Abstract
A 16-year-old male patient reported with swelling of the right submandibular region for 3 months. The patient was asymptomatic and gave a history of fever lasting for 2 days before observing the swelling. Fine-needle aspiration cytology revealed nonspecific lymphadenitis, and since there was no apparent cause detected in the oral cavity or any systemic condition noted, the enlarged lymph node was surgically excised and submitted for histopathologic examination. The inflammatory condition and large numbers of macrophages appeared nonspecific while granuloma formation was not seen. Specific antibody titer against Toxoplasma gondii was carried out and extremely high level of IgG for toxoplasma was detected confirming the diagnosis of toxoplasmosis leading to lymphadenitis.
Keywords: Lymphadenitis, submandibular, toxoplasmosis
INTRODUCTION
Toxoplasmosis is a parasitic disease caused by the protozoan Toxoplasma gondii.[1] The parasite infects most genera of warm-blooded animals, including humans, but the primary host is the felid (cat) family. Infection occurs: (1) By eating infected meat, particularly swine products. (2) By ingesting water, soil or food that has meet-infected animals’ fecal matter. This is most commonly spread in feces by household cats. (3) By transmission from infected mother to fetus during pregnancy. It is more commonly seen in immunocompromised individuals such as in AIDS, transplant recipients and cancer patients.
Symptoms are usually mild and consist of low-grade fever, cervical lymphadenopathy, fatigue and muscle and joint pain.[2]
CASE REPORT
A 16-year-old male patient reported with swelling in the right submandibular region present for 3 months. The patient gave a history of fever lasting for 2 days before the development of swelling in the right submandibular region. The swelling measured approximately 4 cm × 2 cm on palpation which was soft and not fixed to underlying tissues [Figures 1 and 2]. A thorough oral examination did not reveal any dental source of infection, and the swelling persisted even after a course of antibiotics. No organomegaly was detected clinically. Fine-needle aspiration cytology (FNAC) was performed, and the report consisted of an admixture of small and large lymphocytes with occasional polymorph without evidence of granuloma formation or Reed–Sternberg cells or any malignancy. It was reported as reactive lymphadenitis. Subsequently, the enlarged submandibular lymph node was surgically excised and submitted for histopathologic examination.
Figure 1.

Clinical photographs showing swelling in the right submandibular region in a 16 year-old male patient (fornt)
Figure 2.
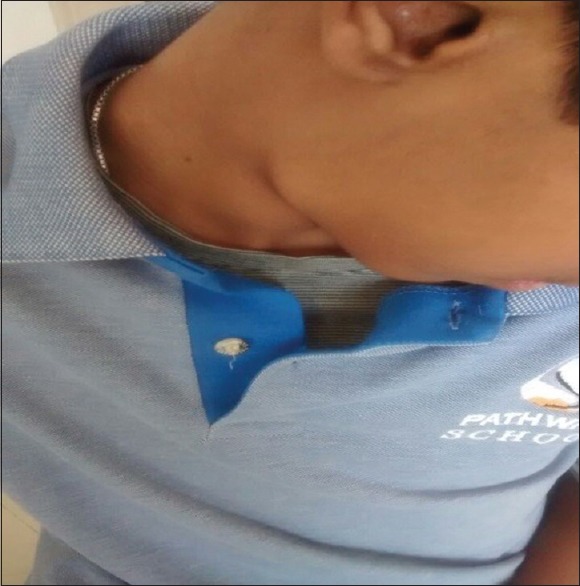
Clinical photographs showing swelling in the right submandibular region in a 16 year-old male patient (side view)
Histopathologic features
Sections taken from the submitted specimen stained with Haematoxylin and Eosin stain showed reactive lymph follicles, focal areas of monocytoid B cells along with many well-formed nonnecrotizing epithelioid granulomas in the paracortex, focally impinging in the germinal centers [Figures 3 and 4]. The germinal centers had ragged “moth-eaten” margins and contained numerous tingible body macrophages. No Hodgkin-Reed-Sternberg cells were seen in the given sections. Focally, the capsule showed evidence of acute and chronic inflammation consistent with prior FNA report.
