify its antioxidant capacity and lay basis for its daily use by elderly people.
Generally, in this study, we investigated the antioxidant effects of WOC in vitro and in vivo. The DPPH scavenging test was selected to explore the antioxidant capacity of WOC in vitro. The biochemical indicators such as T-AOC, SOD, GSH-Px and MDA in serum, liver and brain were detected to evaluate the antioxidant of WOC in vivo. Also, the western blotting assays were employed to detect some proteins which were closely related to oxidative stress and aging. Moreover, the histopathologic examination was observed to discuss the effects of WOC on tissues.
The DPPH scavenging test showed that the scavenging rate of VC was much higher than WOC, but it was the opposite in in vivo research.The reason might be that the instability of VC led to the low bioavailability in vivo. Instead, the bioactive ingredients of WOC might have higher bioavailability.
The dose of D-gal covered a wide range wherein the minimal dose could be 50 mg/kg (25), while the higher dose could be 1,250 mg/kg (26). The modeling period usually ranges from 6 to 8 weeks. In this research, the aging model was established by 1,000 mg/kg D-gal for 8 weeks. Different indicators were selected to evaluate the antioxidant effects of WOC in vivo. T-AOC could reflect the T-AOC of the body. The activity of SOD and GSH-Px and the content of MDA are closely related to aging process and oxidative stress (27–29). Enzymes of SOD and GSH-Px are important antioxidases with strong ability of eliminating free radicals (27, 30). The MDA can reflect the extent of lipid peroxidation and attacks from free radicals to tissues and cells (31). In the results, WOC could obviously increase the levels of T-AOC and the activities of SOD and GSH-Px. It could also decrease the contents of MDA.
HO-1 is an oxidative, stress-induced enzyme belonging to the HO family, which has strong antioxidant capacity (32, 33). Normally, the expression of HO-1 is low in normal organs, such as brain, and high only in the spleen and liver(34). However, the expression of HO-1 will be upregulated under the condition of oxidative stress (35). In the results, the expression of HO-1 in the aging model group was higher than the normal group. WOC, VC, and pure walnut could obviously upregulate the expression of HO-1 and thus enhance the antioxidant capacity.
iNOS belongs to a family of enzymes called nitric oxide synthase (NOS), which is closely related to the production of nitric oxide (NO) (36). NO plays contrasting roles in living organism. It is an important host defense effector in the immune system. It is also a free radical that plays an important role in pathological processes, especially in inflammatory disorders (37–39). In many systems, the oxidative stress could increase cytokine-mediated iNOS expression (40), which is accompanied by increasing production of NO, and the NO could react with superoxide to produce the strong oxidant peroxynitrite, which in turn can increase lipid peroxidation (41). In our results, WOC could alleviate the oxidative stress by obviously decreasing the expression of iNOS.
Klotho is an anti-aging gene that was first discovered in mice by Kuro-o et al. in 1997. Deficiency of Klotho leads to a syndrome resembling aging, including a short lifespan; stunted growth and kyphosis; vascular calcification and atherosclerosis; osteoporosis; pulmonary emphysema; cognitive impairment; deafness; and atrophy of skin, muscles, gonads, and many other organs (42). Conversely, an overexpression of klotho could extend life span (43). Moreover, studies also indicated that Klotho worked as an important factor in the regulation of oxidative stress, cell proliferation, and apoptosis (44, 45). Our results showed that WOC could upregulate the expression of Klotho and protect against the damages of oxidative stress.
All results showed WOC could enhance the antioxidant capacity of the body, but the improvements did not exhibit a dose-dependent behavior. We inferred that there might be two reasons: (1) The dose gradient we designed was small and so there were no obvious differences among WOC groups; (2) The improvements reached a maximum at a low dose of WOC, but the effects did not enhance with an increase in dose. The expression of proteins HO-1, iNOS, and Klotho were related to many factors and could be regulated through different pathways (41, 46–47). So the mechanism of how WOC regulates the expression of these proteins needs further studies. And, the data of weight changes in mice showed that the growth rate in the WOC group was lower than that in the normal group, leading to the inference that WOC might have a weight-loss function.
Conclusion
In conclusion, WOC could improve the antioxidant capacity in aging mice induced by D-gal through increasing the activities of antioxidant enzymes, decreasing the contents of MDA, and regulating the expression of oxidative stress–related proteins. Our study suggests that WOC can be used as a promising anti-aging and weight-loss food.
