Abstract
Background:
Dental pulp inflammation is a very complex process due to its situation in low compliance (confined environment) surrounded by mineralized dentin. Mast cells are one of the mediators of inflammation. Immunohistochemical localization of mast cells by anti-tryptase antibodies in formalin-fixed paraffin-embedded sections has been shown to be highly specific. The aim of the present study is to quantify the mast cells in inflamed and noninflamed human pulp tissue using immunohistochemical analysis.
Materials and Methods:
Immunohistochemical localization of mast cells by anti-tryptase antibody was done in 15 inflamed and 15 noninflamed formalin-fixed paraffin-embedded pulp tissue. A number of mast cell per five high-power fields were performed using three observers and the mean was calculated. Statistical analysis was performed using t-test.
Results:
The results of the present study showed an increased number of mast cells in inflamed pulp in comparison with noninflamed pulp.
Conclusion:
Thus, the presence of mast cells in pulp inflammation could be used as a diagnostic marker. It can also aid in the management of pulpitis as mast cell stabilizers and antihistaminic agents could be used to control pulpal pain and inflammation.
Keywords: Immunohistochemistry, inflamed pulp, mast cell tryptase, mast cells, noninflamed pulp
INTRODUCTION
Injury to the dental pulp results in the activation of inflammatory reactions mediated by histamine, bradykinin and arachidonic acid metabolites.[1] Mast cells are granule-containing secretory cells that contain tumor necrosis factor-alpha (TNF-alpha), which mediates inflammation by promoting leukocyte infiltration.[2] The location of these mast cells is usually confined to the vicinity of blood vessels. Degranulation of mast cells results in the release of its products both into the surrounding tissues and blood stream. The stimuli for degranulation include physical injury, mechanical trauma, certain chemical agents, irradiation, heat, toxins and surfactants. In addition, the mast cells express receptors for neuropeptides which on activation induces cytokine production and release of mediators which include several proteins and angiogenesis factors.[3] Inflammatory tissue changes such as increased vascular permeability, edema and phagocytosis are brought about by biologically active substances released from mast cells.[4] The immunohistochemical localization of mast cells by anti-tryptase antibodies in formalin-fixed paraffin-embedded sections has been shown to be highly specific for mast cell quantification. The aim of the present study is to quantify the mast cells in inflamed and noninflamed human pulp tissue using immunohistochemical analysis.
MATERIALS AND METHODS
The study was approved by the Institutional Ethics Committee – CSP/13/AUG/30/140. Human pulp tissue was obtained from both inflamed and normal healthy tooth. Pulp tissue was extirpated with the help of barbed broach as a regular endodontic procedure in the root canal treated tooth for the inflamed group (n = 15). Healthy pulp tissue was obtained from third molars and premolars extracted for orthodontic reasons (n = 15). Samples were stored in 10% buffered formalin immediately after extraction. Teeth were sectioned apically, and 10% formalin was injected into the root for the fixation of pulp tissue. The tooth was split longitudinally to remove the pulp tissue and stored in 10% formalin. The normal skin was used as a control for mast cells. Then, the specimens were dehydrated in ethanol, immersed in xylene and embedded in paraffin block for further analysis. The 4 μm sections were made and stained with hematoxylin and eosin according to the standard protocol. Human tissue samples were stained with immunohistochemistry (IHC) for mast cell tryptase. In brief, 4 μm sections were made on polylysine-coated slides and were deparaffinized and dehydrated with three changes of xylene and alcohol, respectively, and treated with peroxide block. Following this, antigen retrieval was done, and protein block was applied. The sections were stained with primary mouse monoclonal anti-mast cell tryptase antibody for 30 min at room temperature following which the sections were incubated with horseradish peroxide at room temperature for 30 min. The sections were incubated with diaminobenzidine (DAB) chromogen for 5 min. The DAB was prepared only before use by mixing one drop of chromogen to 1 ml of chromogen buffer in the mixing vial. The tissue sections were counterstained with hematoxylin in a staining bath for 1 min. The anti-mast cell tryptase antibody stained the mast cells reddish brown against a blue background. The number of mast cells in inflamed and noninflamed samples in five fields at a magnification of ×40 at the “hot spots” was counted under the light microscope. Results were expressed as the average number of mast cells per high-power field. Any cluster of mast cell granules appearing reddish-brown and clearly separate from an adjacent cluster was considered to be a single mast cell. All counts were performed by three investigators in a five-headed microscope to eliminate interobserver variation. The results were statistically analyzed using Student's t-test.
