Abstract
Introduction:
Oral cancer has been a scourge on the human population that drastically affects the quality of life-causing chronic anxiety and depression leading to disturbance in hypothalamus pituitary axis reflected by cortisol hormone dysregulation. Stress hormones affect tumor progression at different levels such as initiation, tumor growth and metastasis. Cortisol dysregulation has been reported in other malignancies; however, a thorough review of English literature revealed only anecdotal studies investigating it in patients with oral squamous cell carcinoma (OSCC).
Objectives:
The objective of this study is to evaluate morning plasma and salivary cortisol levels in patients with OSCC, premalignant disorders (PMD's) and smokers and/or drinkers without any lesion (risk group) and control group and its correlation with Hospital Anxiety And Depression Scale (HADS-subjective) and Hamilton Rating Scale for Anxiety and Depression (HRSA, HRSD-objective).
Materials and Methods:
This cross-sectional study was carried out on 25 patients each of OSCC, PMD's, risk and control group. Self-administered HADS and clinician-rated HRSD, HRSA were applied to each patient. Morning unstimulated saliva and venous blood sample were collected between 08:00 and 11:00 h to avoid diurnal variations. Morning salivary and plasma cortisol levels were analyzed using enzyme-linked immunosorbent assay method.
Results:
Both morning plasma and salivary cortisol levels were significantly higher in patients in OSCC group as compared to other three groups in the study which were further supported by higher scores obtained from HADS, HRSA and HRSD scales.
Conclusion:
The study observed that there was marked dysregulation of both morning plasma and salivary cortisol levels along with higher scores of anxiety and depression in OSCC.
Keywords: Cortisol, hamilton rating scale for anxiety, hamilton rating scale for depression, hospital anxiety and depression scale, oral squamous cell carcinoma, plasma, Premalignant disorders, saliva
INTRODUCTION
Tumor progression is a result of interaction within the tumor and its surrounding supporting tissue, tumor stroma and microenvironment.[1] Cancer development is multifactorial which can be influenced by psychoneuroimmunological factors (stress, depression, fear and hopelessness) inherent in the patient.[2]
Chronic stress has been reported to play a role in the development of oral cancer. Long-term exposure of cancer cells to stress hormones (cortisol) causes increased production of free radicals which causes DNA damage and reduces the ability of abnormal cells to undergo apoptosis and DNA repair process which are important in self-regulating anticancer mechanisms. Increased chronic stress stimulates the production of various growth factors including insulin-like growth factor-1, vascular endothelial growth factor that can promote tumor cell growth.[3]
Stress can be measured by physiological and psychological parameters. Physiological measures include biological response to situation as well as biochemical measures including blood pressure, heart rate and increased secretions of stress hormones catecholamine's, cortisol and epinephrine. These methods are direct, highly reliable and easily quantified but have a disadvantage that they induced stress through clinical settings. Psychological measures estimate the levels of stress through self-reports and scales based on life events or daily hassles for stress evaluation and are more preferred over physiological parameters including laboratory assessments.[4]
Hospital Anxiety and Depression Scale (HADS)[5] published by Zigmond and Snaith is used to assess the psychometric properties in hospital outpatients in an adult population. Overreliance of the self-reported questionnaires can lead to overdiagnosis or underdiagnosis of psychiatric conditions. Studies have suggested that the use of clinician-administered probing can provide more sensitive indicators for diagnosing psychiatric disorders/stress.[6]
The Hamilton Rating Scale for Anxiety (HRSA)[7] and Hamilton Rating Scale for Depression (HRSD)[8] are psychological questionnaire used by clinicians to rate the severity of a patient's anxiety and depression respectively, are widely used in both clinical and research settings.[9,10,11]
Cortisol is one of the most important hormones involved in physiological regulation of stress. Cortisol secretion is induced by both physical and psychological stress, which is further controlled by feedback inhibition. Assessment of cortisol in serum has long been used to reflect adrenocortical function and disturbances in the hypothalamic–pituitary axis (HPA).[12]
In plasma, the majority of the cortisol is bound to cortisol bound protein thus; analysis of free cortisol is expensive, difficult and laborious. Moreover, the sampling of blood may be distressing which could further interfere with the experimental stress procedures.[13]
Saliva can be used for the measurement of free cortisol levels in addition to plasma. Salivary cortisol offers additional advantages over plasma cortisol as it is noninvasive, stable at room temperature for a week, easy collection of the sample and majority of cortisol exists in free form which is an indicator of free cortisol or biologically active cortisol.[14]
Increased cortisol levels have shown to be related to worse disease prognosis and poorer response to treatment in patients with breast, ovary, kidney, lungs and colon cancer. Plasma cortisol elevation, a common consequence of stress, is associated with advanced disease in patients with oral cancer.[15]
Although changes in cortisol levels (plasma and saliva) have been reported in many types of cancers, a thorough review of English literature revealed only anecdotal studies investigating this hormonal dysregulation along with psychological parameters in patients with oral squamous cell carcinoma (OSCC).[14,15]
Thus, the aim of the study was to analyze plasma and salivary cortisol levels in patients with OSCC, premalignant disorders (PMD's group), tobacco users and healthy controls and to evaluate their correlation with psychological and physiological parameters.