Figure 3.
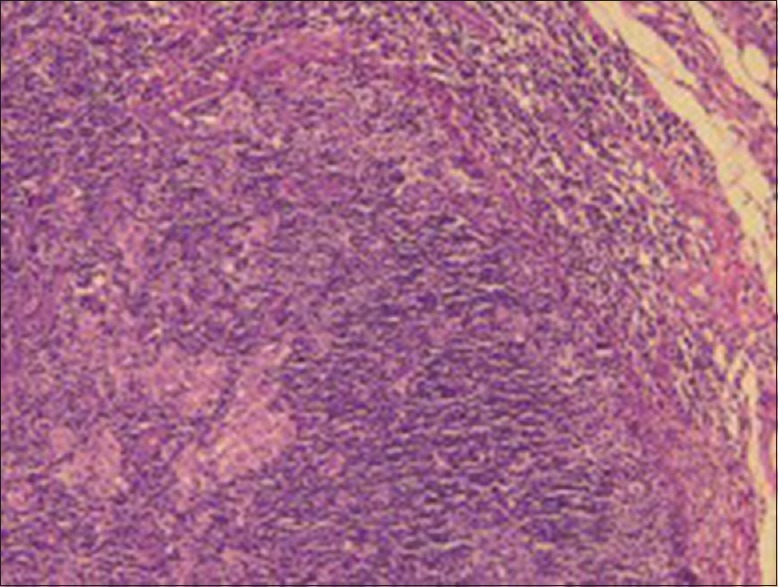
Section of lymph node shows scattered reactive lymphoid follicles with prominent germinal centers (H&E, ×10)
Figure 4.
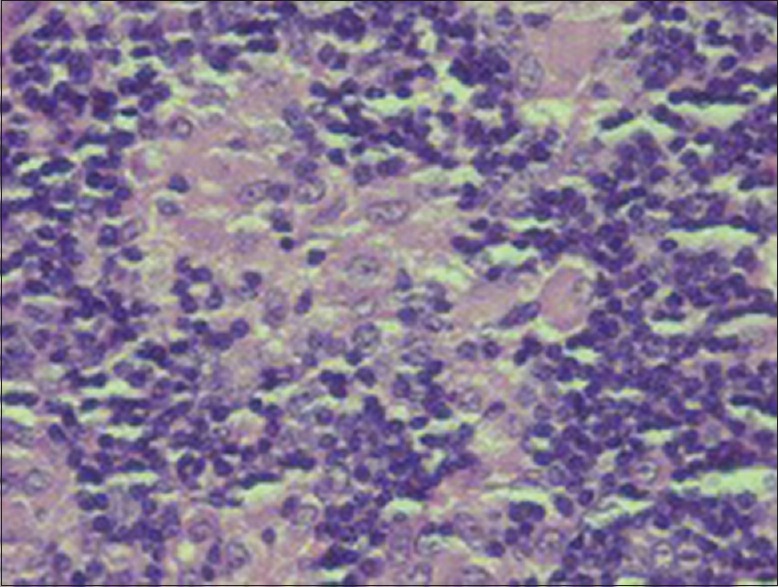
Focal areas of interfollicular expansion by epithelioid cell granulomas and large mononuclear cells (×40)
Laboratory findings
Toxoplasma IgG serum values were 393.6 IU/ml (reference values: <1.6 IU/mL for nonreactive), angiotensin converting enzyme serum values were 144 U/L (reference value: 8–65 U/L) and presence of gamma interferon was detected.
Skin Mantoux test and serum calcium levels were normal.
Overall, the morphology, immunoprofile and serologic studies are supportive of toxoplasma lymphadenitis [Figures 5–7].
Figure 5.
