Abstract
Background:
Many studies have reported that macrophages and eosinophils are involved in the pathogenesis of several diseases. To the best of our knowledge, this is the first study comparing macrophages and eosinophils in oral reactive lesions.
Aims:
In this study, we aimed to determine the contribution of macrophages and eosinophils to the pathogenesis of oral reactive lesions and the relationships between these biomarkers and the diverse histopathologic features.
Methods:
Seventy-five paraffin-embedded tissue samples were assessed in this study. Five categories (15 cases for each group), including peripheral ossifying fibroma, pyogenic granuloma, fibroma, inflammatory fibrous hyperplasia, and peripheral giant-cell granuloma, were considered. Anti-CD68 immunohistochemical and hematoxylin-eosin staining was carried out.
Results:
We found that macrophages, but not eosinophils, were a significant internal component of oral reactive lesions. Macrophages were observed in high densities in all studied groups and diffusely distributed or clustered throughout these lesions. The number of macrophages was increased in peripheral giant-cell granuloma compared with other groups.
Conclusions:
Our findings suggest that macrophages are involved in the pathogenesis and the variation of microscopic features of oral reactive lesions. However, further clinical studies should be conducted to identify the biological process behind macrophages and the molecular interactions of these cells, with the ultimate aim of suggesting a new potential therapeutic target for these lesions. We found that eosinophils were not involved in the fibrotic process and the variation of microscopic features in oral reactive lesions. Our results showed that peripheral giant-cell granulomas highly demonstrated histiocytic characteristics.
Keywords: Eosinophil, macrophage, oral, reactive
INTRODUCTION
Soft-tissue reactive lesions are the most common diseases of the oral cavity. These lesions represent hyperplasia of the connective tissue in response to local irritation or chronic and low-grade trauma. In spite of the presence of chronic irritation as a common etiologic factor, these lesions reveal diverse histopathologic features. However, important questions remain regarding the pathogenesis and the mechanisms of development of these lesions.[1,2,3]
Recent studies regarding the pathogenesis of various lesions have shifted toward the microenvironmental elements (stromal constituent, e.g., fibroblasts and immunological cells) and many researchers believe that the microenvironmental parameters are a more effective constituent in the development of a lesion. A range of factors has been recognized as having an association with the process of development, including interactions between different cell types such as monocytes, lymphocytes, fibroblasts, and epithelial cells with differing kinetics. Inflammatory cells are considered to play an important role in this process. These cells interconnect through a complex network of intercellular signaling pathways that take place during the progression of the diseases. Inflammatory cells act synergistically and cooperatively with both stromal and epithelial cells by local production of stimulating factors.[4,5] Among these, two basic antigen-presenting cells are seen in the oral mucosa: macrophages and dendritic cells. Dissimilar to dendritic cells that move in and out of the oral mucosa, macrophages that are derived from blood monocytes, differentiate into macrophages on delivery to the target tissue and remain within it.[6,7] Macrophages are the main terminally differentiated mononuclear phagocytic cells, generally known as the first line of cells defending against pathogens. These cells are the major and very important immune cells present in the microenvironment cells and exhibit an array of important functions. They recognize pathogens, prime the adaptive immune cells, and initiate and conduct the host response. The function of these cells, therefore, is critical for the survival of the host.[6,8] Furthermore, macrophages have been claimed to be involved in the pathogenesis of various lesions, for example, oral diseases. These cells can exert this function by producing several mediators and growth factors, for example, epidermal growth factor, platelet-derived growth factor, fibroblast growth factor, and chemotactic factors such as interleukin-8.[9,10,11]
Eosinophils are a specific lineage of leukocytes that derive from the bone marrow, circulate in the blood and move into the peripheral tissues. These cells are characterized by distinct granules that contain cationic proteins such as eosinophil cationic protein, eosinophil peroxidase, major basic protein, and eosinophil neurotoxin, which are intensely stained by eosin. Eosinophils have been the target of attention in numerous diseases and have been shown to be increased in a number of oral lesions. Furthermore, these cells have been reported to be present in numerous fibrotic diseases, for example, fibrotic lung diseases, scleroderma, and scleroderma-like disorders as well as wound healing.[12,13,14,15]
Many studies have suggested that macrophages and eosinophils have a significant role in the pathogenesis of various lesions. Considering this, we focused on macrophages and eosinophils in this study. Our aim was to determine the contribution of these cells to the pathogenesis of reactive lesions of the oral cavity and the relationships between these biomarkers and the diverse histopathologic features. Based on the fact that oral reactive lesions interfere with the function and also considering the impossibility of surgical removal in some cases (particularly larger lesions), this could be helpful in managing these lesions. We also hypothesized that eosinophils may take part as one of the critical effector cells in fibrotic conditions of the oral cavity. Antifibrotic agents have been suggested as a nonsurgical treatment strategy for some fibrotic diseases, for example, scleroderma and chronic renal diseases. To the best of our knowledge, the role of macrophages and eosinophils has not been investigated in reactive lesions of the oral cavity to date.
