Abstract
Introduction and Objectives:
Odontogenic cysts are the most common cysts of the jaws and are formed from the remnants of the odontogenic apparatus. Among these odontogenic cysts, radicular cysts (RCs) (about 60% of all diagnosed jaw cysts), dentigerous cysts (DCs) (16.6% of all jaw cysts) and odontogenic keratocysts (OKCs) (11.2% of all developmental odontogenic cysts) are the most common. The behavior of any lesion is generally reflected by its growth potential. Growth potential is determined by measuring the cell proliferative activity. The cell proliferative activity is measured by various methods among which immunohistochemistry (IHC) is the commonly used technique. Most of the IHC studies on cell proliferation have been based on antibodies such as Ki-67 and proliferating cell nuclear antigen.
Materials and Method:
In the present study, the total sample size comprised of 45 cases of odontogenic cysts, with 15 cases each of OKC, RC and DC. Here, an attempt is made to study immunohistochemical (streptavidin-biotin detection system HRP-DAB) method to assess the expression of Ki-67 in different layers of the epithelial lining of OKCs, RCs and DCs.
Observations and Results:
Ki-67 positive cells were highest in epithelium of OKC as compared to DC and RC.
Conclusion:
The increased Ki-67 labeling index and its expression in suprabasal cell layers of epithelial lining in OKC and its correlation with suprabasal cell layers of epithelial lining in DC and RC could contribute toward its clinically aggressive behavior. OKC is of more significance to the oral pathologist and oral surgeon because of its specific histopathological features, high recurrence rate and aggressive behavior.
Keywords: Dentigerous cysts, immunohistochemistry, Ki-67, odontogenic keratocyst, radicular cyst
INTRODUCTION
The term cyst is derived from the Greek word “Kystis” which means a bladder or sac. A cyst is a pathological cavity having fluid, semifluid or gaseous contents and which is not created by the accumulation of pus. It is frequently, but not always, lined by epithelium (Kramer 1974).[1]
Odontogenic cysts are defined as those cysts, which arise from the remnants of the odontogenic apparatus, including cell rests of Malassez (remnants of Hertwig's epithelial root sheath), cell rests of Serres (remnants of dental lamina), reduced enamel epithelium and from the basal cell layer of the oral epithelium.[2,3]
Among odontogenic cysts, radicular cysts (RC) are the most common inflammatory origin (about 60% of all diagnosed jaw cysts) and arise from epithelial rests of Malassez; dentigerous cysts (DC) are the most common developmental cysts (16.6% of all jaw cysts) arising from reduced enamel epithelium and odontogenic keratocyst (OKC) represents 11.2% of all developmental odontogenic cysts and arise from the dental lamina or its remnants.[4,5] Out of these cysts, the OKC is of more significance to the oral pathologist and oral surgeon because of its specific histopathological features, high recurrence rate (20%–62%) and aggressive behavior.[6,7]
Cell proliferation is a biological process which is of vital importance to all living organisms and is fundamental to both embryonic and postembryonic existence.[8] Cell proliferation activity has been investigated in odontogenic cysts previously by various authors using different methods including IHC (Ki-67 labeling index [LI]).[9,10]
Immunohistochemistry (IHC) refers to the process of localizing proteins in cells of a tissue section exploiting the principle of antibodies binding specifically to antigens in biological tissues. Most of the IHC studies on cell proliferation have been based on antibodies such as Ki-67 and proliferating cell nuclear antigen (PCNA). In contrast to PCNA, the Ki-67 antigen is degraded fast as the biologic half-life of the detectable antigen is <1 h. It is consistently absent in quiescent cells and is not detectable during DNA repair processes.[11,12] Therefore, Ki-67 antigen expression is a more reliable immunohistochemical tool for measuring proliferation activity in human tissues.
Here, an attempt is made to assess the expression of Ki-67 in OKC, RC and DCs.
Aim
To study immunohistochemical expression of Ki-67 and its correlation in different layers of the epithelial lining of OKCs, RCs and DCs.
Objectives
To observe Ki-67 LI in different layers of the epithelial lining of OKC, RC and DC
To compare Ki-67 LI in OKC, RC and DC.
