Abstract
Background:
Pemphigus is a group of autoimmune blistering diseases characterized by loss of keratinocyte cell adhesion that leads to blister formation clinically. Induction of apoptosis or of proapoptotic proteins by pemphigus immunoglobulin G (IgG) may be part of the mechanism by which IgG induces acantholysis. Some of the current data suggest that activation of proapoptotic proteins such as bax and caspase cysteine proteinases may sensitize cells to the acantholytic effects of pemphigus IgG. Thus, a central role of apoptosis in the mechanisms of blister induction is well recognized.
Aims:
This study aims (a) To find which pathway of apoptosis is involved in the pathogenesis of pemphigus and (b) to evaluate the expression of bax and caspase-8 and its key role in pemphigus.
Materials and Methods:
The study was conducted on 21 samples of oral pemphigus. The presence of apoptosis was evaluated in the sections taken from histopathologically diagnosed oral pemphigus archival blocks using peroxidase-antiperoxidase immunohistochemical method.
Results:
The expression and staining intensity of pro-apoptotic marker bax and apoptotic marker caspase-8 were observed in the various areas with varying intensity in different samples. The result was subjected to statistical analysis.
Conclusion:
The results obtained in the present study suggest that the process of apoptosis occurs in PV. Hence, inhibition of apoptosis in the patients could reduce the severity of the lesions, and they could also represent new specific targets for pemphigus treatment.
Keywords: Apoptosis, bax, caspase-8, immunohistochemistry, oral pemphigus
INTRODUCTION
Oral mucous membrane may be affected by a variety of blistering mucocutaneous diseases. Oral lesions may occur first or very early in several mucocutaneous disorders which are associated with persistent suffering for months and years. Pemphigus is a group of autoimmune blistering diseases characterized by loss of keratinocyte cell adhesion that leads to clinical blister formation. Apoptosis can follow both as a cell to cell detachment and as a loss of cell-matrix interaction.[1] Induction of apoptosis or of proapoptotic proteins by pemphigus immunoglobulin G (IgG) may be part of the mechanism by which IgG induces acantholysis.[2] Current data recognize a central role of apoptosis in the mechanism of blister induction.[3] Some of the current data suggest that apoptosis is not required for blister induction,[4] but that activation of proapoptotic proteins such as bax and caspase cysteine proteinases may sensitize cells to the acantholytic effects of pemphigus IgG.[5] IHC is an excellent detection technique and has the tremendous advantage of being able to show exactly where a given protein is located within the tissue examined. Using IHC is thus a useful tool in identification of apoptotic proteins in the disease of interest and the present study is carried out to identify the presence of bax and caspase-8 as the markers of apoptosis in oral pemphigus.
Aims and objectives
To find which pathway of apoptosis is involved in the pathogenesis of pemphigus
To evaluate the expression of bax and caspase-8 and its key role in pemphigus.
MATERIALS AND METHODS
The archival samples (21 in number) which were histopathologically diagnosed as oral pemphigus were included for the study. Thus, procured paraffin-embedded samples were sectioned to the thickness of 3–4 μm and mounted on coated (charged) slides. Then, the sections were deparaffinized, and routine immunohistochemical staining procedure using peroxidase-antiperoxidase method was followed.
Immunohistochemistry
Immunohistochemistry was used to detect the expression of caspase-8 and bax in the collected samples. Caspase-8 (mouse monoclonal) primary antibody (1 mL) was used with the dilution of 1:100. The rabbit polyclonal (6ml - ready to use solution) was used as Bax primary antibody. Heat-induced antigen retrieval was used for caspase-8; anti-bax antibody required no antigen retrieval. Caspase 8 was incubated for 1 h and bax for 30 min. 3,3’-Diaminobenzidine was used as the chromogen and Mallory's hematoxylin for counterstaining the nuclei.
The sections were washed and dehydrated with grading alcohols, finally stabilized with the mounting medium and viewed the staining under the microscope for the evaluation of the results [Figures 1–6].
Figure 1.