METHODS
Tissue samples
Seventy-five formalin-fixed, paraffin-embedded surgically resected specimens of the oral reactive lesion were analyzed in this study. Five categories of lesions (15 cases for each group) were evaluated, including peripheral ossifying fibroma, pyogenic granuloma, fibroma, inflammatory fibrous hyperplasia, and peripheral giant-cell granuloma. All cases were obtained from the archives of the Department of Oral and Maxillofacial Pathology, Faculty of Dentistry, Tabriz, Iran, over a 10-year period (from 2005 to 2015). The clinical records and hematoxylin and eosin-stained microscope slides of all of the samples were reviewed to confirm the diagnosis.
The inclusion criteria were: (i) reactive lesions located in the oral cavity; (ii) patients submitted to surgery as the initial treatment; (iii) tissue available for microscopic analysis (glass slides and/or paraffin blocks); and (v) presence of sufficient clinical records. Exclusion criteria were: (i) patients to whom previous treatment other than surgery was performed; (ii) inappropriate paraffin blocks to obtain microscopic sections; (iii) and insufficient microscopic fields to analyze the samples; and (v) absence of an intact and nonulcerated epithelial surface.
Staining procedure
To detect the specific CD68 antigens, the sections were immunohistochemically stained with biotin-streptavidin method according to the manufacturer's guidelines (DakoCytomation, Glostrup, Denmark). Briefly, the principal procedure comprised of the following steps: (1) serial sectioning of the samples in 4-μm thickness; (2) deparaffinization of the sections in xylene; (3) rehydration of the sections through graded alcohol; (4) blocking endogenous peroxidase activity through incubation in 1% hydrogen peroxide; (5) antigen retrieval in 0.01 m sodium citrate buffer solution (pH 6.0) for unraveling of the epitopes, and subsequently rinsing with distilled water; (6) incubation with 1:50 diluted monoclonal mouse anti-human CD68 primary antibody for 30 min at room temperature to detect macrophages, and subsequently rinsing with phosphate buffered saline; (7) amplifying the immune reaction using the secondary antibody and the Streptavidin–Biotin–Peroxidase HRP complex (Envision/HRP); (8) incubation of the section with DAB (3,3’-diaminobenzidine) for 5 min to visualize the reaction products; (9) counterstaining the slides with Harris hematoxylin; and (10) mounting the slides.
To detect eosinophils, the section was stained through hemotoxylin and eosin staining method. The procedure displays the eosinophilic granules (including eosinophil cationic protein, eosinophil peroxidase, major basic protein and eosinophil neurotoxin) that are intensely stained with eosin. The staining method includes the following steps: (1) removing the wax from the sections with xylene; (2) passing through several changes of alcohol to remove the wax and rehydrate the samples, so the aqueous reagent will easily penetrate the cells and tissues; (3) applying the hematoxylin nuclear stain; (4) bluing the sections with a week alkaline solution; (5) applying the eosin to stain nonnuclear elements; and (6) rinsing, dehydrating and mounting the slides.