METHODOLOGY
The present study comprising of 45 samples of odontogenic cysts (OKC, RC, DC) was carried out in the Department of Oral and Maxillofacial Pathology and Microbiology, Ahmedabad Dental College and Hospital, Ahmedabad, Gujarat. Samples were collected from archives of the department and also from private clinics.
The study included histopathologically diagnosed cases of OKC, RC and DC. The paraffin-embedded tissue blocks from the archives were sectioned and stained and tissue samples obtained from the private clinics were subjected to normal tissue processing, embedded in paraffin wax blocks and then stained.
Standardized immunohistochemistry (IHC), streptavidin-biotin detection system HRP-DAB method was used, with standard equipments, reagents and chemicals.
Ki-67 (clone MIB-1) primary antibody, mouse monoclonal antihuman antibody (Product code:-N1633 DAKO, Denmark)
Peroxidase Detection System (streptavidin-biotin detection system HRP-DAB) (peroxidase block, protein block, biotinylated secondary antibody, streptavidin HRP, DAB-chromogen, DAB-substrate buffer, hematoxylin).
Assessment of immunohistochemically stained sections
The positive control was examined for the presence of a colored end product at the site of the target antigen (DAB chromogen brown end product). The presence of these colors was interpreted as positive staining result, indicating proper performance of kit reagents. The absence of nonspecific staining in the negative control confirmed the specificity of primary antibody.
Cells were considered positive for the Ki-67 if there was intranuclear DAB staining (brown color). All stained nuclei were scored positive regardless of intensity of staining. Cells that lacked a clear nucleus were excluded. Minimum of 1000 cells was counted in each section. Tissue sections positive for Ki-67were examined for the presence of brown-stained nuclei and evaluated by locating the epithelial linings most heavily labeled by scanning the sections at a ×100 magnification. Cell counts were made at ×400 magnification with conventional light microscope in 5 randomly selected fields. Ki-67 labeled cells’ counting were done among all groups. The constituent cells of the lining epithelium were divided into basal, suprabasal/intermediate and surface layers in OKC and the mean counted as in complete epithelium. We considered cuboidal cells located from one row at the basement membrane as the basal layer. The surface layer constitutes flattened or polygonal cells consisting of one to five layers localized just underneath the surface of the lining epithelium. The suprabasal/intermediate layer is composed of relatively large round cells between the basal and the surface layers. In RC and DC, counting was done in basal cell layer and suprabasal cell layer and mean counted as in complete epithelium.
The number of positively stained nuclei was expressed as a percentage of the total number counted for individual layer and in complete epithelium.
![]()
RESULTS
In the present study, the total sample size comprised of 45 cases of odontogenic cysts, with 15 cases each of OKC, RC and DC. Out of total 45 cases, 41 (91.11%) shows positivity, among which OKC and RC showed positivity in 14 (93.33%) out of 15 cases and DC showed positivity in 13 (86.66%) out of the 15 cases.
Table 1 shows representation of Ki-67 LI in different layers of the epithelial lining of different groups:
Table 1.
Representation of Ki-67 labeling index in different layers of the epithelial lining in different groups

Ki-67 LI was observed in descending order in basal layer as DC, OKC and RC with values 11.17 (±9.288), 9.18 (±4.796) and 8.43 (±5.203), respectively, in suprabasal layer as OKC, DC and RC with values 19.66 (±7.897), 3.60 (±2.31) and 3.12 (±2.192), respectively, and in complete epithelial layer as OKC, DC and RC with values 12.76 (±4.780), 5.87 (±4.249) and 5.08 (±3.118), respectively.
Table 2 shows head-to-head comparison of Ki-67 LI for basal layer:
Table 2.
Head-to-head comparison of Ki-67 labeling index in the basal layer of the epithelial lining between odontogenic keratocyst, radicular cyst and dentigerous cysts
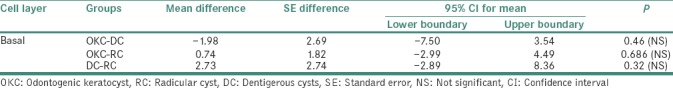
Statistically nonsignificant variation of mean difference of Ki-67 LI was noted among all groups, with − 1.98 (±2.699) in OKC-DC (P = 0.46), 0.74 (±1.827) in OKC-RC (P = 0.686) and 2.73 (±2.748) in DC-RC (P = 0.32).
Table 3 shows head-to-head comparison of Ki-67 LI for suprabasal layer:
Table 3.