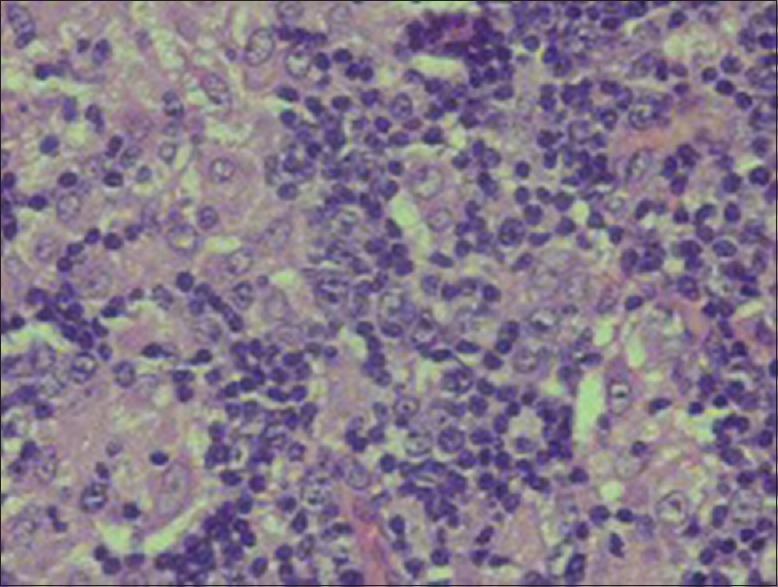
Predominantly mononuclear cells showing prominent nucleoli (×40)
Figure 7.
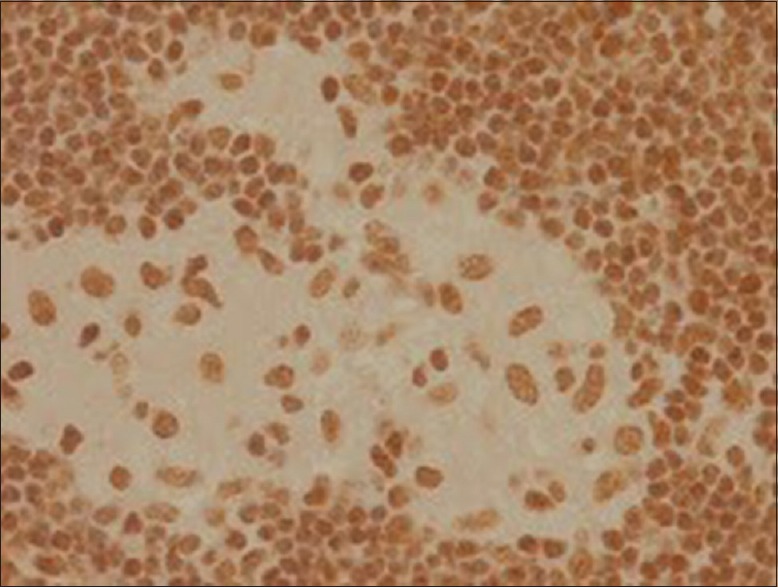
Immunohistochemistry positive for Pax 5
Figure 6.
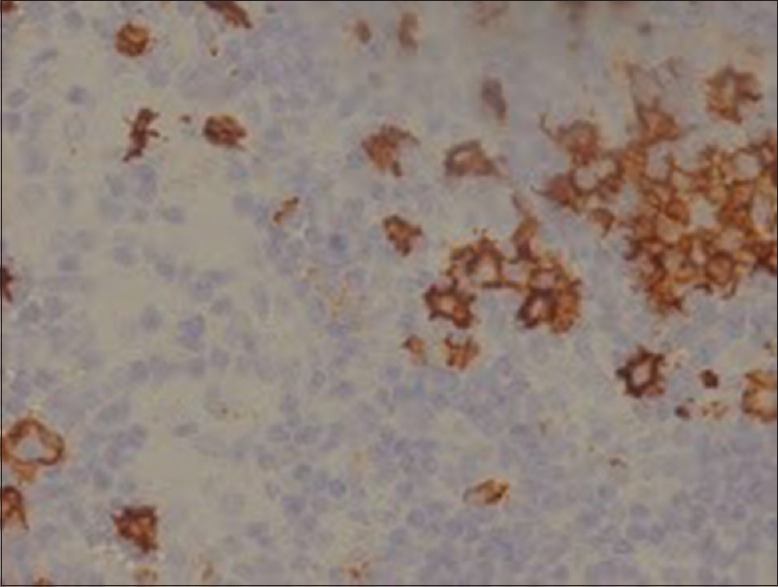
Immunohistochemical staining shows CD30 positive for the large cells
Features supportive of classical Hodgkin lymphoma or an Epstein–Barr virus (EBV)-associated lymphoproliferative disease were not identified in the biopsy.