Quantitative evaluation of macrophages and eosinophils
All the samples were examined through the entire depth at the magnification of ×100 to recognize the hot spot fields, that is, fields with the highest number of macrophages. Five hotspot fields were selected and the obviously stained macrophages were manually counted in each high-power field (at a magnification of ×400, i.e., ×10 eyepiece and ×40 objective). The mean of five fields was the macrophage count for that specimen.[7,8]
In each case, to determine the eosinophil count, the sections were scanned at the magnification of ×100 and hot spot fields were detected. Eosinophils were then counted in 10 hot spot high-power fields, and the mean of 10 fields was the eosinophil count for that case. The samples with the eosinophil count <50 per high-power field were ranked “low;” the samples with the eosinophil count between 50 and 120 in a high-power field were graded “moderate;” and the samples with more than 120 eosinophils per high-power field were considered as “heavy.”[14,16] Only the nucleated cells with strongly red cytoplasmic granules were counted as eosinophils, and care was taken not to count mononuclear and polymorphonuclear leukocytes superimposed on red blood cells or those cells that were located within lymphoid and vascular channels.
Statistical analysis
The macrophage count for each clinical group was expressed as mean value ± standard error of the mean (SEM). The collected data were statistically analyzed using Statistical Package for Social Sciences 20.0 (SPSS, Chicago, IL, USA). To compare the macrophage count between the clinical groups, one-way ANOVA followed by Tukey's HSD test was carried out and statistical significance was established at P < 0.05. To compare the eosinophil count between the clinical groups, Kruskal–Wallis test was used and P < 0.05 was considered statistically significant.
RESULTS
Clinical findings
Clinical records and biopsy materials from 75 samples of reactive lesions of the oral cavity (15 samples for each category) were analyzed in this study. The specimens included peripheral giant-cell granuloma (6 females and 9 males) ranging in age from 6 to 51 (mean 32) years; fibroma (7 males and 7 females) ranging in age from 20 to 66 (mean 44.4) years; peripheral ossifying fibroma (6 females and 9 males) ranging in age from 14 to 42 (mean 25); pyogenic granuloma (6 males and 9 females) ranging in age from 13 to 68 (mean 43.7) and inflammatory fibrous hyperplasia and (2 males and 13 females) ranging in age from 14 to 66 (mean 49.6). Totally, 41 out of 75 cases (54%) occurred in females. The mean age distributions were as follows in descending order: inflammatory fibrous hyperplasia (49.6y), fibroma (44.4 y), pyogenic granuloma (43.7y), peripheral giant-cell granuloma (32 y), and peripheral ossifying fibroma (25 y). We observed that the sex distribution was relatively similar in these lesions. Nine cases of peripheral ossifying fibroma (60%), seven cases of fibroma (46.6%), eight cases of pyogenic granuloma (53.3%), seven cases of inflammatory fibrous hyperplasia (46.7%) and seven cases of peripheral giant-cell granuloma (46.7%) were located in the maxillary gingiva. These patterns of distribution showed that peripheral ossifying fibroma and pyogenic granuloma occurred more commonly in the maxillary gingiva.
Histopathological and immunohistochemical findings
In this study, we found that macrophages were an important integral component of reactive lesions of the oral cavity. Regarding the pattern of distribution, macrophages were diffusely distributed or clustered throughout these lesions [Figure 1]. These cells were present not only in the connective tissue but also in the epithelium. Macrophages were observed in high densities in all of the studied groups. The mean values and SEMs for macrophage counts in the oral reactive lesions are illustrated in Figure 2. Between-group differences in mean macrophage counts were analyzed using one-way ANOVA followed by Tukey's statistical tests. The number of macrophages was increased in peripheral giant-cell granuloma compared with all other groups, and this increase was statistically significant when compared with pyogenic granuloma, fibroma and inflammatory fibrous hyperplasia (P < 0.05).
Figure 1.