Head-to-head comparison of Ki-67 labeling index in the suprabasal layer of the epithelial lining between odontogenic keratocyst, radicular cyst and dentigerous cysts
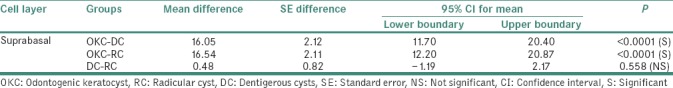
Statistically significant variation of mean difference of Ki-67 LI was noted in groups, with values 16.05 (±2.124) in OKC-DC (P < 0.0001) and 16.54 (±2.116) in OKC-RC (P < 0.0001). Statistically nonsignificant variation of mean difference of Ki-67 LI was noted in group with value 0.48 (±0.82) in DC-RC (P = 0.558).
Table 4 shows head–to-head comparison of Ki-67 LI for complete epithelium:
Table 4.
Head-to-head comparison of Ki-67 labeling index in the complete epithelium between odontogenic keratocyst, radicular cyst and dentigerous cysts
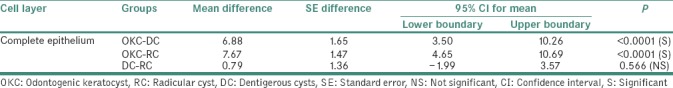
Statistically significant variation of mean difference of Ki-67 LI was noted with values 6.88 (±1.651) in OKC-DC (P < 0.0001) and 7.67 (±1.473) in OKC-RC (P < 0.0001). Statistically nonsignificant variation of mean difference of Ki-67 LI was noted in group DC-RC 0.79 (±1.36) (P = 0.566).
DISCUSSION
Odontogenic cysts are the most common cysts of the jaws. The epithelium involved in odontogenesis and/or its remnants are the origin of different odontogenic lesions, with distinct clinical behavior. Several studies have reported differences in the proliferative potential of odontogenic epithelial cells, with significant impacts on the formation of odontogenic cysts.[13]
The epithelial lining of RCs is presumed to arise from epithelial rests of Malassez, DCs from reduced enamel epithelium and that of keratocysts from the dental lamina or its remnants.[14] Out of these cysts, the OKC is of more significance to the oral pathologist and oral surgeon because of its specific histopathological features, high recurrence rate (20%–62%) and aggressive behavior.[6,7]
The behavior of any lesion is generally reflected by its growth potential. This growth potential is determined by measuring the cell proliferative activity. The growth potential and its correlation with the clinical aggressiveness and recurrence suggest the nature of lesion. Experimental evidence suggests that the degree of cellular proliferation within a tumor can estimate or predict its biological aggression.[15] The growth potential is therefore a reliable guide to predict prognosis and warrant the treatment modality.
It is a well-known fact that the basal and parabasal cell layers represent the normal cell proliferative compartment of oral epithelium. The suprabasal layers are cellular maturation spaces where cellular alterations show the expression of keratin. Therefore, any sign of proliferative cellular activity beyond the parabasal layer should be considered as perturbing.[16]
Immunohistochemical technique is easy, relatively inexpensive in comparison with other methods (e.g., flow cytometry) for studying cell proliferation. Immunohistochemical techniques also have advantage over other techniques because of the maintenance of cellular and tissue architectures, simplicity of methodology and rapidity of results. Thus, immunohistochemical demonstration of proliferation-related marker is a valuable method for showing the proliferative potential of cell.
Most of the IHC studies on cell proliferation have been based on antibodies such as Ki-67 and PCNA. In contrast to PCNA, the Ki-67 antigen is degraded fast as the biologic half-life of the detectable antigen is <1 h. It is consistently absent in quiescent cells and is not detectable during DNA repair processes.[11,12] Therefore, Ki-67 antigen expression is a more reliable immunohistochemical tool for measuring proliferation activity in human tissues.
In the present study, immunohistochemistry for Ki-67 antigen was employed to evaluate cell proliferation in epithelial lining of OKC (n = 15), RC (n = 15) and DC (n = 15).