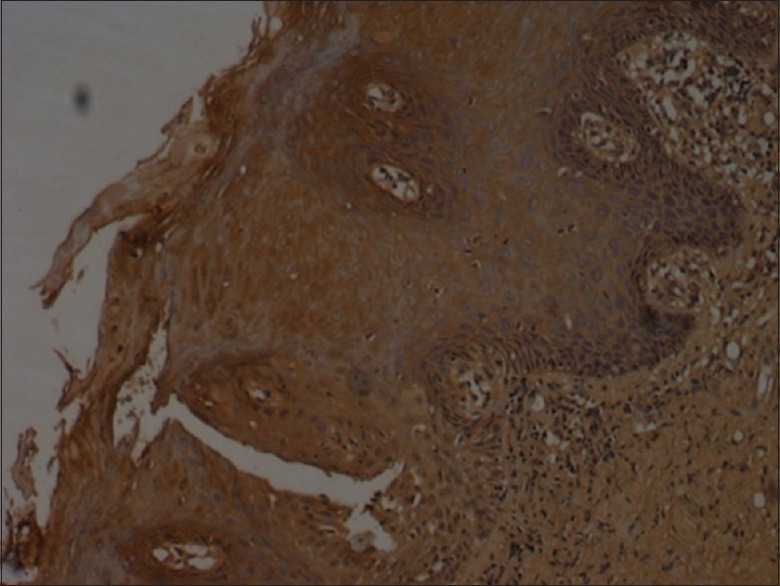
Bax staining in pemphigus - mild (×10)
Figure 6.
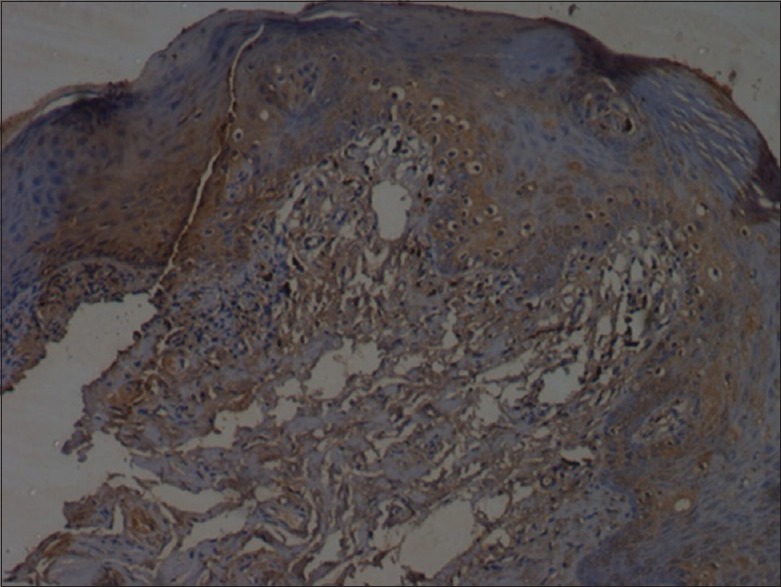
Moderate caspase-8 staining (×10)
Figure 2.
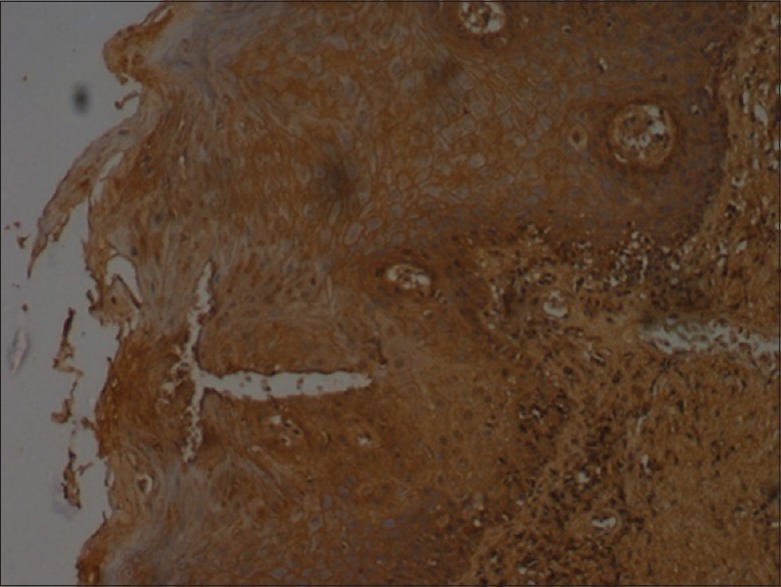
Bax staining in pemphigus - moderate (×10)
Figure 3.