RESULTS
Mast cells were observed in both inflamed and noninflamed pulps with an increased number of mast cells in the inflamed group in comparison with the control group. The results were statistically significant (P < 0.001). The results are tabulated in Table 1. Figure 1 shows H & E-stained noninflamed tissue section under ×40 magnification. Figure 2 shows H & E-stained inflamed tissue section under ×40 magnification. Figure 3 shows the presence of mast cells in an IHC-stained inflamed pulp tissue section under ×40 magnification. Figure 4 shows the absence of mast cells in an IHC-stained noninflamed pulp tissue section under ×40 magnification. Figure 5 shows the presence of degranulating mast cells in an IHC-stained pulp tissue section under ×40 magnification. Figure 6 shows normal skin tissue used as a positive control under ×40 magnification. Figure 7 shows normal skin tissue used as a negative control under ×40 magnification.
Table 1.
Statistical analysis showing comparison of mast cells in inflamed and noninflamed pulp tissue
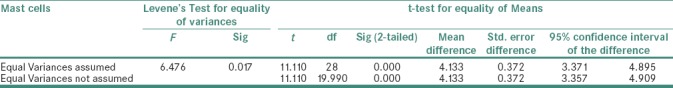
Figure 1.
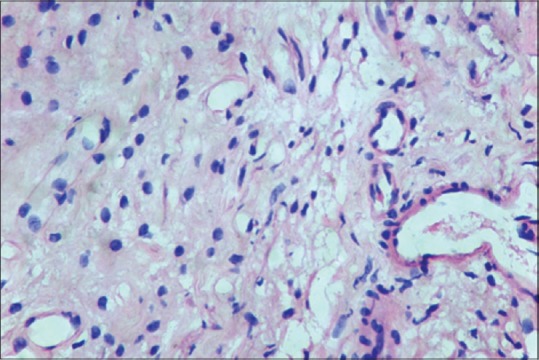
Noninflamed pulp tissue (H&E, ×40)
Figure 2.
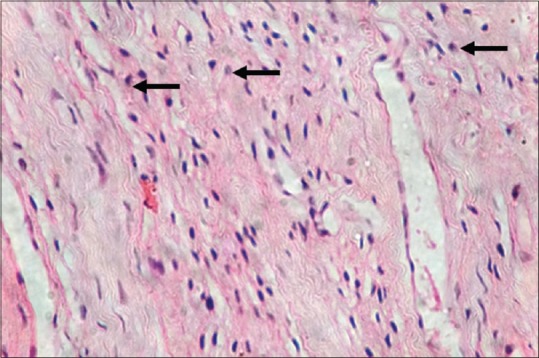
Inflamed pulp tissue (H&E, ×40)
Figure 3.
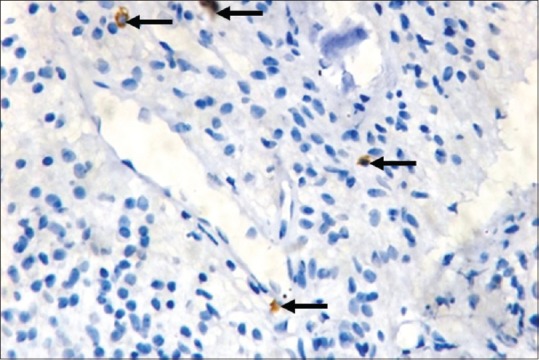
Presence of mast cells in inflamed pulp tissue (IHC, ×40)
Figure 4.
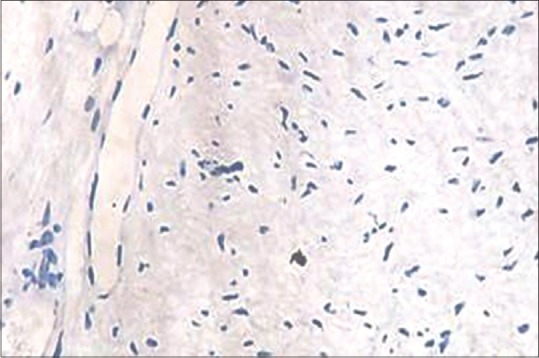
Absence of mast cells in noninflamed pulp tissue (IHC, ×40)
Figure 5.