In our study, overall Ki-67 LI was noted highest in OKC (12.76 ± 4.78) than DC (5.87 ± 4.24) and RC (5.08 ± 3.11) when all layers of epithelial lining were taken into consideration. The Ki-67 LI was significantly higher in suprabasal cell layer (19.66 ± 7.89) than in basal cell layer (9.18 ± 4.79) in OKC [Figures 1 and 2], whereas in RC [Figures 3 and 4] and DC [Figures 5 and 6], most of the Ki-67 positive cells were mainly located in basal cell layer (RC = 8.43 ± 5.20 and DC = 11.17 ± 9.28) and very few were noted in the suprabasal cell layer (RC = 3.12 ± 2.19 and DC = 3.60 ± 2.31). These results suggest that proliferative activity was highest in the suprabasal cell layer in OKC. These findings are in accordance with few of the previous studies done by Slootweg,[10] Li et al.,[17] Saraçoǧlu et al.[18] and Kichi et al.[19] that used Ki-67 antigen. This unusual proliferation of OKC epithelial lining represents an epithelial disorganization and also reflects a unique epithelial differentiation process in which the basal cells assume some characteristics of preameloblasts.
Figure 1.
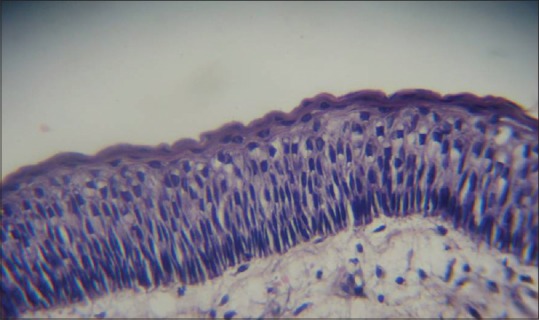
Photomicrograph showing odontogenic keratocyst epithelial lining. (H & E, ×40)
Figure 2.
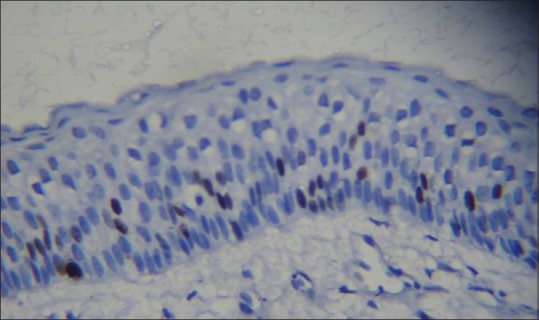
Photomicrograph showing distribution of Ki-67-positive nuclei in odontogenic keratocyst lining. Majority of the labeled nuclei are in suprabasal position (hematoxylin – anti-Ki-67, ×40)
Figure 3.
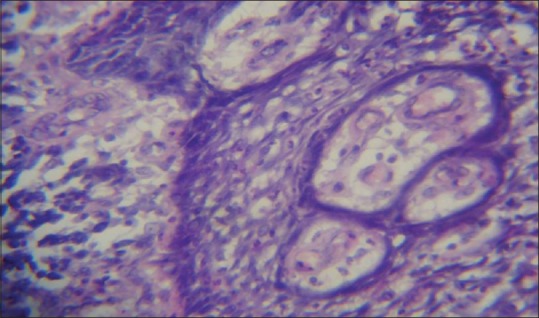
Photomicrograph showing radicular cyst epithelial lining (H & E, ×10)
Figure 4.
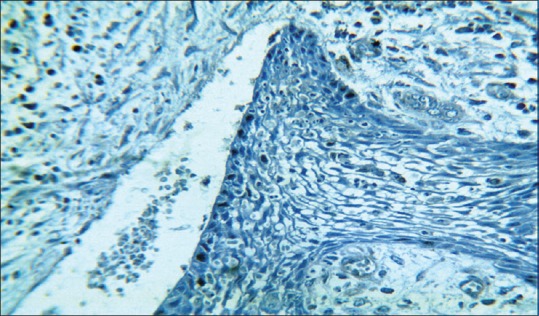
Photomicrograph showing distribution of Ki-67-positive nuclei in radicular cyst lining (hematoxylin – anti Ki-67, ×10)
Figure 5.