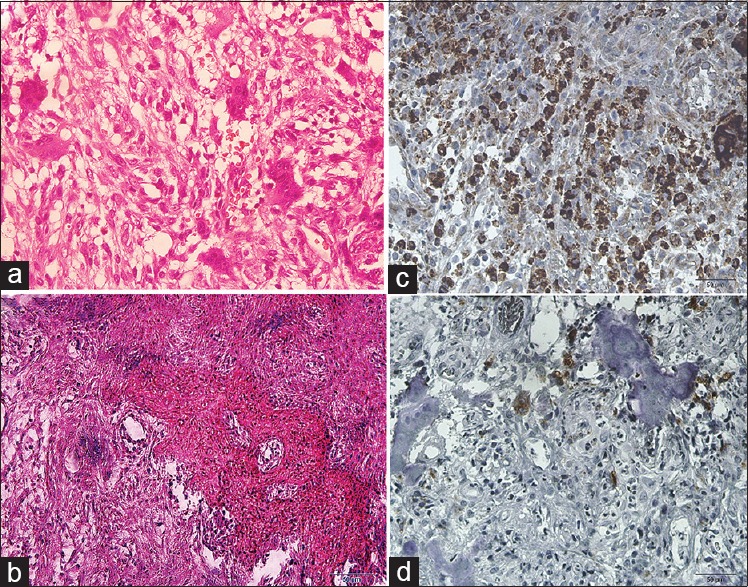
(a) Peripheral giant-cell granuloma (H&E, ×400), (b) peripheral ossifying fibroma (H&E, ×400), (c) peripheral giant-cell granuloma (IHC anti-CD68 staining, ×400), (d) peripheral ossifying fibroma (IHC anti-CD68 staining, ×400)
Figure 2.
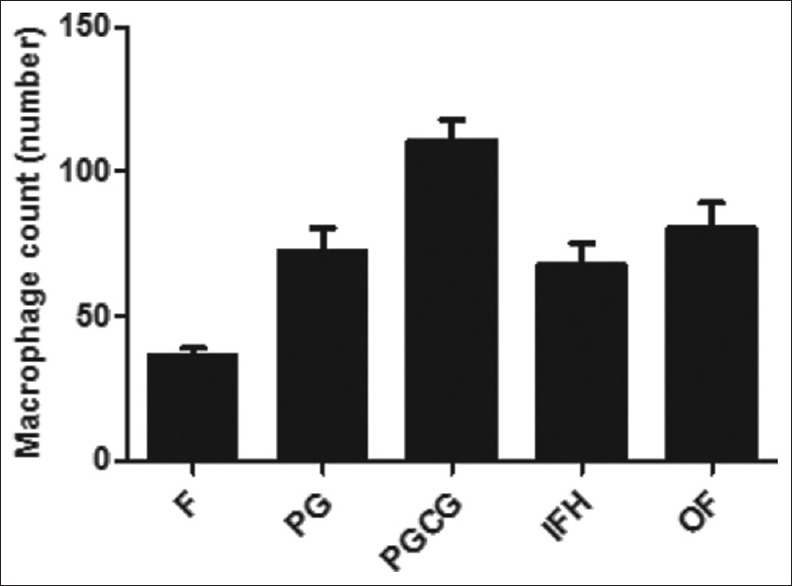
Histogram of macrophage counts in F: Fibroma, PG: Pyogenic granuloma, PGCG: Peripheral giant-cell granuloma, OF: Peripheral ossifying fibroma, IFH: Inflammatory fibrous
Our findings revealed that eosinophils were an insignificant constituent of reactive lesions of the oral cavity. Eosinophils were identified at low densities in almost all samples (under 50 eosinophils per high-power field in almost all cases). Kruskal–Wallis test showed no statistically significant difference between the groups (P > 0.05).
DISCUSSION
The immunohistochemical evidence of this study showed that CD68-positive cells were an important component of reactive lesions of the oral cavity. CD68 is a glycoprotein that is expressed on macrophages and monocytes and is also a lysosome-related protein of membrane; it is a selective marker of monocyte-macrophage lineage.[17] Our results are in accordance with previous studies that have suggested macrophages to be involved in the pathogenesis of different lesions in various sites of the body. They have suggested that macrophages have a critical role in the pathogenesis by producing several mediators and growth factors.[18,19,20]
Our results showed that macrophages were observed in high densities in all of the reactive lesions of the oral cavity, and these cells were an important internal component of oral reactive lesions. The highest number of macrophages was observed in peripheral giant-cell granuloma, and then in peripheral ossifying fibroma, pyogenic granuloma, inflammatory fibrous hyperplasia and fibroma in descending order.