MATERIALS AND METHODS
This randomized cross sectional study was approved by ethical committee of the institution and included 100 subjects over a period of two years (October 2013–September 2015). These subjects were shortlisted after examining 8496 subjects of which 8396 subjects were eliminated. Included patients were categorized into four groups of 25 subjects each OSCC, PMD's, risk and control.
Group I: Participants with histopathologically established the diagnosis of OSCC graded according to the criteria laid by WHO System (2005) (OSCC group).
Group II: Participants with histopathologically established diagnosis of premalignant disorders (PMD's) graded according to criteria laid by WHO System (2005) (PMD's group).
Group III: Participants with no history of OSCC/PMD's but who are smokers and/or alcohol abusers.(Risk group).
Group IV: Healthy age- and sex-matched controls (Control group).
Inclusion criteria
Participants in the age group of 35–60 years were taken up for the study to avoid age-related bias in the cortisol level as higher hypothalamic drive is found in young men which tends to decrease with age; however, there is an overall higher range of plasma basal cortisol levels in older age group.[16,17]
Only male participants were selected for the study as hormonal changes and frequent use of oral contraceptives among females have shown to induce cognitive bias in the plasma cortisol levels. Further, studies have shown that menstrual cycle phase and oral contraceptive use exert effects on HPA responsiveness affecting cortisol levels.[16,17]
Criteria for oral squamous cell carcinoma group
Subjects with histopathological diagnosis of OSCC without any previous treatment, location of primary tumor of OSCC in anterior two thirds of tongue, floor of mouth, gingiva, lip, hard palate, buccal mucosa or retromolar area, history of smokeless tobacco consumption (≥5 Gutka sachets/Zarda/Betel quids per day), history of smoking tobacco consumption (≥5 cigarettes or bidis per day) and history of alcohol abuse (drinking 5 or more alcoholic drinks on the same occasion on at least 1 day in the past 30 days).
Criteria for PMD group
Participants with histopathologically established premalignant lesions and conditions, history of smokeless tobacco consumption (≥5 Gutka sachets/Zarda/Betel quids per day), history of smoking tobacco consumption (≥5 cigarettes or bidis per day) and history of alcohol abuse (drinking 5 or more alcoholic drinks on the same occasion on at least 1 day in the past 30 days).
Criteria for risk group
Participants with a history of smokeless tobacco consumption (≥5 Gutka sachets/Zarda/Betel quids per day), history of smoking tobacco consumption (≥5 cigarettes or bidis per day) and history of alcohol abuse (drinking 5 or more alcoholic drinks on the same occasion on at least 1 day in the past 30 days).
Criteria for healthy group
Age- and sex-matched apparently healthy controls who had no recent history of systemic conditions/diseases.
Exclusion criteria
Participants taking any medication which interferes with HPA, history of systemic (diabetes mellitus, hypertension, cardiovascular diseases, renal dysfunction, liver disorders, etc.) endocrinal, autoimmune and metabolic (gout, amyloidosis) disorders (medication involves corticosteroids administration) other than OSCC. Participants under medication affecting the level of salivary cortisol such as corticosteroids, participants with history of AIDS, bleeding dyscrasias and ulcerative conditions to prevent saliva contamination with blood, Participants undergoing radiotherapy so as to avoid hormonal changes associated with the treatment, patients who used alcoholic mouth washes on regular basis.