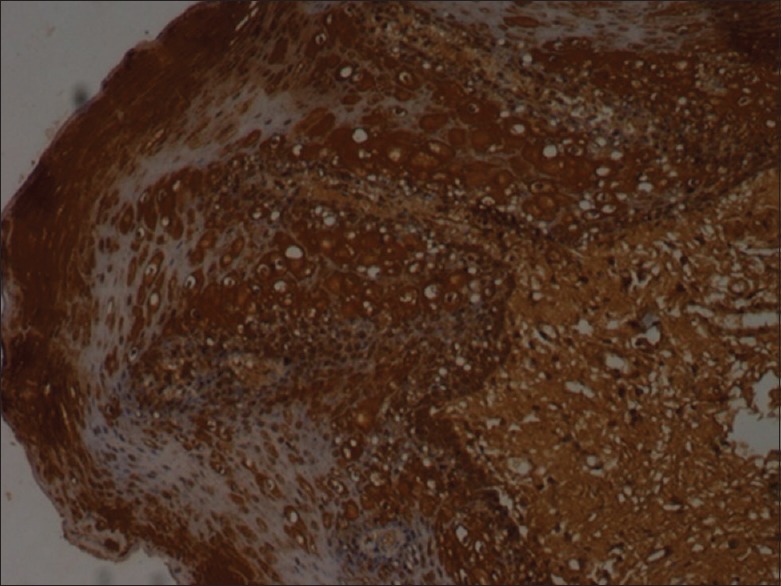
Intense bax staining (×10)
Figure 4.
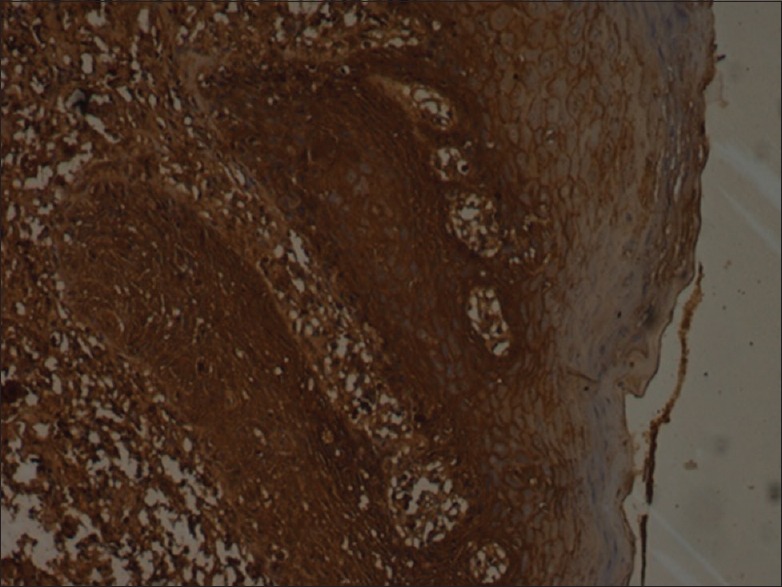
Intense staining of caspase-8 (×10)
Figure 5.
