Abstract
Cytoskeleton of a cell is made up of microfilaments, microtubules and intermediate filaments. Keratins are diverse proteins. These intermediate filaments maintain the structural integrity of the keratinocytes. The word keratin covers these intermediate filament-forming proteins within the keratinocytes. They are expressed in a specific pattern and according to the stage of cellular differentiation. They always occur in pairs. Mutations in the genes which regulate the expression of keratin proteins are associated with a number of disorders which show defects in both skin and mucosa. In addition, there are a number of disorders which are seen because of abnormal keratinization. These keratins and keratin-associated proteins have become important markers in diagnostic pathology. This review article discusses the classification, structure, functions, the stains used for the demonstration of keratin and associated pathology. The review describes the physiology of keratinization, pathology behind abnormal keratin formation and various keratin disorders.
Keywords: Keratinization, disorder, marker
INTRODUCTION
Keratin is a multigene family of proteins. The word kera is derived from the Greek word meaning horn. Historically the term “keratin” stood for all of the proteins extracted from skin modifications, such as horns, claws and hooves. Subsequently, it was realized that keratin is actually a mixture of keratins, keratin filament-associated proteins and other enzyme proteins derived from epithelial cells. These keratins are characteristically found only in the epithelial cells. In humans, keratins are encoded by 54 genes.
Keratins are broadly divided into the following:[1]
Based on the preferential synthesis
Primary keratins – keratins which are always synthesized by the epithelial cells on a regular basis. For example, – K 8/18 in simple epithelia and K 5/14 in the stratified epithelia
Secondary keratins – Produced by epithelial cells in addition to primary keratins, K 7/19 in the simple epithelia and 6/16 in the stratified epithelia.
Based on biochemical properties
Type I – Acidic (9–20)
Type II – Basic (1–8).
Based on molecular weight
Low – Glandular and simple
Intermediate – Stratified epithelia
High – Keratinized stratified epithelia.
Based on distribution
Soft – Skin and mucosa
Hard – Nails and hair.
CHEMICAL STRUCTURE OF KERATIN
Each keratin is characterized by a chain of amino acids as the primary structure of the keratin protein, which may vary in the number and sequence of amino acids, as well as in polarity, charge and size (Brown, 1950; Makar et al. 2007). The amino acid sequence of a keratin influences the properties and functions of the keratin filament (Roop et al. 1984).
All proteins that form intermediate filaments have a tripartite secondary structure consisting of an N-terminal head domain, a central α - helical rod domain and C-terminal tail domain, and all proteins can self-assemble into filaments. The secondary structure of keratins is also divided into three parts, i.e., the head domain (toward the N-terminal of the molecule), the rod domain in the center and the tail domain. Each of these three domains is divided into subdomains. Domains and subdomains are determined by the amino acid sequence of the keratin and serve various functions in the assembly of keratin filaments and in the binding of keratins and keratin filaments to cell adhesion complexes or to signaling molecules.[2]
FUNCTIONS OF KERATIN
Keratins fundamentally influence the architecture and mitotic activity of epithelial cells[3]
Keratins and associated filaments provide a scaffold for epithelial cells and tissues to sustain mechanical stress, maintain their structural integrity, ensure mechanical resilience, protect against variations in hydrostatic pressure and establish cell polarity[3]
Keratins and its filaments are involved in cell signaling, cell transport, cell compartmentalization and cell differentiation[4]
Keratin proteins regulate the response to pro-apoptotic signals and have the ability to modulate protein synthesis and cell size in epithelial cells[5]
Keratins also participate in wound healing.
DIFFERENT STAINS USED FOR KERATINS
Routine H and E staining demonstrates keratin, but it is difficult to distinguish between different connective tissue structures with this routine stain. Some special stains help to detect and differentiate keratins better from other connective tissue components. These special stains are summarized in Table 1.[6,7]
Table 1.