Authors’ contributions
Huandong, Zhao, postgraduate student of Xiangya Hospital, majored in Biomedical Engineering; Jian, Li, professor of surgery in Xiangya hospital; Juan, Zhao, postgraduate student of Xiangya hospital, majored in Clinical Laboratory Diagnostics; Yang, Chen, postgraduate student of Xiangya hospital, majored in Biomedical Engineering; Caiping, Ren, professor of Oncology in School of Basic Medical Science, Central South University; Yuxiang, Chen, professor of pharmacy in School of Pharmaceutical Sciences, Central South University.
Conflict of interest and funding
All authors declared no conflict of interest.
Funding
This work was supported by the Development and Research of Compound Walnut Oil Soft Capsule (2014.10–2018.09), National Basic Research Program of China (2010CB833605), and Hunan Provincial Science Department Program (2014FJ6006).
References
- 1.Dillin A, Gottschling DE, Nystrom T. The good and the bad of being connected: the integrons of aging. Curr Opin Cell Biol 2014; 26: 107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E, Dattilo M, Macut D, Duntas L, Gonos E, Goulis DG, et al. Mechanisms in endocrinology: aging and anti-aging: a combo-endocrinology overview. Eur J Endocrinol 2017; 176: R283–308. [DOI] [PubMed] [Google Scholar]
- 3.Forman HJ. Redox signaling: an evolution from free radicals to aging. Free Radic Biol Med 2016; 97: 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jyoti A, Mishra N, Dhas Y. Ageing: consequences of excessive free radicals and inflammation. Curr Sci India 2016; 111: 1787–93. [Google Scholar]
- 5.Lei L, Ou L, Yu X.. The antioxidant effect of Asparagus cochinchinensis (Lour.) Merr. shoot in D-galactose induced mice aging model and in vitro. J Chin Med Assoc 2016; 79: 205–11. [DOI] [PubMed] [Google Scholar]
- 6.An F, Yang GD, Tian JM, Wang SH. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen Res 2012; 7: 2565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol 2006; 44: 198–206. [DOI] [PubMed] [Google Scholar]
- 8.Laubertova L, Konarikova K, Gbelcova H, Ďuračková Z, Žitňanová I. Effect of walnut oil on hyperglycemia-induced oxidative stress and pro-inflammatory cytokines production. Eur J Nutr 2015; 54: 291–9. [DOI] [PubMed] [Google Scholar]
- 9.Arranz S, Perez-Jimenez J, Saura-Calixto F. Antioxidant capacity of walnut (Juglans regia L.): contribution of oil and defatted matter. Eur Food Res Technol 2008; 227: 425–31. [Google Scholar]
- 10.Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 2001; 73: 285–90. [Google Scholar]
- 11.Park M, Cho H, Jung H, Lee H, Hwang KT. Antioxidant and anti-inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J Food Biochem 2014; 38: 259–70. [Google Scholar]
- 12.Szeto YT, Lee KY, Kalle W, Pak SC. Protective effect of grape seed extracts on human lymphocytes: a preliminary study. Appl Physiol Nutr Metab 2013; 38: 275–9. [DOI] [PubMed] [Google Scholar]
- 13.Gong G, Xu F. Study of aging model in mice. J China Pharmaceut Univ 1991; 2: 101–3. [Google Scholar]
- 14.Zhu Y. Establishment and measurement of D-galactose induced aging model. Fudan Univ J Med Sci 2007; 34: 617–19. [Google Scholar]
- 15.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD.. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 2002; 277: 44784–90. [DOI] [PubMed] [Google Scholar]
- 16.Wei H, Li L, Song Q, Ai H, Chu J, Li W. Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav Brain Res 2005; 157: 245–51. [DOI] [PubMed] [Google Scholar]
- 17.Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem 2009; 113: 1202–5. [Google Scholar]
- 18.Chen J, Li Y, Zhu Q, Li T, Lu H, Wei N, et al. Anti-skin-aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by d-Galactose. Mech Ageing Dev 2017; 164: 1–7. [DOI] [PubMed] [Google Scholar]
- 19.Martin GM. Aging and cell structure. Hum Pathol 1981; 14: 96. [Google Scholar]
- 20.Pannese E. Neuroglial cells: morphological changes during normal aging. Rend Lincei Sci Fis 2013; 24: 101–106. [Google Scholar]
- 21.Go Y, Jones DP. Redox theory of aging: implications for health and disease. Clin Sci 2017; 131: 1669–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glassock RJ, Denic A, Rule AD. The conundrums of chronic kidney disease and aging. J Nephrol 2017; 30: 477–83. [DOI] [PubMed] [Google Scholar]