Application of stress scales
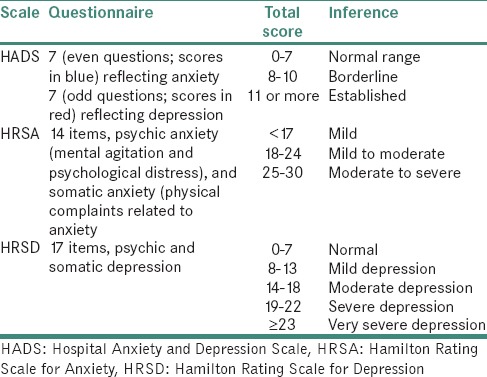
After taking informed written consent, participants were instructed to relax applying the Benson relaxation technique.[18] HADS was applied for the assessment of anxiety and depression in hospital outpatients for an adult population. The validated translated Punjabi version of HADS was used for non-English speaking participants participating in the study.[6] Each question was answered by the patient on a four point (0–3) response category, with higher scores indicating greater anxiety or depression. The total score thus calculated. The HRSA[7] is a clinicometric index which focuses on the patient's gravity of the disease. Nine of the items are scored on a five-point scale, ranging from 0 to 4. HRSD[8] is 17-item version, nine of the items are scored on a five-point scale, ranging from 0 to 4 [Table 1].
Table 1.
Scoring criteria for Hospital Anxiety And Depression Scale, Hamilton Rating Scale for Anxiety and Hamilton Rating Scale for Depression
Sample collection
For an accurate diagnosis of cortisol levels, timely sampling is mandatory as basal cortisol secretion fluctuates throughout the day. ACTH and cortisol are secreted in short pulsatile episodes, concentrated in the morning hours and declining over the afternoon and throughout the evening until sleep onset. Thus, the morning blood and salivary samples were collected from all the participants and controls between 08:00 and 11:00 h to avoid diurnal variations.
Collection of salivary sample
Stimulated whole saliva is less suitable for diagnostic applications because the foreign substances used to stimulate saliva tend to modulate the fluid pH and generally stimulate the water phase of saliva secretion, resulting in a dilution in the concentration of molecules of interest.
For collection of morning unstimulated whole salivary sample, the participants were asked to refrain from eating, drinking, chewing gums and brushing at least 2 h before the sampling procedure for collecting unstimulated whole saliva at predetermined time. To avoid temperature induced changes in cortisol level, all samples were collected in air-conditioned environment. The participants were made to rinse the oral cavity with deionized water for 20 s. Participants were asked to sit comfortably in an upright position and tilt their heads down slightly forward to pool saliva in the floor of the mouth. The intraorally retained saliva was expectorated into a graduated sterile container placed on crushed ice to a volume of 10 ml or for a maximum of 20 min of collection (whichever was earliest).
Determination of blood contamination in the saliva samples was done in OSCC group, a test commonly used to analyzed the presence of free hemoglobin in the saliva (Human free hemoglobin Enzyme-linked immunosorbent assay [ELISA] kit, Wkea Med Supplies Corp, China.), according to the manufacturer's protocol. Before analysis, the samples were centrifuged at 3500 rpm for 10 min, and then, the 2 ml supernatant clear fluid was used for the detection of cortisol immediately or stored at −20°C until analysis was performed.
Collection of blood sample
Salivary sample before blood sample to avoid stress-induced increase in plasma cortisol levels. Venous blood sample (3 ml) was collected in a sterile test tube. Moreover, centrifuged at 3500 rpm for 5 min, and then, 2 ml of plasma was used immediately for the cortisol detection or stored at −20°C until analysis was performed.
Analysis of sample for cortisol evaluation
The plasma and salivary samples were analyzed using ELISA with a commercial kit (DetectX, Cortisol Enzyme immunoassay kit, Arbor Assays USA), following the manufacturer's instructions. Optical density generated from each well was read at 450 nm on a microplate reader within 30 min, and values were interpreted at logit graph.
Calibration and statistical analysis
Data obtained was subjected to statistical analysis which was performed using the SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc. T Spearman's Rank correlation coefficient was used to identify and test the strength of a relationship. Tukey's Honest Significant Difference test in conjunction with an ANOVA (post hoc analysis) was applied to determine and identify whether there is a significant difference between the means and the observed means in all the four categories. The P < 0.05 was considered to be significant and P < 0.01 was taken as highly significant [Tables 2–4].[19]
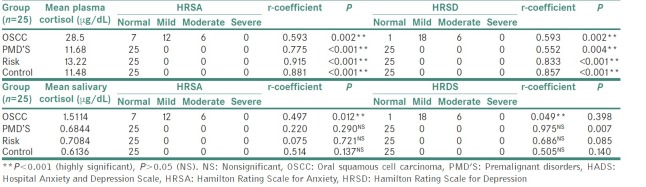
Table 2.