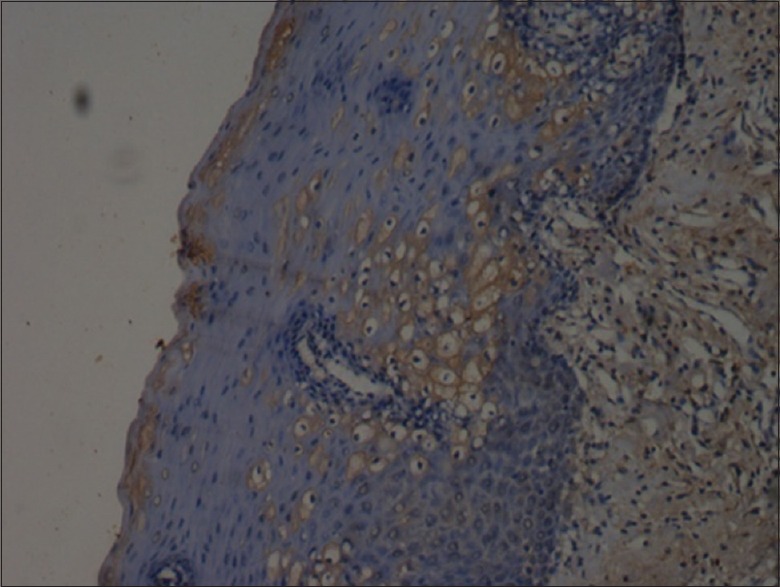
Mild staining of caspase-8 (×10)
Evaluation of immunostaining
On light microscope, each section was examined for the expression of bax and caspase-8 individually based on the following criteria: intensity of staining (mild, moderate and severe); distribution pattern of the stain (localized or generalized/diffuse) and location (layers of epithelium stained). The results were then subjected to statistical analysis.
RESULTS
Positivity of results (staining) was based on 3 features related to staining – intensity, spread and location and the following score pattern was used
Intensity was graded as mild (1), moderate (2) and intense (3)
Distribution pattern were scored as generalized (1) and localized (2)
Locations (layers) involved were scored as basal only (1), spinous only (2), basal and parabasal or basal and spinous (3), all layers (4).
Out of 21 oral pemphigus samples, all samples were immunopositive for bax and 16 samples were immunopositive for caspase-8 with only 5 samples showing immunonegativity. 15 out of 21 samples showed immunopositivity for both the antibodies [Table 1 and Figure 7].
Table 1.
Data depicting the number of samples showing positive and negative results with both the markers

Figure 7.
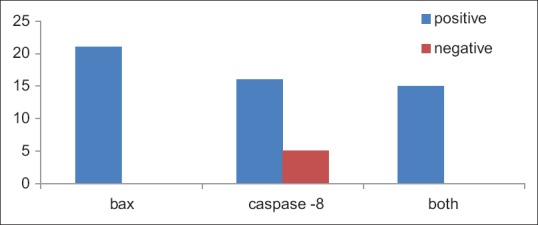
Bar diagram depicting the immunoreactivity of bax and caspase-8
Data were tabulated for intensityof staining: With the 21 samples of oral pemphigus, 7 were stained mildly with bax, 4 took moderate staining and 10 showed intense staining with bax. With caspase-8 staining in oral pemphigus, only 1 sample showed mild staining, 2 with moderate uptake and 13 were intensely stained [Table 2 and Figure 8].
Table 2.
Data depicting the intensity of staining uptake by both the markers

Figure 8.
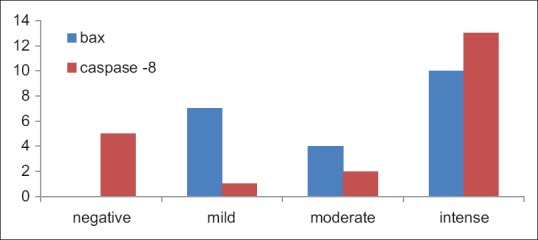
Column bar chart indicating the intensity of stain uptake
Data were tabulated for the distribution pattern of the stain, and out of the 21 samples, 18 samples showed generalized distribution pattern for bax and 3 showed localized distribution of the stain. Whereas in case of caspase-8, two samples exhibited generalized pattern and one exhibited localized staining in distribution [Table 3 and Figure 9].
Table 3.
Distribution pattern of the stain

Figure 9.