Different stains for keratin

PHYSIOLOGY OF KERATINIZATION
The oral epithelium is stratified squamous type consisting of cells called keratinocytes. These cells undergo a process of maturation during which the cells produced by the mitotic division in the basal layer migrate to the surface where they are shed off and are replaced by the maturing cell population. This process of maturation follows two patterns: keratinization and nonkeratinization.
There are two types of cell populations: progenitor population and maturing population. The progenitor population consists of stem cells and amplifying cells. After each cell division, the daughter cell either enters the progenitor population again or enters the maturing compartment. Once it enters the maturing compartment, the keratinocyte undergoes differentiation and becomes committed to biochemical and morphologic changes. At the end of the differentiation process, a dead cell filled with densely packed protein contained within a toughened cell membrane is formed. After reaching the surface, it is shed off, a process called desquamation. This process of migration of an epithelial cell from the basal cell layer to the surface is called maturation. The time taken by a cell to divide and pass through the entire epithelium is called turnover time. It is estimated as 52–75 days in the skin, 4–14 days in the gut, 41–75 days in the gingiva and 25 days in the cheek.
These epithelial cells are composed of a cytoskeleton which forms a structural framework of the cell. Cytokeratins (CKs) along with microfilaments and microtubules form this framework. These CKs are called intermediate filaments because their diameter is intermediate (7–11 nm) between the larger microtubules (25 nm) and the smaller microfilaments (4–6 nm). The cells of the oral epithelium which contain these CKs are described as the keratinocytes. The cells of the basal layer are the least differentiated and contain the typical cellular organelles and are called the stratum basale. These cells synthesize DNA and undergo mitosis, thus providing new cells. The cells once they leave the basal layer become determined for maturation. The adjacent cells are connected to each other by desmosomes which are specialized intercellular junctions. These desmosomes contain two types of proteins – the transmembranous proteins and proteins of the attachment plaque. The transmembranous proteins are the desmogleins and desmocollins which are the members of the cadherin family. The attachment plaque proteins are desmoplakin, plakoglobin, plakophilin, envoplakin and periplakin. Above the basal cell layer rest polyhedral cells which occupy larger volume and this layer is called the stratum spinosum. The tonofilaments become denser in the spinous layer than the basal cell layer. The tonofilaments turn and loop into the attachment plaque (intracellular portion of the desmosomes). The protein-synthesizing activity of the spinous cell layer is more, indicating its biochemical changes and commitment to keratinization. The cells of the stratum granulosum are flatter and wider than the spinous cells and contain basophilic keratohyalin granules. The nuclei show signs of degeneration and pyknosis. Tonofilaments are more dense and seen in association with the keratohyalin granules. Lamellar granule or Odland body or keratinosome, a small organelle, forms in the upper spinous and granular cell layers. Keratohyaline granules discharge their contents at the junction of the granular and cornified cell layers and form the permeability barrier. At the same time, the inner unit of the cell membrane thickens forming the cornified cell envelope. Several proteins contribute to this structure such as involucrin, loricrin, periplakin and envoplakin. The stratum corneum is made up of keratinized squame which are larger and flatter than the granular cells. Here, all nuclei and other organelles such as ribosomes and mitochondria have disappeared. The keratohyalin granules have disappeared. Ultrastructurally, the cells of the cornified layer are composed of densely packed filaments developed from the tonofilaments, altered and coated by the basic protein of the keratohyalin granule, filaggrin. The keratinized cell becomes compact, dehydrated and covers a greater surface area than does the basal cell from which it developed and finally it desquamates.[8,9] The superficial acidophilic layer of the keratinized epithelium consists largely of insoluble fibrous protein with a high proportion of the sulfur-containing amino acid cysteine. This protein is termed keratin, although it is important to remember that keratins differ widely in composition between tissues and between species and the term refers to a family of proteins (Iqbal and Gerson, 1971; Baden and Goldsmith, 1972). Electron micrographs show that keratin consists of aggregates of fine fibrils, essentially similar to the tonofilaments in the deeper cells of the epithelium but appearing as light structures against a darker background of the matrix in fully keratinized cells, an appearance that has been called the “keratin pattern” (Brody, 1964). The filaments are probably bound together by the same attractive forces which operate between all polypeptide chains, although the presence of appreciable number of disulfide linkages is an important characteristic. The rate and extent of keratinization of the epithelium depends on a number of different processes during differentiation such as synthesis, breakdown and dehydration which determine the classification of the epithelium into keratinized or nonkeratinized.[10] Thus, keratin is not a cellular secretion, but it is the end result of transformation of ectoderm-derived epithelial cells called keratinocytes into squame of keratin. When these squame are worn away or desquamate from the surface, they are replaced by the process of keratinization.