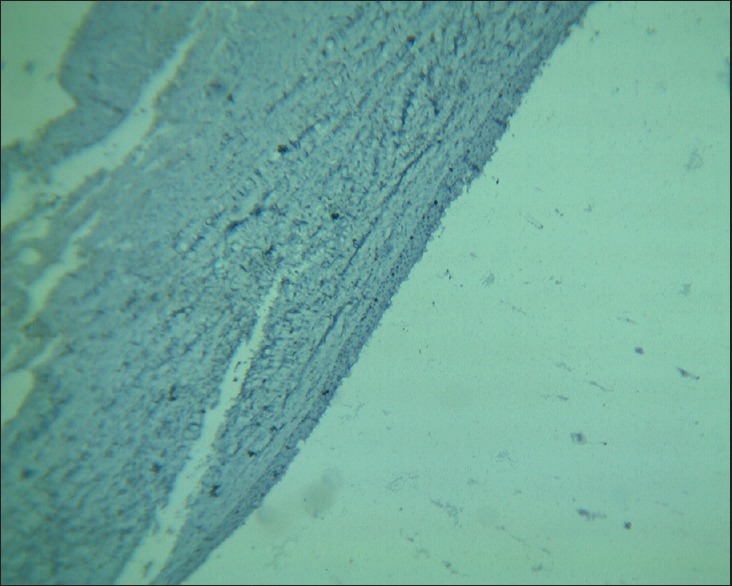
Photomicrograph showing dentigerous cyst epithelial lining (H & E, ×10)
Figure 6.
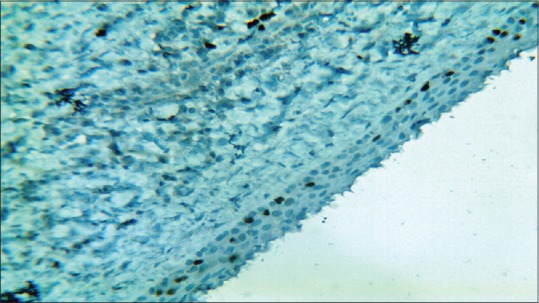
Photomicrograph showing distribution of Ki-67-positive nuclei in dentigerous cyst lining (hematoxylin – anti Ki-67, ×10)
In the present study, the Ki-67 LI was mainly noted in basal cell layer in DC (11.17 ± 9.28) and RC (8.43 ± 5.20) with no statistically significant difference of values between DC and RC. These findings for DC correlate with other studies done by Tosios et al.,[20] Kim et al.,[21] Edamatsu et al.[13] and Piattelli et al.[22]
These quantitative and qualitative differences of the proliferative activity of the epithelium in OKC compared to other cysts suggest the abnormal control of the cell cycle and are considered suggestive of an intrinsic growth potential that could play a role in its development and biological behavior. These molecular findings suggest that the intrinsic growth potential of epithelium and biological aggressive behavior of OKC has given rise to the hypothesis that OKCs may represent a low-grade neoplasm rather than developmental cyst.
CONCLUSION
Ki-67 positive cells were highest in epithelium of OKC as compared to DC and RC. The increased Ki-67 LI and its expression in suprabasal cell layers of epithelial lining in OKC and its correlation with suprabasal cell layers of epithelial lining in DC and RC could contribute toward its clinically aggressive behavior. Hence, the study of OKC using cell proliferation marker Ki-67 suggests that OKC could be neoplastic in origin rather than a developmental cyst.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shear M, Speight PM. Cysts of the Oral and Maxillofacial Regions. 4th ed. Oxford, UK: Blackwell Munksgaard; 2007. [Google Scholar]
- 2.Hume WJ, Moore JK, Main DM. Differences in in vitro growth of epithelium from inflammatory and developmental odontogenic cysts. Br J Oral Maxillofac Surg. 1990;28:85–8. doi: 10.1016/0266-4356(90)90127-7. [DOI] [PubMed] [Google Scholar]
- 3.Stenman G, Magnusson B, Lennartsson B, Juberg-Ode M. In vitro growth characteristics of human odontogenic keratocysts and dentigerous cysts. J Oral Pathol. 1986;15:143–5. doi: 10.1111/j.1600-0714.1986.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 4.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 3rd ed. India: Elsevier; 2005. Odontogenic cysts; pp. 590–7. [Google Scholar]
- 5.Rajendran R. Shafer's Textbook of Oral Pathology. 6th ed. India: Elsevier; 2009. Cysts and tumors of odontogenic origin; pp. 254–310. [Google Scholar]
- 6.Brannon RB. The odontogenic keratocyst: A clinicopathological study of 312 cases. Part II. Histologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1977;43:233–55. doi: 10.1016/0030-4220(77)90161-x. [DOI] [PubMed] [Google Scholar]
- 7.Blanas N, Freund B, Schwartz M, Furst IM. Systematic review of the treatment and prognosis of the odontogenic keratocyst. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:553–8. doi: 10.1067/moe.2000.110814. [DOI] [PubMed] [Google Scholar]
- 8.Tumuluri V, Thomas GA, Fraser IS. The relationship of proliferating cell density at the invasive tumour front with prognostic and risk factors in human oral squamous cell carcinoma. J Oral Pathol Med. 2004;33:204–8. doi: 10.1111/j.0904-2512.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 9.Allison RT, Spencer S. Nucleolar organiser regions in odontogenic cysts and ameloblastomas. Br J Biomed Sci. 1993;50:309–12. [PubMed] [Google Scholar]