Mean plasma and salivary cortisol levels in comparison to Hospital Anxiety and Depression Scale parameters
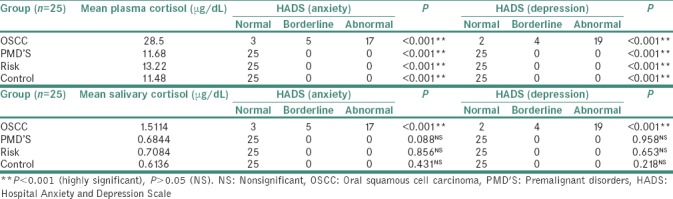
Table 4.
Comparison of self-administered (Hospital Anxiety and Depression Scale) and clinician-rated scales (Hamilton Rating Scale for Anxiety, Hamilton Rating Scale for Depression)
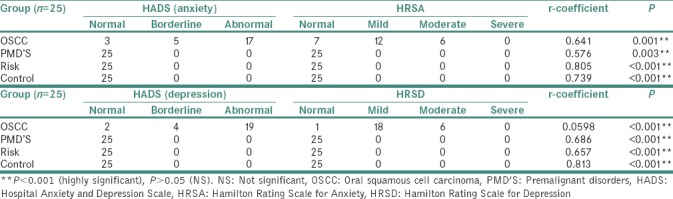
Table 3.
Mean plasma and salivary cortisol levels in comparison to Hamilton Rating Scale for Anxiety, Hamilton Rating Scale for Depression
RESULTS
Group I (oral squamous cell carcinoma group)
The mean plasma cortisol level and mean salivary cortisol level in OSCC group (27.52 μg/dL) were found to be two times higher as compared to mean plasma and salivary cortisol levels in PMD's, risk and control group, which was found to be statically significant. The scores of HADS, HRSA and HRSD were also higher in OSCC group as compared to other 3 groups showing higher levels of anxiety and depression in OSCC patients.
Group II: PMD's group
The mean plasma cortisol levels (11.68 μg/dL) and mean salivary cortisol levels (0.68 μg/dL) in PMD's group was found to be comparable to risk and control groups, and this difference was found to be statically nonsignificant. The scores of HADS, HRSA and HRSD showed normal values indicating low levels of anxiety and depression in PMD's group.
Group III: Risk group
The mean plasma cortisol (13.22 μg/dL) and mean salivary cortisol levels (0.70 μg/dL) in risk group were found to be comparable to PMD's and control groups, and this difference found to be not statistically significant. The scores of HADS, HRSA and HRSD showed low levels of anxiety and depression which was found to be similar to PMD's and control groups.
Group IV: (Control group)
The mean plasma cortisol (11.48 μg/dL) and mean salivary cortisol level (0.63 μg/dL) in risk group was found to be comparable to PMD's and control groups and this difference was found to be statistically nonsignificant. The scores HADS, HRSA and HRSD showed low levels of anxiety and depression.
DISCUSSION
Stress jeopardizes the constant state of homeostasis by the action of various external and internal stressors.[20] Stress can be measured by physiological and psychological parameters. Studies measuring physiological parameters chiefly focus on increased secretions of stress hormones catecholamines, cortisol and epinephrine.[21]
Cortisol being the major component of stress response is responsible for the fight or flight behavior and regulates immediate physiological processes such as immune function rendering cortisol as an indicator in stress evaluation studies.
Psychosocial oncology researchers have emphasized a “quality of life” with framework in the studies with assessment focused on psychological (depression/anxiety, social support) and physical (fatigue/low energy, pain and general health) outcomes related to cancer diagnosis and treatment.[22]
Clinician-rated (HRSA, HRSD) and self-reported scales (HADS) have been used for psychological stress evaluation. Studies have shown that relying only on self-reported scales often leads to overdiagnosis of stress. Clinician-rated scales which are more reliable and sensitive in the assessment of stress should be used in conjugation with patient based self-reported scales for providing a better analysis of stress.