Aging is associated with decreased rate of metabolic activity, but studies on epithelial proliferation and rate of tissue turnover in healthy tissue are inconclusive. Histologically, the epithelium appears thinner, and a smoothing of the epithelium connective tissue interface results from the flattening of epithelial ridges.[9]
Factors regulating keratinocyte differentiation.[11]
Numerous factors control keratinocyte differentiation.
Active metabolites of Vitamin D3 act in an autocrine pathway to decrease keratinocyte proliferation and to increase cell differentiation
Epidermal growth factor (EGF) and transforming growth factor-α exert a mitogenic effect on basal cells through interaction with EGF receptors. The receptor for EGF has been localized in basal cells of the oral mucosa
Keratinocyte growth factor (KGF), a member of the fibroblast growth factor family, is produced by lamina propria fibroblasts. Acting in a paracrine pathway, KGF exerts a powerful stimulus for epithelial cell proliferation
Interleukin 1-β and interleukin-6 can increase keratinocyte proliferation by stimulating the production of KGF
Hepatocyte growth factor is another paracrine factor originating in connective tissue that elicits keratinocyte proliferation and migration
TGF-β inhibits DNA synthesis in basal cells and promotes terminal differentiation. It is secreted by the basal and suprabasal cells
High levels of Vitamin A cause normally cornified epithelia to undergo mucous metaplasia, a condition wherein cornified surface cells are replaced by noncornified cells of lining mucosa. Vitamin A deficiency can cause an opposite effect, i.e., squamous metaplasia
Calcium plays an important role in keratinocyte differentiation.
HOW APOPTOSIS AND DESQUAMATION DIFFER?
The process of cornification involves the programmed death of the keratinocytes (Houben et al., 2007). This epidermal programmed cell death is different from the programmed cell death in apoptosis because the extra- and intra-cellular signaling cascade that is characteristic of apoptosis is not activated (Lippens et al., 2005; Denecker et al., 2007). The cornification of keratinocytes also differs from apoptosis because the terminally differentiated cells are dead but intact and not cell fragments as in apoptotic bodies (Weil et al., 1999). The only genuine apoptosis in the epidermis occurs in the so-called sunburn cells, which are basal cells that are damaged by ultraviolet B rays (Denecker et al., 2007). Apoptosis can take place at any stage of cell differentiation, but the process of cornification can start only after a cell has already gone through a certain differentiation. Hence, cornification is not a type of apoptosis (Denecker et al., 2008). However, apoptotic enzymes, such as caspase-3, are activated in cornifying cells in the transition zone between the granular layer and the layer of cornified cells (Weil et al., 1999). The enzyme caspase-3 may be involved in the dismantling of the cell nucleus and of the organelles (Weil et al., 1999). The apoptotic protease caspase-14, which is also activated in the cornifying keratinocytes of the epidermis (Demerjian et al., 2008), is not expressed in the keratinizing but not cornifying keratinocytes of the oral epithelium (Lippens et al., 2000). Caspase 14 is not activated in the cornifying cells of the nail matrix (Jäger et al., 2007), indicating differences in the processes of soft versus hard keratinization and cornification.[2]
Cytokeratin expression in normal oral mucosa
All stratified oral epithelia possess keratins 5 and 14. The keratinized oral epithelium expresses keratins 1, 6, 10 and 16 and nonkeratinized epithelium expresses keratins 4, 13 and 19[9,12] [Figure 1].
Figure 1.