DISCUSSION
There is a considerable variety of protozoal diseases which may occasionally manifest oral involvement. The protozoa are unicellular animals which are usually divided into two subphyla: the plasmodroma-protozoa which move by means of pseudopodia or flagella, and the Ciliophora-protozoa which move by means of cilia.[3] Toxoplasmosis is a protozoal disease which may involve oral structures occasionally. The essential reservoir host of T. gondii is the common house cat and other felines. The organisms from the intestinal cycle are passed in cat feces and mature into infective cysts within 3–4 days in the external environment[4] [Figure 8].
Figure 8.
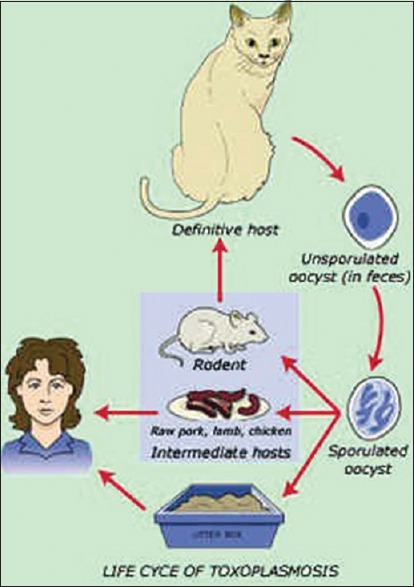
Life-cycle of Toxoplasma organism. Source-internet images
Some infective forms (trophozoites) of the oocyst develop as slender, crescentic types called tachyzoites [Figure 9].
Figure 9.
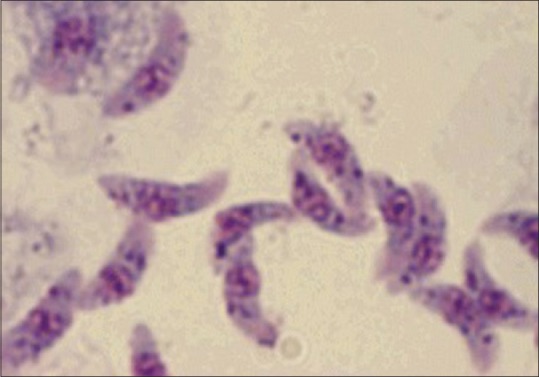
Tachyzoites of Toxoplasma gondii. Source-internet images
These rapidly multiplying forms are responsible for the initial infection and tissue damage. Slow-growing, shorter forms called bradyzoites also develop and form cysts in chronic infections.[4] It is increasingly apparent that certain immunocompromised individuals (like patients with AIDS) are more likely to have severe manifestations. Humans become infected from two sources: (1) Ingestion of improperly cooked meat from animals that serve as intermediate hosts and (2) Ingestion of infective oocysts from contaminated cat feces.[4] Transplacental infection can occur in pregnancy, either from infection acquired from meat and meat juices or from contact with cat feces. Transplacental infection from an infected mother has a devastating effect on the fetus.