The nervous, endocrine and immune systems are closely related by humoral mediators and various receptor sites sensitive to these signals. Numerous studies on organs such as skin,[15] breast and[23] lung[24] validated the effect of the neurohormonal products derived from chronic stress on cancer progression. However, there is a paucity of information regarding the influence of cortisol on evolution of oral cancer.
Thus, the present study was designed to analyze morning plasma and salivary cortisol levels and to evaluate their correlation with psychological parameters using subjective (HADS) and objective (HRSD, HRSA) scales in patients with OSCC, PMD's, risk and control group.
The present study found a considerable difference in plasma and salivary cortisol levels of OSCC group as compared to PMD’S, risk and control group. Paleri et al.[25] found that elevated plasma cortisol levels are a constant feature in head-and-neck cancer patients from period of diagnosis to 6 months later. In contrast, several studies found no significant difference in plasma cortisol levels between cancer patients and healthy controls. Mean plasma and salivary cortisol levels were twice in OSCC group as compared to other groups in the study which could be attributed to the presence of high levels of psychological stress associated with the awareness of lesion progression, no improvement, visibility of oral cancer, impaired esthetics and function as well as the fear of death. Moreover, there is a stimulatory effect of certain cytokines (interleukin-1 [IL-1], 2, 6) on the neuroendocrine system which may act in conjunction with psychological factors to induce chronic hypercortisolemia in cancer patients.[15,26]
Stress-related OSCC progression in OSCC cell lines have been related to upregulated IL-6 production in response to the stress hormone. Individuals who experience emotional stress display high IL-6 circulating levels.[25,27,28] Study conducted by Bernabe et al. 2011 found that IL-6 plays a key role in tumor angiogenesis, attachment and invasion of tumour cells.[15] Series of studies also supported the view that chronic stress is related to tumor progression as IL-6 stimulates cell proliferation and bone invasion of OSCC cells.[15,25,26,27,28] High IL-6 levels in OSCC tissue and plasma are associated with recurrence, lymph node involvement, a poor prognosis and survival.[25] Thus, increase in cortisol level upregulates IL-6 which is directly related to chronic stress and further to cancer progression.
In our study, there was no significant increase in cortisol levels in PMD's group compared to risk and healthy group. Kirschbaum et al.[29] showed that nicotine is the main component of cigarette smoke and can affect HPA axis, thereby disrupting cortisol secretion which attenuates the cortisol response to acute psychosocial stressors. On the contrary, Direk et al.[30] supported that smoking has short-term effect on HPA axis and its consequences on cortisol secretion patterns at a time is small to moderate. Studies conducted by Badrick et al.[31] and Bernabe et al. also found no statistical difference in cortisol levels among PMD's, risk and healthy groups and suggested that alcohol alters the cortisol circadian rhythm during the day besides the morning.[14]
Mean values of serum cortisol levels in the study were significantly higher as compared to salivary cortisol level in the same subject. The difference in the findings can be ascribed to the fact that cortisol in the plasma is bound to larger molecular proteins including transcortin and albumin. Plasma cortisol includes both bound and free form of cortisol present in individual.[32] The large molecules (e.g., binding globulins) cannot penetrate the acinar cells of the salivary gland, thereby only free form of cortisol is present in the saliva which accounts for about only 70% of the total cortisol concentration in serum.[33]
Results of the study showed higher HADS, HRSA and HRSD scores in OSCC group as compared to PMD, risk and control group interpreting that cancer patients were associated with major depression and anxiety, thus resulting in poorer quality of life in these patients suffering from the debilitating disease. Results of the present study were similar to series of studies conducted on various cancers which also show higher levels of anxiety and depression owing to poorer quality of life, bodily pain, decreased vitality and social functioning.[34,35,36]
On comparison of self-administered (HADS) and clinician-rated scales (HRSA/HRSD) for measuring stress, the study found statistically significant difference (P = 0.001) highlighting the significance of clinician-rated scales in obtaining accurate measurement of severity in psychological levels of stress. Hence, clinician-rated scales should be combined with self-reported questionnaire to provide an accurate evaluation since each assessment modality provides unique but redundant information that complements the other in predicting treatment outcomes.
Previous studies have shown that in the presence of psychological factors (depression, anxiety and stress); there is activation of HPA axis leading to raise in serum cortisol levels. In the present study, mean plasma cortisol levels in OSCC group has a significantly higher correlation to total anxiety and depression scores calculated by all the three scales as compared to other three groups.