Column bar chart depicting distribution pattern of both the stains
Location of the staining pattern is explained in Table 4: In the 21 samples stained with bax, 2 samples exhibited immunopositivity in the basal layer, 7 samples in the spinous layer, 7 in the basal and spinous layers and 5 in all the layers. With caspase-8 staining, 1 sample showed immunopositivity in the basal layer only, 10 were positive in the spinous layer and 5 were positive in the basal and spinous layers [Figure 10].
Table 4.
Location of the staining pattern

Figure 10.
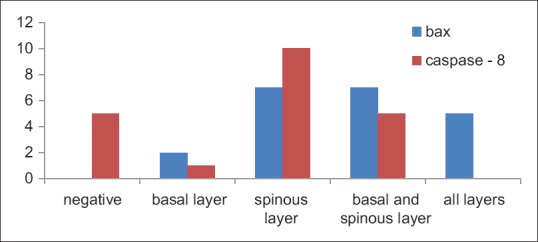
Column bar chart depicting location of the staining pattern for both the stains
Statistical analysis
The main outcome of the study was analyzed using Chi-square test.
With all the samples showing immunopositivity for bax and 76.2% of samples being immunopositive for caspase-8, the statistical significance was obtained with P = 0.017 [Table 5].
Table 5.
Statistical analysis

Further, the results obtained using the staining features such as intensity of staining (mild, moderate and severe); distribution pattern of the stain (localized or generalized/diffuse) and location (layers of epithelium stained) were tabulated and statistically analyzed using nonparametric test called Mann-Whitney test for each antibody. The test result was statistically significant in relation to the staining location with P = 0.007 but was statistically insignificant for staining intensity and distribution [Table 6].
Table 6.
Mann-Whitney test

DISCUSSION
Oral mucosa is affected by several mucocutaneous lesions and can sometimes be an initial manifestation of the disease process. Oral pemphigus is one such lesion associated with cutaneous manifestations and may be an initial manifestation of the disease process, even before cutaneous manifestations appear. It is a well-known fact that apoptosis plays a key role in the pathogenesis of pemphigus. Numerous studies have shown the role of apoptotic markers through the disease process.[6] However, very few studies have been conducted to elaborate which pathway of apoptosis is involved in these disease processes. Knowing the exact pathway involved might help in blocking the required molecules of that pathway so as to interfere in the progression of the disease and reducing the symptoms, course and suffering of the patient.[7,8]
Apoptosis occurs in a variety of settings. It is widespread during development, and it is often in that situation that it actually represents a prelethal phase of programmed reaction to injury. It is often difficult to estimate the extent because of the rapidity with which the apoptotic cells and fragments are taken up by adjacent parenchymal and/or mesenchymal phagocytes. Understanding of the mechanisms of cell death is rapidly increasing. The sequence of biochemical events leads to cell death following a variety of injuries with the overall aim of determining the least number of pathways or possibly a final common pathway. While the activation of “death receptors” on the cell surface initiates and activates extrinsic pathway through the activation of caspase-8, the initiation of intrinsic pathway requires the activation of bax on the outer membrane of the mitochondria leading to subsequent apoptosis.[9,10]
This study hence has been an attempt to add value to such a finding as to which pathway of apoptosis has been mostly adopted in oral pemphigus. As pemphigus is known to have an autoimmune etiology, assessment of apoptosis through immunohistochemical analysis was useful.
Caspase-8 was used in this study as a marker of extrinsic pathway as it is one of the molecules acting as an initiator caspase leading later in activation of the executioner caspase-3. Bax is a pro-apoptotic protein which activates caspase-9, an initiator caspase found in the intrinsic pathway, which further activates the executioner caspase-3. Thus, both the pathways cause activation of the executioner caspase, the caspase-3, ultimately ending in the apoptosis of the cell involved.
The main result inferred from this study showed that bax antibody staining showed more immunopositive results than caspase-8 antibody [Table 1]. However, immunopositivity for bax antibody was 100%, immunopositivity for caspase-8 antibody was found to be 76.2% and immunopositivity for both the markers was about 71.4%. This result was found to be nonspecific as to which pathway of apoptosis was more favored.