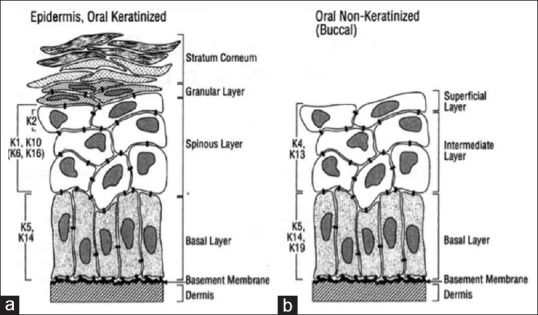
(a) Expression of cytokeratins in the oral keratinized epithelium. (b) Expression of cytokeratins in oral Nonkeratinized (Buccal) epithelium
Cytokeratin expression in odontogenic tissues
Odontogenic epithelium shows positivity for CK14, but it is gradually replaced by CK19 in preameloblasts and secreting ameloblasts.[13] Hertwig's epithelial root sheath and stellate reticulum cells show positivity for CK7. Cell rest of Malassez shows positivity for CK5/19.[1]
Cytokeratin expression in normal salivary gland tissues
In normal salivary gland, both luminal and abluminal cells show positivity for pan-CK AE1/AE3. Myoepithelial cells show positivity for CK14. Acinar cells show positivity for CK18. Intercalated, striated and excretory ducts show positivity for CK 6, 7, 18 and 19.[14,15]
PATHOLOGY OF KERATINIZATION
Pathology of keratinization shows various patterns. These pathologies can be due to defect in genes which code for keratin proteins or there are lesions which demonstrate abnormal keratinization histopathologically due to different etiological factors. There could be increased keratinization, decreased keratinization, or abnormal keratinization.
Hyperkeratinization is the defect of epithelial cells. Normally, these epithelial cells shed or de-squamate at regular intervals. In hyperkeratinization, this process is disturbed because of an excess of keratin formation and accumulation due to lack of adequate desquamation. It occurs as a secondary reaction to chronic irritation or some infection or malignancy. Hyperkeratinization which occurs because of chronic irritation is due to higher rate of proliferation of the epithelial cells.
Decreased keratinization or lack of keratin production is due to failure of the epithelial cells to undergo complete differentiation and maturation to the point of keratin formation.
Dyskeratosis is premature keratinization which occurs in individual cells or group of cells in different strata of the epithelium, before they reach the surface. These cells become separated from the adjacent cells. These dyskeratotic cells are large and round with a deep eosinophilic cytoplasm and a hyperchromatic nucleus.
A benign keratin pearl is surrounded by cells which are not dysplastic in nature; for example, – dyskeratosis. When there is lack of cohesion among the epithelial cells due to malignant changes, the cells get arranged in a concentric manner. As the fate of a squamous cell is to form keratin, these cells lay down keratin in a concentric manner and then appear as keratin pearls which are known as malignant keratin pearls. Keratin pearls are thus whorl-shaped accumulations of keratin made by malignant squamous cells and are present in concentric layers in between the squamous epithelium.
These different patterns of keratin formation depend on the amount and the nature of the inciting stimulus. In frictional keratosis or in mild leukoplakia if the underlying stimulus is removed, the change in the mucosa is reverted back to normal; while in squamous cell carcinoma, there is premature keratinization of the cells before they undergo complete differentiation.
ORAL KERATINIZATION DISORDERS
A number of lesions occur in the oral cavity which show an abnormal pattern of keratinization. These include oral geno-dermatosis to mild self-limiting lesions to cysts and tumors. All these lesions show some defect in keratinization [Flow Chart 1].[1]
Flow Chart 1.