Thus, increased psychological (HADS, HRSA and HRSD) and physiological (plasma and salivary cortisol levels) parameters in cancer patients, thereby strengthen the hypothesis that the presence of OSCC is associated with a dysregulation of cortisol secretion.
The limitations of the present study include analysis of salivary and plasma cortisol levels being performed in samples collected only at one time of day (in the morning). For a complete evaluation of the HPA axis, cortisol rhythms should be measured during 24 h as it is important to identify the fluctuations of cortisol levels over a day (analysis of circadian rhythms).[33] Another shortcoming of present study was lack of analysis of interleukins as upregulation of IL-6 expression, induced OSCC cell proliferation and could be a better indicator of disease progression.[37,38]
On the basis of the results of the study, OSCC patients exhibit significant increase in the levels of cortisol (plasma and saliva) compared with PMD's, risk and healthy controls showing dysregulation of HPA axis induced due to increased levels of anxiety and depression, having a significant negative impact on quality of life and tumor progression.
CONCLUSION
The study concluded that levels of depression and anxiety along with cortisol levels (plasma and salivary) should be evaluated in cancer patients as these frequently remain undiagnosed and untreated in such patients which results in a significant negative impact on quality of life and tumor progression.
However, despite significant progress in the past decade, further research is needed to understand mechanism underlying the stress hormones which modulate the interplay between tumor and stromal cells in the tumor microenvironment, resulting in regulation of signaling pathways with important implications on cancer progression. Stress management should be an important part of cancer treatment. Therefore, psychological assessment and intervention with psychopharmacological and psychotherapeutic modalities should be an essential part of the comprehensive treatment approach to ongoing cancer treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaker PH, Lutgendorf SK, Sood AK. The neuroendocrine impact of chronic stress on cancer. Cell Cycle. 2007;6:430–3. doi: 10.4161/cc.6.4.3829. [DOI] [PubMed] [Google Scholar]
- 3.Denaro N, Tomasello L, Russi EG. Cancer and stress: What's matter. From epidemiology: The psychologist and oncologist point of view? J Cancer Ther Res. 2014;3:6. [Google Scholar]
- 4.Brannon L, Feis J. 7th ed. United States of America: Cengage Learning; 2009. Health Psychology: An Introduction to Behavior and Health. [Google Scholar]
- 5.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 6.Sandhu SV, Sandhu JS, Bansal H, Dua V. Oral lichen planus and stress: An appraisal. Contemp Clin Dent. 2014;5:352–6. doi: 10.4103/0976-237X.137946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 8.Adapted from: Hamilton, M. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 10.Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton depression rating scale: Has the gold standard become a lead weight? Am J Psychiatry. 2004;161:2163–77. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- 11.Clark DB, Donovan JE. Reliability and validity of the Hamilton anxiety rating scale in an adolescent sample. J Am Acad Child Adolesc Psychiatry. 1994;33:354–60. doi: 10.1097/00004583-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Rödström PO, Jontell M, Hakeberg M, Berggren U, Lindstedt G. Erosive oral lichen planus and salivary cortisol. J Oral Pathol Med. 2001;30:257–63. doi: 10.1034/j.1600-0714.2001.300501.x. [DOI] [PubMed] [Google Scholar]
- 13.Shah B, Ashok L, Sujatha GP. Evaluation of salivary cortisol and psychological factors in patients with oral lichen planus. Indian J Dent Res. 2009;20:288–92. doi: 10.4103/0970-9290.57361. [DOI] [PubMed] [Google Scholar]
- 14.Bernabé DG, Tamae AC, Miyahara GI, Sundefeld ML, Oliveira SP, Biasoli ÉR, et al. Increased plasma and salivary cortisol levels in patients with oral cancer and their association with clinical stage. J Clin Pathol. 2012;65:934–9. doi: 10.1136/jclinpath-2012-200695. [DOI] [PubMed] [Google Scholar]
- 15.Bernabé DG, Tamae AC, Biasoli ÉR, Oliveira SH. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav Immun. 2011;25:574–83. doi: 10.1016/j.bbi.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Seeman TE, Robbins RJ. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr Rev. 1994;15:233–60. doi: 10.1210/edrv-15-2-233. [DOI] [PubMed] [Google Scholar]
