Abstract
Mast cells (MCs) have been discovered over 130 years ago; their function was almost exclusively linked to allergic affections. At the time being, it is well known that MCs possess a great variety of roles, in both physiologic and pathologic conditions. In the oral tissues, MCs release different pro-inflammatory cytokines and tumor necrosis factor-alpha that promote leukocyte infiltration in various inflammatory states of the oral cavity. These cells play a key role in the inflammatory process and, as a consequence, their number changes in different pathologic conditions of the oral cavity, such as gingivitis and periodontitis. By understanding the role of MCs in the pathogenesis of different inflammatory diseases of the oral cavity, these cells may become therapeutic targets that could possibly improve the prognosis. Therefore, this review summarizes the current understanding of the role of MCs in various inflammatory pulpal, periapical and periodontal pathophysiological conditions.
Keywords: Gingival inflammation, mast cells, periapical cyst, periapical granuloma, periodontitis
INTRODUCTION
Mast cells (MCs) are important cells of the immune system and are of the hematopoietic lineage.[1] MCs are found in all connective tissue types of the oral cavity, including the periodontal ligament, the dental pulp and the gingiva.[2] The induction of inflammation by MCs is consequent upon the release of preformed biological mediators as well as secondary mediators.[3]
Most of the periapical and gingival lesions are inflammatory in origin, and these lesions are a response to periapical tissues to the microbial and chemical stimuli coming from the pulp through root canal system. Among the cells found in periapical lesions, MCs have been detected in inflammatory infiltrate of periapical granulomas and cysts, suggesting a role of MCs in inflammatory mechanism of these lesions.
Chronic generalized inflammatory gingival hyperplasia and pyogenic granuloma are common inflammatory gingival lesions. They represent inflammation and repair attempts that are stymied due to ongoing etiological stimulation.[4]
Among the cells found in periodontal tissues, MCs are detected both in healthy and inflamed gingiva.[5]
The role of MCs in allergic diseases, anaphylaxis and autoimmunity has been well documented. However, their role in the pathogenesis of oral pathologies is still debatable. Hence, the present review article aims to explore the role of MCs in the initiation and progression of inflammatory pulpal, periapical and periodontal conditions.
MAST CELL MEDIATORS
Each MC typically contains between 80 and 300 granules. When activated (induced by a range of stimuli [Table 1]), they may either undergo explosive degranulation and then re-synthesize their granules or they may release solitary granules into their environment on an ongoing basis, a process termed “piecemeal degranulation” that has been observed in both the oral mucosa and skin.[6]
Table 1.

Following degranulation, MC mediators [Table 2] are deposited in large quantities in the extracellular environment, where they exert effects on endothelial cells and other cell types.
Table 2.
Mast cell mediators[9]
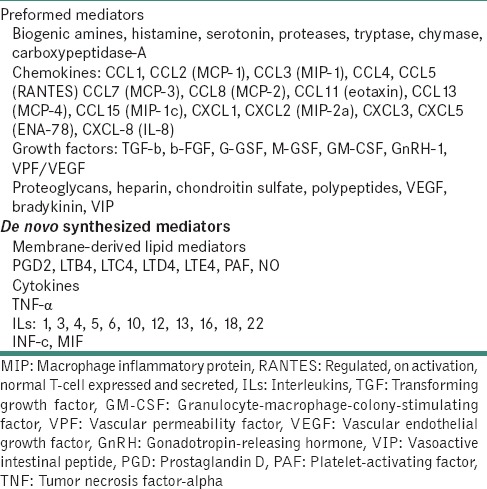
MC may subsequently synthesize and secrete additional mediators that are not preformed in their granules. Key mediators that are preformed in MCs are the serine proteases like tryptase, chymase, and cathepsin G. Histamine, histamine, heparin, serotonin, acid hydrolases the cytokines: tumor necrosis factor-alpha (TNF-α) and interleukin-16 (IL-16) are also preformed. Following activation, MCs can synthesize a range of mediators, like IL-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13 and IL-16, together with granulocyte-macrophage-colony-stimulating factor, platelet-activating factor, RANTES(regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein (MIP 1a) and the arachidonic acid metabolites like prostaglandin (PG) 2 and leukotriene C4.[7]
PULP INFLAMMATION AND MAST CELLS
The inflammatory process in human dental pulp consists in vascular changes and migration of inflammatory cells to the site of inflammation. No MCs are normally present in human dental pulp, but they are known as active cells in the inflammatory response.[10] According to the studies of Miller et al. in 1978, MCs are occasionally found in inflammed pulp.[11]
Some evidence concerning the oral cavity suggested that neurogenic inflammation involved the participation of MCs.[2] In the human dental pulp tissue that has been subject to inflammation, high concentrations of TNF-α have been detected. The source of TNF-α may be oral MCs granules, which is released upon degranulation process.[12]