- 10.Slootweg PJ. P53 protein and Ki-67 reactivity in epithelial odontogenic lesions. An immunohistochemical study. J Oral Pathol Med. 1995;24:393–7. doi: 10.1111/j.1600-0714.1995.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 11.Schlüter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, et al. The cell proliferation-associated antigen of antibody Ki-67: A very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993;123:513–22. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu SC, Klein-Szanto AJ. Markers of proliferation in normal and leukoplakic oral epithelia. Oral Oncol. 2000;36:145–51. doi: 10.1016/s1368-8375(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 13.Edamatsu M, Kumamoto H, Ooya K, Echigo S. Apoptosis-related factors in the epithelial components of dental follicles and dentigerous cysts associated with impacted third molars of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:17–23. doi: 10.1016/j.tripleo.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Matthews JB, Mason GI, Browne RM. Epithelial cell markers and proliferating cells in odontogenic jaw cysts. J Pathol. 1988;156:283–90. doi: 10.1002/path.1711560403. [DOI] [PubMed] [Google Scholar]
- 15.Battifora H. P53 immunohistochemistry: A word of caution. Hum Pathol. 1994;25:435–7. doi: 10.1016/0046-8177(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 16.Hofstädter F, Knüchel R, Rüschoff J. Cell proliferation assessment in oncology. Virchows Arch. 1995;427:323–41. doi: 10.1007/BF00203402. [DOI] [PubMed] [Google Scholar]
- 17.Li TJ, Browne RM, Matthews JB. Epithelial cell proliferation in odontogenic keratocysts: A comparative immunocytochemical study of Ki67 in simple, recurrent and basal cell naevus syndrome (BCNS)-associated lesions. J Oral Pathol Med. 1995;24:221–6. doi: 10.1111/j.1600-0714.1995.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 18.Saraçoǧlu U, Kurt B, Günhan O, Güven O. MIB-1 expression in odontogenic epithelial rests, epithelium of healthy oral mucosa and epithelium of selected odontogenic cysts. An immunohistochemical study. Int J Oral Maxillofac Surg. 2005;34:432–5. doi: 10.1016/j.ijom.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Kichi E, Enokiya Y, Muramatsu T, Hashimoto S, Inoue T, Abiko Y, et al. Cell proliferation, apoptosis and apoptosis-related factors in odontogenic keratocysts and in dentigerous cysts. J Oral Pathol Med. 2005;34:280–6. doi: 10.1111/j.1600-0714.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 20.Tosios KI, Kakarantza-Angelopoulou E, Kapranos N. Immunohistochemical study of bcl-2 protein, Ki-67 antigen and p53 protein in epithelium of glandular odontogenic cysts and dentigerous cysts. J Oral Pathol Med. 2000;29:139–44. doi: 10.1034/j.1600-0714.2000.290306.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim DK, Ahn SG, Kim J, Yoon JH. Comparative Ki-67 expression and apoptosis in the odontogenic keratocyst associated with or without an impacted tooth in addition to unilocular and multilocular varieties. Yonsei Med J. 2003;44:841–6. doi: 10.3349/ymj.2003.44.5.841. [DOI] [PubMed] [Google Scholar]
- 22.Piattelli A, Fioroni M, Santinelli A, Rubini C. P53 protein expression in odontogenic cysts. J Endod. 2001;27:459–61. doi: 10.1097/00004770-200107000-00006. [DOI] [PubMed] [Google Scholar]