Wang et al. in 2004, Roh et al. and Avalos-Díaz et al. in 2007 in their individual studies on role of apoptosis in pemphigus showed that apoptosis may play an important role in the pathogenesis of pemphigus with the positivity for apoptotic markers expressed in extrinsic and intrinsic pathways with their studies including various apoptotic proteins involved in this complex mechanism.[11,12,13] Our study results also show the positivity for both the markers.
Deyhimi and Tavakoli in 2013 studied apoptosis in oral pemphigus vulgaris using immunohistochemical marker bax and the evaluation of the pro-apoptotic molecule, bax, showed its weak expression in keratinocytes[14] which is contradictory to our study result. This difference in the result outcome could be attributed to the technical differences or the complexities involved in the process of apoptosis.
Puviani et al. in 2003 had conducted a study to find levels of FasL in pemphigus sera through ELISA method and found that the elevated levels of FasL triggers the extrinsic apoptotic pathway through the activation of caspase-8.[15]
Gil et al. in experiment inhibited FAK, a nonreceptor tyrosine kinase, which is required for the activation of bax and then caspase-9 involved in intrinsic pathway and prevented the blister formation in mice induced with pemphigus sera. This showed that intrinsic pathway of apoptosis was involved in acantholysis leading to the blister formation.[16]
Our study result is indicative of the fact that in oral pemphigus, either of the pathways may be activated in the process of apoptosis and hence may not be specific. The previous study results are also inadequate in this aspect to conclude specifically which caspase pathway may be activated in the pathogenesis of oral pemphigus.
With respect to intensity of staining, the staining intensity for both the antibodies was mostly intense [Table 2].
With regard to the distribution, diffuse or generalized distribution with both the antibodies was seen in most of the samples [Table 3].
The location or the layers of the epithelium which showed immunopositivity for the antibodies were analyzed [Table 5]. With both the antibodies, positivity was seen either only in spinous layer or involved both the basal and spinous layers. This is justified by the fact that in oral pemphigus, acantholysis in spinous layer induces the basal keratinocytes to undergo degeneration. The test result was statistically significant for the immunopositivity of the antibodies in relation to the location or layers involved, with statistically insignificant result for other two aspects of staining.
The variations in the staining intensity, distribution and the location may not directly affect the outcome of the result; however, it may be the indication of the complexity of interactions through which the apoptotic proteins initiate the process and may be indicative of the present stage of the disease process. Thus, it may be helpful to monitor the progression of the disease process.
The studies done by Pacheco-Tovar et al. have highlighted the caspase pathway as a possible therapeutic target in experimental pemphigus. They, in their experiment, explored an alternative treatment approach for experimental pemphigus based on modifying caspase-related events and they concluded from their study that caspase inhibition prevents apoptosis and acantholysis in vivo.[7] Other studies using caspase inhibitors were also found effective in blocking acantholysis.
However, there have been no adequate studies to elaborate the pathways taken up in the pathogenesis of the disease. Apoptotic inhibitors if used generally may affect the other normal physiologic process. Hence, knowing the specific pathway may enlighten the study to use specific peptide inhibitor to combat the involved protein in the disease mechanism. This needs more such studies to support or clarify this hypothesis. By additional supportive studies directed at knowing the specific pathway involved, the study result can be utilized for the patient's benefit and thus reducing their sufferings.