Symptoms of acute disease include chills, fever, headaches, myalgia, lymphadenitis and fatigue; the symptoms occasionally resemble those of infectious mononucleosis. In chronic disease, the signs and symptoms include lymphadenitis, occasionally a rash, evidence of hepatitis, encephalomyelitis and myocarditis.[4] In the present case, the patient had a history of fever following which the right submandibular lymph node was enlarged. It was a mobile and painless swelling measuring approximately 4 cm × 3 cm in size. Similar asymptomatic submandibular lymph node swelling was observed in two previously reported cases.[5,6] In both the cases, the patients were asymptomatic with submandibular lymphadenopathy and the patients were young of 13 and 9 years of age, respectively. FNAC of the lesion had revealed an admixture of small and large lymphocytes with scattered histiocytes and occasional polymorphs and the impression was of reactive lymph node hyperplasia. In the case reported by Macey-Dare et al.,[6] acquired toxoplasmosis of submandibular lymph node was diagnosed by FNAC thus avoiding biopsy or surgical intervention. Diagnosis is made by correlating histologic and serologic data, but the history too is important.
Histologic analysis will reveal that the lymph node architecture is preserved and that hyperplastic follicles are present. Multiple mitoses are seen in the germinal centers. Also present are many epithelioid cells with pale eosinophilic cytoplasm. Serologic investigations include the Sabin-Feldman dye test and the enzyme-linked immunosorbent assay (ELISA).[5] The Sabin-Feldman dye test is a neutralization test that entails lysis of organisms in the presence of antibody and complement; it measures IgG antibodies. The ELISA involves the use of antigens of killed toxoplasmosis organisms to detect antitoxoplasmosis antibodies. Measuring IgM, IgE and IgG levels can help differentiate chronic from acute infection. An IgM level indicates an acute infection if it rises within a few days of inoculation. IgM levels then normalize in 3–4 months. IgG elevation indicates a previous infection; these levels rise 2–3 weeks after inoculation, and this antibody remains positive for the rest of the patient's life. An elevated IgE level is an indication that the infection is more acute than an infection that is associated with only an IgM level; IgE levels normalize within 2 months.[7] The patient may also be infected following cat scratch disease as reported in a Japanese study.[8] It has been estimated that 15% of unexplained cases of lymphadenopathies are of toxoplasmosis and cervical lymph nodes are commonly affected.[9] Serological examinations are most trustworthy in confirming recent or old toxoplasmosis infection.[10]
CD30 is a transmembrane receptor with restricted expression on activated T and B cells in normal lymphoid tissues.[11] Pax5, or B-cell-specific activator protein, is a nuclear protein in the paired-box containing (PAX) family of transcription factors involved in control of organ development and tissue differentiation. Pax5 is mostly expressed in B lymphocytes and B-cell lymphomas. Pax5 immunohistochemistry is fairly specific for B-cell lineage and is a valuable addition to the armamentarium of markers available for lymphoma subtyping.[12]
Epstein–Barr encoding region (EBER) in situ hybridization is positive for EBV-infested Hodgkin's lymphoma. The immunohistochemistry markers and EBER in situ hybridization were used to rule out malignant lymphomas.[13]
When positive results are present in suspected acquired toxoplasmosis, conservative management should be the treatment of choice. Patients suffering from lymphadenopathy in the head and neck regions, without any other systemic manifestation, should be followed up with no complementary drug therapy. Lymphadenectomy must be considered in cases of growing lymph nodes. In more severe cases, pyrimethamine and sulfadiazine are the treatment of choice.[5]
CONCLUSION