Some data suggest that MC histamine, which is a strong vessel dilator and mediator of vascular permeability, may play a role in the initiation of dental pulp inflammation.[13]
Substance P may mediate MC degranulation following MC activation at the level of the dental pulp.[14]
Bacterial invasion of the pulp during caries formation may also provoke MC activation. In 2009, Karapanou et al. came up with a hypothesis that MC activation may take place through the neuropeptides that are locally released in the pulp, and, afterward, pro-inflammatory mediators that are liberated from the MC granules may participate in the inflammatory process of the dental pulp and may serve as diagnostic markers for inflammatory pulpal diseases.[15]
INFLAMMATORY PERIAPICAL LESIONS
Cyst and granulomas are chronic periapical lesions mediated by a set of inflammatory mediators that develop to contain a periapical infection.[16]
Inflammatory infiltrate of periapical lesions is composed mostly of plasmacytes, lymphocytes, macrophages and MCs.[17]
In a study by de Oliveira Rodini et al., MCs were present in greater number in periapical cysts than in granulomas. In cysts MCs were more numerous in the region of active inflammation. Furthermore, MCs tended to be more common in peripheral regions of both the periapical lesions and were often found in close proximity to lymphocytes.[18]
Role of mast cells in lymphocyte recruitment
Following activation, MCs induce T-cell migration either directly by release of exosomes and chemokines such as lympholactin, IL-16 and MIP-1 or indirectly by induction of adhesion molecule expression on endothelial cells.[18]
Histamine increases vascular permeability through structural changes which include endothelial contraction and intercellular gap formation. In addition, histamine promotes leukocyte adhesion to endothelium through transient mobilization of adhesion molecule P selectin to the endothelial surface.[19]
This functional relationship between MC and T-lymphocytes has been shown to be bidirectional, fulfilling mutually regulatory and/or modulatory roles, including influences on cellular processes such as growth, proliferation, activation and antigen presentation. In addition, T-cell-derived mediators, such as β-chemokines, directly induce MC degranulation. These findings led to propose a functional relationship between these two cell population that may facilitate elicitation of an immune response contributory to the initiation of pathogenesis of periapical lesions.[18]
CYST INITIATION
Gao et al. stated that the presence of immune cells in periapical granuloma and cysts initiates cell-mediated and humoral immunoreactions in these lesions which may be associated with epithelial proliferation within periapical lesions.[20]
Role of mast cells in epithelial proliferation
The stimulated MCs may release IL-1, which causes increased epithelial proliferation. This property of MC-derived IL-1 may act as one of the factors in the proliferation of epithelium (cell rests of Malassez) in periapical granuloma leading to cyst formation.[21]
CYST ENLARGEMENT/EXPANSION
The hydrostatic pressure of the luminal fluid is important in cyst enlargement, and MC activity might contribute to this by increasing the osmotic pressure.[22]
Teronen et al. stated that MCs were found to be located in inflammatory cell-rich tissue areas and just beneath the cyst epithelium. MCs located at the border of bone were observed to be degranulated and release tryptase at the regions of early bone destruction, suggesting that the MC tryptase may contribute significantly to jaw cyst tissue remodeling during growth of cyst and to the destruction of surrounding bone, resulting in jaw cyst expansion.[23]
Smith et al. observed high staining activity of heparin in connective tissue just beneath the epithelium of cysts and suggested that local tissue metabolism and inflammation will contribute to the release of glycosaminoglycans from connective tissue into the luminal fluid and are thought to be important in the expansile growth of cysts.[24]
Role of mast cells in increasing the hydrostatic pressure of cystic luminal fluid
The increase in osmotic pressure could be in three ways as follows:
By direct release of heparin into luminal fluid
By release of hydrolytic enzymes which could degrade capsular extracellular matrix (ECM) components, thereby facilitating their passage into the fluid
By the action of histamine on smooth muscle contraction and vascular permeability, encouraging transudation of serum proteins.[22]
Role of mast cells in degradation of extracellular matrix and bone resorption
MCs contain tryptase and chymases (proteolytic enzymes) that take part in the degradation of ECM.[25] Thus, they help in the breakdown of proteoglycans of connective tissue capsule of periapical lesions leading to expansion.