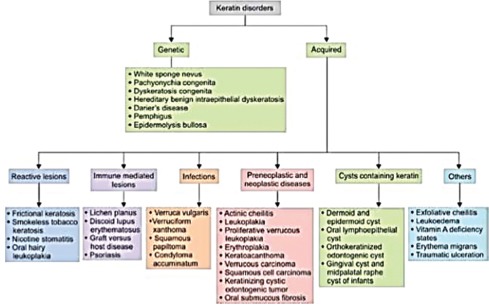
Working classification of oral keratin disorders[1]
The genetic disorders of keratin formation are due to mutations in genes which code for different keratin proteins. These lesions and the associated mutations are mentioned in Table 2.[16,17]
Table 2.
Genetic disorders of keratin formation
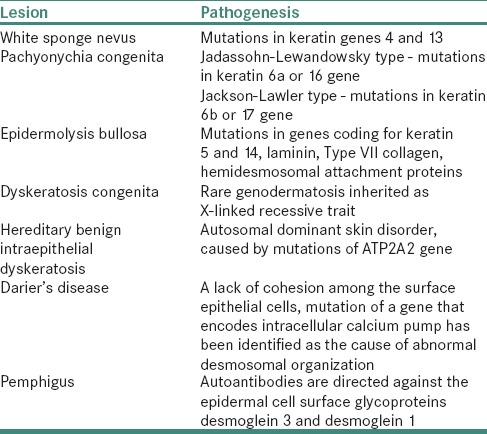
ACQUIRED KERATIN DISORDERS
There are various lesions which demonstrate abnormal keratinization histopathologically. They include reactive lesions, immune-mediated lesions, infections, preneoplastic and neoplastic diseases as well as cysts containing keratin and miscellaneous lesions. There is a considerable histopathological similarity between these disorders. These lesions show a broad spectrum of histopathological changes such as hyperkeratosis, lack of keratinization, individual cell keratinization and keratin pearl formation, while some odontogenic lesions show the presence of ghost cells which are believed to be an abnormal form of keratinization.
EXPRESSION OF CYTOKERATINS IS STUDIED BY MANY RESEARCHERS AND IS EVIDENT IN PATHOLOGIES SUCH AS ODONTOGENIC CYSTS, ODONTOGENIC TUMORS AND SALIVARY GLAND TUMORS
Cytokeratin expression in odontogenic cysts
CK 19 is absent in odontogenic keratocyst and shows positive expression in dentigerous and radicular cysts. Majority of odontogenic keratocysts are positive for CK19 and negative for dentigerous and radicular cysts. CK 5/6 is expressed in all three odontogenic cysts. CK10 is more significantly expressed in odontogenic keratocysts as compared to other odontogenic cysts. CK13 is expressed in suprabasal layers of all odontogenic cysts.[18]
Cytokeratin expression in odontogenic tumors
Odontogenic tumors with epithelial component frequently express CK 14 and 19. Immunohistochemical studies by Crivelini et al. proved that most of the tumors of mesenchymal origin such as odontogenic myxoma do not express CK 14 and 19. Thus, CK 14 and 19 can be used as markers for tumors of odontogenic epithelial origin.[19,20]
Cytokeratin expression in salivary gland tumors
Pan-CK (AE1/AE3) is an epithelial marker used for differential diagnosis between myoepithelioma/myoepithelial carcinoma or “undifferentiated carcinoma” and nonepithelial tumors.[14] According to a study conducted by Nikitakis et al., immunoreactivity for CK 7 was evident in all malignant salivary gland tumors.[21]
SUMMARY AND CONCLUSION
A cell synthesizes different subsets of keratin during the process of maturation, for example., basal cells of keratinized epithelia express K5 and 14, while suprabasal cells express K1 and K10. These epithelia can be classified according to CK expression. This pattern of keratin expression of a particular cell allows one to identify the origin of the cell and its stage of differentiation and thus helps to characterize the neoplasm. In immunohistochemistry, antibodies to keratin proteins are used routinely as markers for diagnosis of carcinomas.