In this study, we found that that macrophage count in case of peripheral giant-cell granuloma was significantly higher than all other reactive lesion of the oral cavity. Peripheral giant-cell granuloma of the oral cavity occurs peripherally in the periodontal ligament. Histologically, peripheral giant-cell granulomas are characterized by the presence of multinucleated giant-cells in a proliferative stroma of mononuclear mesenchyme cells. The histogenesis of giant-cell granuloma has been controversial for many years. However, the understanding of the possible origin of this lesion is a very important issue to manage and control these disease.[1,2,21,22] Studies on giant-cell granulomas have recently shifted toward mononuclear cells, since many investigators believe that mononuclear cells are the proliferative component of these lesions and are responsible for their biological behavior. Several studies have suggested that the cellular component shows characteristics of osteoclast progenitor cells, macrophages, myofibroblasts endothelial cells, or fibroblasts.[23,24,25] However, in this study, peripheral giant-cell granuloma showed the highest macrophage count between the reactive lesions of the oral cavity that have similar etiology. Thus, our results suggest that histiocytes are involved in the pathogenesis of these lesions. To the best of our knowledge, this is the first study comparing macrophage count between peripheral giant-cell granuloma and other oral reactive lesions, which is important in understanding the pathogenesis of this lesion.
Eosinophils have been shown to take part in the pathogenesis of various lesions, in reactions against parasites, and in autoimmune diseases. The involvement of inflammatory cells, especially mast cells and eosinophils, in the modulation of the fibrotic process and in the zones of tissue remodeling and repair, was first noticed by Paul Ehrlich in 1878. The role of eosinophils in the fibrotic process has been assessed in many studies, and the relation between these cells and fibrosis has been reported, for example, in salivary gland tissue. Eosinophils can affect fibroblasts’ functional behavior (the target cells in fibrotic disorders) by synthesizing a wide spectrum of biologically active compounds that have either fibrogenic or fibrinolytic function. Therefore, it is difficult to define the exact role of eosinophils in the fibrotic process and they have been reported to exhibit pro-fibrosis or anti-fibrosis actions in different anatomical locations.[12,14] In the present study, eosinophils were not a significant internal component of the reactive lesions of the oral cavity, and a relation between these cell and the variation of microscopic features and the degree of fibrosis was not observed.
CONCLUSIONS
In this study, we found that macrophages were a significant internal component of the oral reactive lesions and these cells were observed in high densities in all of the studied groups. Our results revealed that these cells could be involved in the pathogenesis and variation of microscopic features in these lesions. However, the biological process behind macrophages is not clearly established to date. Further clinical studies should be conducted to identify the molecular interactions of macrophages, with the ultimate aim of suggesting a new potential therapeutic target for these lesions. Our results showed that eosinophils were not involved in the modulation of the fibrotic process and the variation of microscopic features of oral reactive lesions. Furthermore, we found that peripheral giant-cell granulomas highly demonstrated histiocytic characteristics. No study has compared macrophages and eosinophils between oral reactive lesions to date.
Financial support and sponsorship
This paper was financially supported by the research council of Tabriz University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This paper was financially supported by the research council of Tabriz University of Medical Sciences.