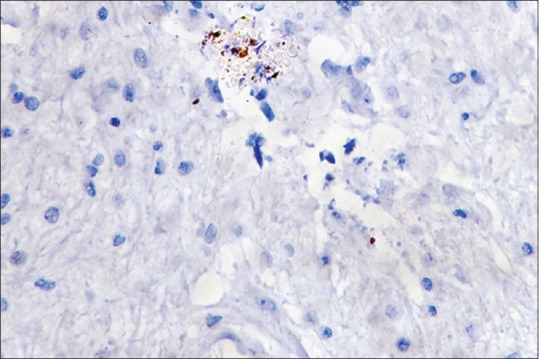
Presence of degranulating mast cells (IHC, ×40)
Figure 6.
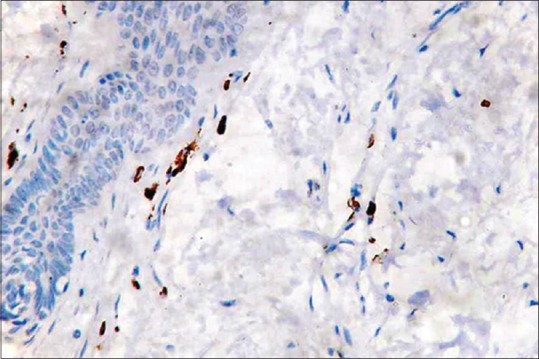
Positive control - normal skin tissue (IHC, ×40)
Figure 7.
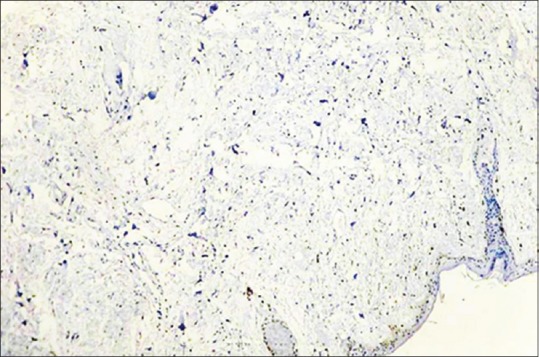
Negative control - normal skin tissue (IHC, ×40)
DISCUSSION
Mast cells are present in most tissues such as skin, mucosa of the lungs, digestive tract, oral cavity, conjunctiva and nose surrounding blood vessels and nerves.[5] Studies have shown that mast cells play a role in neurogenic inflammation in addition to their role in allergic reactions.[6] However, the presence of mast cells in the dental pulp and the role of mast cells in pulpal inflammation have been a controversial issue as studies in the past have revealed the absence of mast cells in normal tissue and few cells in the inflamed tissue.[7] With the available information, the present study was conducted to quantify mast cells in the inflamed and noninflamed pulp.
The demonstration of mast cells in tissue sections is very technique sensitive. Ali Farnoush suggested that the method used for obtaining dental tissue, preservation and fixation process could alter mast cell integrity.[4] To circumvent these problems, we obtained pulp tissue from healthy teeth using a tooth split technique and from pulpitis cases using barbed broach technique. Since dehydration of the pulp affects mast cell integrity, fresh extracted teeth were used in this study. The fixation of the samples was done immediately in 10% neutral buffered formalin to prevent dehydration.
With regard to the marker, we used a monoclonal antibody against tryptase to detect mast cells. Pereira et al. identified that tryptase represents a subfamily of trypsin-like proteinases that are stored in secretory granules of mast cells.[8] The enzyme is a specific marker for mast cells. The immunohistochemical method using tryptase as marker is considered more superior than metachromatic staining with toluidine blue which is not specific and can also stain macrophages.
In this study, skin tissue was used as positive control. The results of this study showed that very few mast cells were present in the healthy pulp tissue sections while distinctly more numbers of mast cells were present in the inflamed pulp tissue. The difference was found to be statistically significant.