In this article, we have seen various phases of a cell transforming itself into keratin, both physiologically and pathologically. Numerous studies have been conducted to test its significance, making it an important characteristic while studying the oral epithelium. Studies on CKs have opened a Pandora's box, which is of great importance in diagnosis as well as prognosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rao RS, Patil S, Ganavi BS. Oral cytokeratins in health and disease. J Contemp Dent Pract. 2014;15:127–36. doi: 10.5005/jp-journals-10024-1502. [DOI] [PubMed] [Google Scholar]
- 2.Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–59. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shetty S, Gokul S. Keratinization and its disorders. Oman Med J. 2012;27:348–57. doi: 10.5001/omj.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaidya MM, Kanojia D. Keratins: Markers of cell differentiation or regulators of cell differentiation? J Biosci. 2007;32:629–34. doi: 10.1007/s12038-007-0062-8. [DOI] [PubMed] [Google Scholar]
- 5.Gu LH, Coulombe PA. Keratin function in skin epithelia: A broadening palette with surprising shades. Curr Opin Cell Biol. 2007;19:13–23. doi: 10.1016/j.ceb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Ramulu S, Kale AD, Hallikerimath S, Kotrashetti V. Comparing modified Papanicolaou stain with ayoub-shklar and haematoxylin-eosin stain for demonstration of keratin in paraffin embedded tissue sections. J Oral Maxillofac Pathol. 2013;17:23–30. doi: 10.4103/0973-029X.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bancroft JD, Cook HC, Stirling RW. New York: Churchill Livingstone; 1994. Manual of Histological Techniques and their Diagnostic Application; pp. 419–20. [Google Scholar]
- 8.Kumar GS. Orban's Oral Histology and Embryology. 13th ed. Elsevier; 2011. pp. 245–52. [Google Scholar]
- 9.Nanci A. Ten Cate's Oral Histology Development, Structure and Function. 8th ed. Elsevier; 2012. pp. 284–9. [Google Scholar]
- 10.Squier CA, Johnson NW, Hackemann M. Oral Mucosa in Health and Disease, 1:3;2. Blackwell Scientific Publications; 1975. Cell differentiation and keratin synthesis. Structure and function of normal human oral mucosa; p. 27. [Google Scholar]
- 11.Garant PR. Oral Cells and Tissues. CarolStram, Illinois 60188: Quintessence Publishing Co, Inc; 2003. [Google Scholar]
- 12.Premalatha BR, Patil S, Rao RS, Reddy NP, Indu M. Odontogenic tumor markers-an overview. J Int Oral Health. 2013;5:59–69. [PMC free article] [PubMed] [Google Scholar]
- 13.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: Function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 14.Nagao T, Sato E, Inoue R, Oshiro H, H Takahashi R, Nagai T, et al. Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem Cytochem. 2012;45:269–82. doi: 10.1267/ahc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azevedo RS, de Almeida OP, Kowalski LP, Pires FR. Comparative cytokeratin expression in the different cell types of salivary gland mucoepidermoid carcinoma. Head Neck Pathol. 2008;2:257–64. doi: 10.1007/s12105-008-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. Philadelphia: W.B. Saunders Company; 1995. [Google Scholar]
- 17.Shafer, Hine, Levy . Shafer's Textbook of Oral Pathology. B. Sivapathasundharam. Elsevier; 2016. [Google Scholar]
- 18.Chatterjee S. Cytokeratins in health and disease. Oral Maxillofac Pathol J. 2012;3:198–202. [Google Scholar]
- 19.Crivelini MM, de Araújo VC, de Sousa SO, de Araújo NS. Cytokeratins in epithelia of odontogenic neoplasms. Oral Dis. 2003;9:1–6. doi: 10.1034/j.1601-0825.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 20.Mosqueda-Taylor A. New findings and controversies in odontogenic tumors. Med Oral Patol Oral Cir Bucal. 2008;13:E555–8. [PubMed] [Google Scholar]
- 21.Nikitakis NG, Tosios KI, Papanikolaou VS, Rivera H, Papanicolaou SI, Ioffe OB, et al. Immunohistochemical expression of cytokeratins 7 and 20 in malignant salivary gland tumors. Mod Pathol. 2004;17:407–15. doi: 10.1038/modpathol.3800064. [DOI] [PubMed] [Google Scholar]