The MC-derived mediators such as histamine, heparin, tryptaes, TNF, IL-1, IL-6, PGs and other arachidonic acid metabolites are known to cause bone resorption.[24] TNF-α and IL-1 and IL-6 increase the bone resorption by intensifying the osteoclastic activity.[25] Heparin is involved in bone resorption and also has been associated with the inhibition of collagen synthesis.[26] MC-derived tryptase can activate matrix metalloproteinase (MMP) which help in ECM degradation and bone resorption for the enlargement of the lesions.[25]
GINGIVA AND MAST CELLS
Gingival inflammation
It has been reported that MCs were constantly present in healthy gingival tissue and located between epithelial cells and connective tissues.[27] In gingival lesions, the products from the bacterial plaque at the gingival margin may directly or indirectly induce proliferation of MCs. The proposed source of allergens includes not only bacteria and their products but also denatured host tissue.[28]
Pro-inflammatory cytokines that are released during the initial stage of the inflammation infuence the migration of MCs. Following degranulation, MC mediators are deposited in large quantities in the extracellular environment, where they exert effects on endothelial cells and other cell types.
Early events in this process include key growth factors, transforming growth factor-ββ and fibroblast growth factor as well as inflammatory cytokines and chemokines, facilitating MC recruitment and activation.
The release of proteolytic enzymes such as MMPs mediates fibrogenic injury, and the overall balance of activators and inhibitors is altered in a manner favoring net matrix deposition. MC effectively supports this process by elaborating the cytokines and chemokines.
MC produces mediators such as histamine, heparin and TNF-α which can influence fibroblast proliferation, ECM synthesis and degradation. TNF-α also upregulates C-C chemokine receptor Type 1 and RANTES expression which in turn triggers further MC degranulation.
MC chymase is also known to stimulate MMP-9 which mediates basement membrane disruption. On the whole, the cyclic activity of inflammatory mediator secretion and MC degranulation may result in the chronicity of gingival inflammation and fibrosis.[27]
Role of mast cells in collagen synthesis and fibrosis
MC-derived IL-1 causes increased fibroblastic response and tryptase causes increased production of Type 1 collagen and fibronectin, thereby leading to increased fibrosis.[21,29] Histamine may also interact with and promote increased fibroblastic proliferation. This could suggest a possible role of MC in enhanced synthesis and fibrosis seen in later stages of inflammatory periapical and gingival lesions [Figure 1].[30,31,32]
Figure 1.