- 23.Bickford PC, Flowers A, Grimmig B. Aging leads to altered microglial function that reduces brain resiliency increasing vulnerability to neurodegenerative diseases. Exp Gerontol 2017; 94: 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian L, Hui CW, Bisht K, Tan Y, Sharma K, Chen S, et al. Microglia under psychosocial stressors along the aging trajectory: consequences on neuronal circuits, behavior, and brain diseases. Prog Neuro Psychoph 2017; 79: 27–39. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Li S, Yang D, Qiu L, Wu Y, Wang D, et al. A novel strain of Lactobacillus mucosae isolated from a Gaotian villager improves in vitro and in vivo antioxidant as well as biological properties in D-galactose-induced aging mice. J Dairy Sci 2016; 99: 903–14. [DOI] [PubMed] [Google Scholar]
- 26.Wang PP, Sun HX, Liu CJ, Hu MH, He XQ, Yue S, et al. Racemic oleracein E increases the survival rate and attenuates memory impairment in D-galactose/NaNO2-induced senescent mice. Phytomedicine 2016; 23: 460–7. [DOI] [PubMed] [Google Scholar]
- 27.Selvaratnam JS, Robaire B. Effects of aging and oxidative stress on spermatozoa of superoxide-dismutase 1- and catalase-null mice. Biol Reprod 2016; 95: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiab NR, King N, McMillan M, Almashhadany A, Jones GL. Age-related protein and mRNA expression of glutathione peroxidases (GPx) and Hsp-70 in different regions of rat kidney with and without stressor. Aims Mol Sci 2016; 3: 125–37. [Google Scholar]
- 29.Li G, Chen Y, Hu H, Liu L, Hu X, Wang J, et al. Association between age-related decline of kidney function and plasma malondialdehyde. Rejuv Res 2012; 15: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mari M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Sign 2009; 11: 2685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonny ME, Garcia EM, Lopez A, Arroquy JI, Nazareno MA. Measurement of malondialdehyde as oxidative stress biomarker in goat plasma by HPLC-DAD. Microchem J 2016; 129: 281–5. [Google Scholar]
- 32.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 1968; 61: 748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher J, Yamamoto M. The rise of antioxidant signaling – the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol 2010; 244: 4–15. [DOI] [PubMed] [Google Scholar]
- 34.Maines MD. The heme oxygenase system and its functions in the brain. Cell Mol Biol 2000; 46: 573–85. [PubMed] [Google Scholar]
- 35.Ryter SW, Alam J, Choi A. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006; 86: 583–650. [DOI] [PubMed] [Google Scholar]
- 36.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci 2004; 75: 639–53. [DOI] [PubMed] [Google Scholar]
- 37.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 2001; 357: 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Da Cunha NV, Cortegoso Lopes FN, Panis C, Cecchini R, Pinge-Filho P, Martins-Pinge MC. iNOS inhibition improves autonomic dysfunction and oxidative status in hypertensive obese rats. Clin Exp Hypertens 2017; 39: 50–7. [DOI] [PubMed] [Google Scholar]
- 39.Bogdan C. Nitric oxide and the immune response. Nat Immunol 2001; 2: 907–16. [DOI] [PubMed] [Google Scholar]
- 40.Kuo PC, Abe KY, Schroeder RA. Oxidative stress increases hepatocyte iNOS gene transcription and promoter activity. Biochem Biophys Res Commun 1997; 234: 289–92. [DOI] [PubMed] [Google Scholar]
- 41.Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, González-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr 2005; 135: 2299–304. [DOI] [PubMed] [Google Scholar]
- 42.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51. [DOI] [PubMed] [Google Scholar]
- 43.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science 2005; 309: 1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kokkinaki M, Abu-Asab M, Gunawardena N, Ahern G, Javidnia M, Young J, et al. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci 2013; 33: 16346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao Y, Wang Y, Zhang Y, Liu C. Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Health Dis 2017; 16: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y, Lu S, Dong X, Xu L, Sun G, Sun X. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis 2017; 22: 1013–24. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 2012; 11: 410–17. [DOI] [PMC free article] [PubMed] [Google Scholar]