Toxoplasmosis lymphadenitis is an uncommon disease affecting humans caused by infection by the parasite T. gondii, which are passed in cat feces producing acute and chronic infection of various tissues in the host by infective forms or trophozoites. Immunocompromised individuals are more likely to have severe manifestations. Symptoms include chills, fever, headaches, myalgia, lymphadenitis and fatigue. Chronic case may present as apparent unexplained lymphadenopathy and superficial lymphadenopathy is a common manifestation in children. Finding of cyst in histology of lymph node is extremely rare.[14] Microscopic examination reveals chronic lymphadenitis with marked lymphoid hyperplasia as commonly seen in reactive hyperplasia. Pale staining histiocytes are seen scattered throughout the lymph node. Confusion with malignant lymphomatous conditions is liable to arise from (1) the apparent disorganization of the architecture of the node, (2) the active proliferation of large cells, (3) the simulation of neoplastic infiltration and (4) the prominent histiocyte clusters which resemble those sometimes found in association with Hodgkin's disease and less often, with lymphosarcoma and reticulosarcoma.[14] Positive serological results confirm the diagnosis of toxoplasmosis infection. The Sabin-Feldman dye test is one of the most specific tests and when positive confirms a previous infection. Patients suffering from lymphadenopathy in the head and neck regions, without any other systemic manifestation, should be followed up with no complementary drug therapy.[5] Toxoplasmosis should be considered in the differential diagnosis of any head and neck lymphadenopathy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ryan KJ, Ray CG, editors. Sherris Medical Microbiology. 4th ed. New York: McGraw Hill; 2004. pp. 723–7. [Google Scholar]
- 2.Ghom AG, Mhaske S. Textbook of Oral Pathology. 2nd ed. India: Jaypee Brothers Medical Publishers (P) Ltd; 2013. p. 502. [Google Scholar]
- 3.Rajendran R, Sivapathasundaram B. Shafer's Textbook of Oral Pathology. 7th ed. India: Elsevier; 2012. p. 378. [Google Scholar]
- 4.Murray PR, Rosenthal KS, Pfaller MA. Medical Microbiology. 6th ed. Philadelphia: Mosby Elsevier; 2009. p. 841. [Google Scholar]
- 5.Azaz B, Milhem I, Hasson O, Kirsch G. Acquired toxoplasmosis of a submandibular lymph node in a 13-year-old boy: Case report. Pediatr Dent. 1994;16:378–80. [PubMed] [Google Scholar]
- 6.Macey-Dare LV, Kocjan G, Goodman JR. Acquired toxoplasmosis of a submandibular lymph node in a 9-year-old boy diagnosed by fine-needle aspiration cytology. Int J Paediatr Dent. 1996;6:265–9. doi: 10.1111/j.1365-263x.1996.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 7.Shashy RG, Pinheiro D, Olsen KD. Toxoplasmosis lymphadenitis presenting as a parotid mass: A report of 2 cases. Ear Nose Throat J. 2006;85:666–8. [PubMed] [Google Scholar]
- 8.Wakisaka N, Yoshizaki T, Furukawa M. Toxoplasmic lymphadenopathy: A case report. Pract Oto Rhino Laryngol. 2005;98:233–7. [Google Scholar]
- 9.Toxoplasmosis, World Health Organization Technical Report Series No. 431. 1969:5–31. [PubMed] [Google Scholar]
- 10.Montoya JG, Remington JS. Studies on the serodiagnosis of toxoplasmic lymphadenitis. Clin Infect Dis. 1995;20:781–9. doi: 10.1093/clinids/20.4.781. [DOI] [PubMed] [Google Scholar]
- 11.Horie R, Watanabe T. CD30: Expression and function in health and disease. Semin Immunol. 1998;10:457–70. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 12.Feldman AL, Dogan A. Diagnostic uses of pax5 immunohistochemistry. Adv Anat Pathol. 2007;14:323–34. doi: 10.1097/PAP.0b013e3180ca8a49. [DOI] [PubMed] [Google Scholar]
- 13.Gulley ML, Glaser SL, Craig FE, Borowitz M, Mann RB, Shema SJ, et al. Guidelines for interpreting EBER in situ hybridization and LMP1 immunohistochemical tests for detecting epstein-barr virus in hodgkin lymphoma. Am J Clin Pathol. 2002;117:259–67. doi: 10.1309/MMAU-0QYH-7BHA-W8C2. [DOI] [PubMed] [Google Scholar]
- 14.Stansfeld AG. The histological diagnosis of toxoplasmic lymphadenitis. J Clin Pathol. 1961;14:565–73. doi: 10.1136/jcp.14.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]