REFERENCES
- 1.Neville B, Damm D, Allen C, Bouquot J, Neville B. Hematologic disorders. Oral Maxillofac Pathol. 2009;2:526–7. [Google Scholar]
- 2.Regezi JA, Sciubba JJ, Jordan RC. Oral Pathology: Clinical Pathologic Correlations. St. Louis: Elsevier Health Sciences; 2012. [Google Scholar]
- 3.Farahani SS, Navabazam A, Ashkevari FS. Comparison of mast cells count in oral reactive lesions. Pathol Res Pract. 2010;206:151–5. doi: 10.1016/j.prp.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Souza LR, Fonseca-Silva T, Santos CC, Oliveira MV, Corrêa-Oliveira R, Guimarães AL, et al. Association of mast cell, eosinophil leucocyte and microvessel densities in actinic cheilitis and lip squamous cell carcinoma. Histopathology. 2010;57:796–805. doi: 10.1111/j.1365-2559.2010.03721.x. [DOI] [PubMed] [Google Scholar]
- 5.Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother Pharmacol. 1999;43(Suppl):S69–71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- 6.Merry R, Belfield L, McArdle P, McLennan A, Crean S, Foey A, et al. Oral health and pathology: A macrophage account. Br J Oral Maxillofac Surg. 2012;50:2–7. doi: 10.1016/j.bjoms.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, et al. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752–9. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
- 8.Bôas DS, Takiya CM, Coelho-Sampaio TL, Monção-Ribeiro LC, Ramos EA, Cabral MG, et al. Immunohistochemical detection of ki-67 is not associated with tumor-infiltrating macrophages and cyclooxygenase-2 in oral squamous cell carcinoma. J Oral Pathol Med. 2010;39:565–70. doi: 10.1111/j.1600-0714.2010.00883.x. [DOI] [PubMed] [Google Scholar]
- 9.Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, et al. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102:1424–31. doi: 10.1111/j.1349-7006.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang XB, Tian XY, Li Y, Li B, Li Z. Elevated expression of macrophage migration inhibitory factor correlates with tumor recurrence and poor prognosis of patients with gliomas. J Neurooncol. 2012;106:43–51. doi: 10.1007/s11060-011-0640-3. [DOI] [PubMed] [Google Scholar]
- 11.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J, et al. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015;51:90–5. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT, et al. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41:152–7. doi: 10.1046/j.1365-2559.2002.01437.x. [DOI] [PubMed] [Google Scholar]
- 13.Levi-Schaffer F, Weg V. Mast cells, eosinophils and fibrosis. Clin Exp Allergy. 1997;27:64–70. doi: 10.1111/j.1365-2222.1997.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 14.Tadbir AA, Ashraf MJ, Sardari Y. Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg. 2009;20:287–9. doi: 10.1097/SCS.0b013e318199219b. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith MM, Belchis DA, Cresson DH, Merritt WD, 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg. 1992;106:27–33. doi: 10.1177/019459989210600124. [DOI] [PubMed] [Google Scholar]
- 16.Alkhabuli JO, High AS. Significance of eosinophil counting in tumor associated tissue eosinophilia (TATE) Oral Oncol. 2006;42:849–50. doi: 10.1016/j.oraloncology.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–13. [PubMed] [Google Scholar]
- 18.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews JB, Basu MK, Potts AJ. Macrophages in oral lichen planus. J Oral Pathol. 1985;14:553–8. doi: 10.1111/j.1600-0714.1985.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 21.Kruse-Lösler B, Diallo R, Gaertner C, Mischke KL, Joos U, Kleinheinz J, et al. Central giant cell granuloma of the jaws: A clinical, radiologic, and histopathologic study of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:346–54. doi: 10.1016/j.tripleo.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 22.Khiavi MM, Aghbali AA, Halimi M, Kouhsoltani M, Hamishehkar H. Immunohistochemical expression of src protein in peripheral and central giant cell granulomas of the jaws. J Oral Maxillofac Pathol. 2013;17:358–62. doi: 10.4103/0973-029X.125197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B, Yu SF, Li TJ. Multinucleated giant cells in various forms of giant cell containing lesions of the jaws express features of osteoclasts. J Oral Pathol Med. 2003;32:367–75. doi: 10.1034/j.1600-0714.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 24.Flórez-Moreno GA, Henao-Ruiz M, Santa-Sáenz DM, Castañeda-Peláez DA, Tobón-Arroyave SI. Cytomorphometric and immunohistochemical comparison between central and peripheral giant cell lesions of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:625–32. doi: 10.1016/j.tripleo.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho YR, Loyola AM, Gomez RS, Araújo VC. Peripheral giant cell granuloma. An immunohistochemical and ultrastructural study. Oral Dis. 1995;1:20–5. doi: 10.1111/j.1601-0825.1995.tb00152.x. [DOI] [PubMed] [Google Scholar]