CONCLUSION
In this study, the results show the confirmed role of apoptosis in pemphigus. Long-term immunosuppressive or immunomodulatory agents being the main line of treatment for this disease condition specifically blocking the proteins involved in the disease process could in future reduce the dependency on steroids, thus reducing their side effects. More such studies may throw light in general, on the exact pathway chosen by this disease in specific, which may also help in generalizing the treatment plan by blocking the specific protein involved. However, efficiency of such therapeutic agents in vivo has to be checked and if needed each patient's treatment can be tailor made, after confirming the exact pathway and the protein involved in the disease process. Immunohistochemistry may then be an indispensable technique in providing such information toward knowing the exact protein involved in the particular apoptotic pathway taken up by these autoimmune diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schmidt E, Waschke J. Apoptosis in pemphigus. Autoimmun Rev. 2009;8:533–7. doi: 10.1016/j.autrev.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Pelacho B, Natal C, España A, Sánchez-Carpintero I, Iraburu MJ, López-Zabalza MJ, et al. Pemphigus vulgaris autoantibodies induce apoptosis in haCaT keratinocytes. FEBS Lett. 2004;566:6–10. doi: 10.1016/j.febslet.2004.03.107. [DOI] [PubMed] [Google Scholar]
- 3.Grando SA. Pemphigus autoimmunity: Hypotheses and realities. Autoimmunity. 2012;45:7–35. doi: 10.3109/08916934.2011.606444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt E, Gutberlet J, Siegmund D, Berg D, Wajant H, Waschke J, et al. Apoptosis is not required for acantholysis in pemphigus vulgaris. Am J Physiol Cell Physiol. 2009;296:C162–72. doi: 10.1152/ajpcell.00161.2008. [DOI] [PubMed] [Google Scholar]
- 5.Bektas M, Jolly P, Rubenstein DS. Apoptotic pathways in pemphigus. Dermatol Res Pract. 2010;2010:456841. doi: 10.1155/2010/456841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation duration apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco-Tovar D, López-Luna A, Herrera-Esparza R, Avalos-Díaz E. The caspase pathway as a possible therapeutic target in experimental pemphigus. Autoimmune Dis. 2011;2011:563091. doi: 10.4061/2011/563091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotti R, Marconi A, Pincelli C. Apoptotic pathways in the pathogenesis of pemphigus: Targets for new therapies. Curr Pharm Biotechnol. 2012;13:1877–81. doi: 10.2174/138920112802273236. [DOI] [PubMed] [Google Scholar]
- 9.Kantari C, Walczak H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim Biophysica Acta. 2011;1813:558–63. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 10.D’Angelis CA. The pathology of pemphigus: A mini-review. North Am J Med Sci. 2013;6:37–40. [Google Scholar]
- 11.Wang X, Brégégère F, Frusić-Zlotkin M, Feinmesser M, Michel B, Milner Y, et al. Possible apoptotic mechanism in epidermal cell acantholysis induced by pemphigus vulgaris autoimmunoglobulins. Apoptosis. 2004;9:131–43. doi: 10.1023/B:APPT.0000018795.05766.1f. [DOI] [PubMed] [Google Scholar]
- 12.Roh BH, Whang KU, Cho MK, Park YL, Lee JS, Lee CW. A study of apoptosis in Pemphigus vulgaris. Korean J Dermatol. 2007;45:650–8. [Google Scholar]
- 13.Avalos-Díaz E, Pacheco-Tovar MG, Vega-Memije E, Bollain-y-Goytia JJ, López-Robles E, Hojyo-Tomoka MT, et al. The Fas pathway mediates the acantholytic process of pemphigus. FASEB J. 2007;21:713–4. [Google Scholar]
- 14.Deyhimi P, Tavakoli P. Study of apoptosis in oral pemphigus vulgaris using immune histochemical marker Bax and TUNEL technique. J Oral Pathol Med. 2013;42:409–14. doi: 10.1111/jop.12022. [DOI] [PubMed] [Google Scholar]
- 15.Puviani M, Marconi A, Cozzani E, Pincelli C. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activation of caspase-8. J Invest Dermatol. 2003;120:164–7. doi: 10.1046/j.1523-1747.2003.12014.x. [DOI] [PubMed] [Google Scholar]
- 16.Gil MP, Modol T, Espana A, Lopez-Zabalza MJ. Inhibition of FAK prevents blister formation in the neonatal mouse model of pemphigus vulgaris. Exp Dermatol. 2012;21:254–9. doi: 10.1111/j.1600-0625.2012.01441.x. [DOI] [PubMed] [Google Scholar]