The presence of few numbers of mast cells in the healthy pulp could be attributed to its presence as resident leukocytes in various connective tissue compartments of the body. However, previous studies by Martins et al.[7] and Nica et al.[9] have shown a complete absence of mast cells in human and rat dental pulp. They hypothesize that the pulp is a unique connective tissue encapsulated within mineralized dentin and is considered a low compliance environment. In this situation, the presence of mast cells could generate the release of vasoactive substances that could produce pain. However, our findings could be explained on the basis that no healthy tissue of the body is histologically perfect. Hence, there could be a low-grade inflammatory infiltrate even in the healthy pulp which could account for the scattered numbers of few mast cells. Zachrisson has also reported the presence of few intact mast cells around small blood vessels and adjacent to plasma cells in young permanent teeth; although, pulp tissue with very few inflammatory cells was devoid of mast cells.[10]
With regard to inflamed pulp samples, the present study revealed the present of significant numbers of mast cells. Our finding justifies that mast cells are found in high numbers in inflammation and are known to participate in innate immune mechanisms. They are activated during inflammation and release various vasoactive amines such as histamine and heparin and also cytokines such as TNF-alpha and interleukin-8 from their granules. In addition, mast cells synthesize and release mediators which exert potent immunomodulatory effects through induction of endothelial leukocyte adhesion molecule. T-lymphocyte-derived cytokines influence mast cell migration and release, which facilitates mast cell degranulation and release in response to a range of immunological and nonimmunological stimuli.[2] Martins et al. also postulated that blood flow during inflammation is controlled by neuropeptides such as substance P and calcitonin gene-related peptide in pulp tissue and periapical regions. In addition, substance P interacts with the mast cells to trigger their degranulation and release vasoactive substances.[7]
Heat conduction into the pulp through carious dentin could result in mast cell activation and degranulation in the inflamed pulp. In addition, the role of microbes should be considered. Microbial components such as lipopolysaccharide could trigger the alternative pathway of complement which could in turn cause production of C3b. C3b is an activator of mast cell degranulation as C3b receptors are present on mast cell surfaces. Based on all these phenomena that are known to operate, our findings of the presence of increased mast cells in inflamed pulp shed light on the fact that mast cells are important in regulating pulpal pathology. Moreover, in a few samples of inflamed pulp, we could also demonstrate actively degranulating mast cells.
The results of our study are important as we have shown increased number of mast cells in the inflamed pulp. These cells could be protective as well as destructive as they could cause connective tissue breakdown in inflammation.[11] As mast cells play a pivotal role in inflammation, therapies that target mast cell function could have value in the management of chronic inflammation of the pulp.
CONCLUSION
Thus, the presence of mast cells in pulp inflammation could be used as a diagnostic marker. Moreover, this could also help in treatment planning in pulpitis as mast cell stabilizers and antihistaminic agents could be used in the future to control pulpal pain and inflammation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. V. Saranaya, Senior Resident, Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, Sri Ramachandra University, for her valuable contribution.
REFERENCES
- 1.Torabinejad M, Walton RE. Endodontics: Principles and Practic. St. Louis, Missouri: WB Saunders, Saunders Elsevier; 2009. Pulp and periapical pathosis; pp. 49–67. [Google Scholar]
- 2.Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003;14:188–98. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- 3.Freitas P, Novaretti CP, Rodini CO, Batista AC, Lara VS. Mast cells and lymphocyte subsets in pulps from healthy and carious human teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e95–102. doi: 10.1016/j.tripleo.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Farnoush A. Mast cells in human dental pulp. Journal of Endodontics. 1984;10:250–2. doi: 10.1016/S0099-2399(84)80057-6. [DOI] [PubMed] [Google Scholar]
- 5.Prussin C, Metcalfe DD. 4.IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2003;111:S486–94. doi: 10.1067/mai.2003.120. [DOI] [PubMed] [Google Scholar]
- 6.Karapanou V, Kempuraj D, Theoharides T. Oral neuroimmune network and mast cells. European Journal of Inflammation. 2009;7:1–8. [Google Scholar]
- 7.Queiroz-Júnior CM, da Fonseca Pacheco CM, Marçal SV, Costa de Melo PC, de Melo Maltos KL. Mast cell in dental pulp: Does it have a role. Odontol. Clin. Cient, Recife. 2012;11:71–4. [Google Scholar]
- 8.Pereira PJ, Bergner A, Macedo-Ribeiro S, Huber R, Matschiner G, Fritz H, et al. Human beta-tryptase is a ring-like tetramer with active sites facing a central pore. Nature. 1998;392:306–11. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- 9.Nica LM, Raica M. Normal and inflammatory human dental pulp: A morphohistochemical approach. TMJ. 2004;54:70–3. [Google Scholar]
- 10.Zachrisson BU. Mast cells in human dental pulp. Arch Oral Biol. 1971;16:555–6. doi: 10.1016/0003-9969(71)90202-0. [DOI] [PubMed] [Google Scholar]
- 11.Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–90. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]