- 17.Collier S, Hadlow N, Wardrop R, Henley D. P11 Variation of Serum Cortisol with Age and Gender CBR. Clin Biochem Rev. 2012;33:S29–30. [Google Scholar]
- 18.Benson H, Kotch JB, Crassweller KD. The relaxation response: A bridge between psychiatry and medicine. Med Clin North Am. 1977;61:929–38. doi: 10.1016/s0025-7125(16)31308-6. [DOI] [PubMed] [Google Scholar]
- 19.Bakan D. The test of significance in psychological research. Psychology Bulletin. 1960;66:423–37. doi: 10.1037/h0020412. [DOI] [PubMed] [Google Scholar]
- 20.Giese-Davis J, Sephton SE, Abercrombie HC, Durán RE, Spiegel D. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol. 2004;23:645–50. doi: 10.1037/0278-6133.23.6.645. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi L, Valle F, Coco M, Calciati A, Sleight P. Physical activity influences heart rate variability and very-low-frequency components in Holter electrocardiograms. Cardiovasc Res. 1996;32:234–7. doi: 10.1016/0008-6363(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 22.Merswolken M, Deter HC, Siebenhuener S, Orth-Gomér K, Weber CS. Anxiety as predictor of the cortisol awakening response in patients with coronary heart disease. Int J Behav Med. 2013;20:461–7. doi: 10.1007/s12529-012-9233-6. [DOI] [PubMed] [Google Scholar]
- 23.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–7. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichter I, Sirett NE. Serial measurement of plasma cortisol in lung cancer. Thorax. 1975;30:91–4. doi: 10.1136/thx.30.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paleri V, Wight RG, Silver CE, Haigentz M, Jr, Takes RP, Bradley PJ, et al. Comorbidity in head and neck cancer: A critical appraisal and recommendations for practice. Oral Oncol. 2010;46:712–9. doi: 10.1016/j.oraloncology.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto M, Hiura K, Ohe G, Ohba Y, Terai K, Oshikawa T, et al. Mechanism for bone invasion of oral cancer cells mediated by interleukin-6 in vitro and in vivo . Cancer. 2000;89:1966–75. doi: 10.1002/1097-0142(20001101)89:9<1966::aid-cncr13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Heikkilä K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44:937–45. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 28.Chakravarti N, Myers JN, Aggarwal BB. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int J Cancer. 2006;119:1268–75. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- 29.Kirschbaum C, Wüst S, Strasburger CJ. ’Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50:435–42. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- 30.Direk N, Newson RS, Hofman A, Kirschbaum C, Tiemeier H. Short and long-term effects of smoking on cortisol in older adults. Int J Psychophysiol. 2011;80:157–60. doi: 10.1016/j.ijpsycho.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92:819–24. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 32.Katayama S, Yamaji T. A binding-protein for aldosterone in human plasma. J Steroid Biochem. 1982;16:185–92. doi: 10.1016/0022-4731(82)90166-2. [DOI] [PubMed] [Google Scholar]
- 33.Lewis JG. Steroid analysis in saliva: An overview. Clin Biochem Rev. 2006;27:139–46. [PMC free article] [PubMed] [Google Scholar]
- 34.Vora A, Parikh PM, Shanthi N, Pai VR, Prasad N, Goswami S, Shah S. Role of hospital anxiety and depression scale in reducing need of a formal psychiatric referral in cancer patients’. J Clin Oncol. 2005;23(16):8037–8037. [Google Scholar]
- 35.Pandey M, Sarita GP, Devi N, Thomas BC, Hussain BM, Krishnan R, et al. Distress, anxiety, and depression in cancer patients undergoing chemotherapy. World J Surg Oncol. 2006;4:68. doi: 10.1186/1477-7819-4-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atesci FC, Baltalarli B, Oguzhanoglu NK, Karadag F, Ozdel O, Karagoz N, et al. Psychiatric morbidity among cancer patients and awareness of illness. Support Care Cancer. 2004;12:161–7. doi: 10.1007/s00520-003-0585-y. [DOI] [PubMed] [Google Scholar]
- 37.Derogatis LR, Morrow GR, Fetting J, Penman D, Piasetsky S, Schmale AM, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–7. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 38.Brenes GA. Anxiety, depression, and quality of life in primary care patients. Prim Care Companion J Clin Psychiatry. 2007;9:437–43. doi: 10.4088/pcc.v09n0606. [DOI] [PMC free article] [PubMed] [Google Scholar]