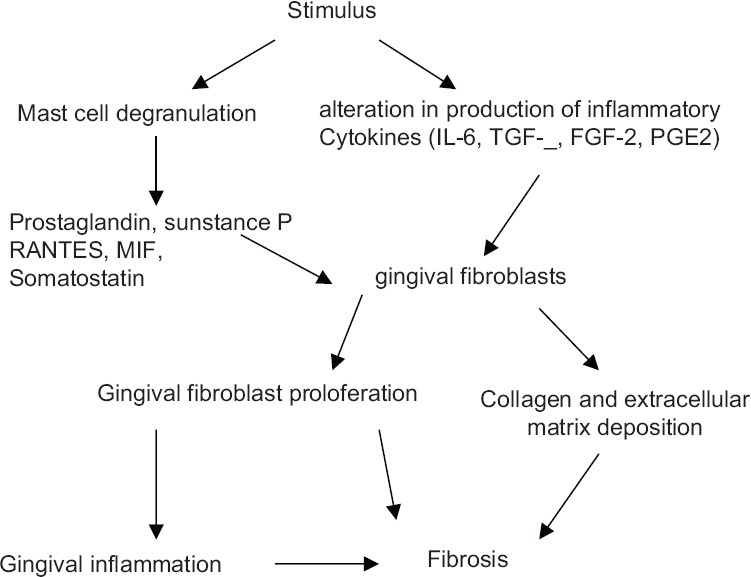
Schematic representation showing mechanisms of mast cells in collagen synthesis and fibrosis
PYOGENIC GRANULOMA
Pyogenic granuloma represents a reactive lesion resulting from local etiological factors such as gingival inflammation, calculus or trauma, which activates MC resulting in the release of mediators, which leads to subsequent changes in tissue leading to the formation of pyogenic granuloma.
MC on stimulation undergoes degranulation and causes inflammatory and vascular changes leading to the formation of pyogenic granuloma [Figure 2].[33]
Figure 2.
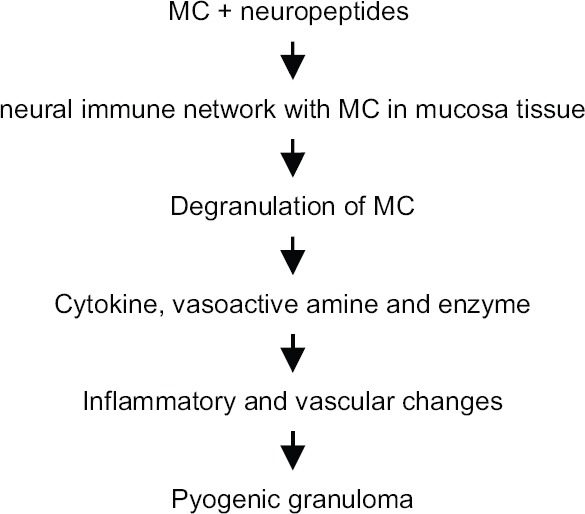
Schematic representation showing mechanisms of mast cells in the pathogenesis of pyogenic granuloma
Haritanont et al. stated that the number of MCs and blood vessel counts in oral pyogenic granuloma was significantly higher than those in normal oral tissues. This suggests that MCs may function as one of the factors for angiogenesis in oral pyogenic granulomas.[34]
Role of mast cells in angiogenesis
MCs play a significant role in angiogenesis, probably by secreting several potent angiogenic factors including heparin, histamine, vascular endothelial growth factor and tryptase. Tryptase, in particular, could directly induce proliferation of human dermal microvascular endothelial cells.[35] Heparin increases the half-life of basic fibroblastic growth factor, which is a potent angiogenic substance. These findings indicate that the MC may function as one of the factors for angiogenesis in pyogenic granuloma, chronic inflammatory gingival hyperplasia and also in periapical lesions.[21,36]
INFLAMMATORY PERIODONTAL DISEASES
Bacterial plaque has been implicated as the primary etiological factor in inflammatory periodontal disease, but recently, several studies have focused on the role of the immune system in the development of periodontal disease, indicating that bacterial antigens trigger an immunopathological reaction.[37]
Among the cells found in the periodontal tissues, MCs have been detected in both healthy and inflamed gingiva.[38]
When triggered by locally produced cytokines or bacterial products, for example, lipopolysaccharides, the cells can release a large number of prestored mediators.
One of the biological and biochemical factors is histamine, which breaks down the tissue barrier, causes edema and helps cellular infiltration.[39]
Another reason is that the expression of MMPs 1, 2 and 8 is strongest in MCs. MMPs are crucial in the degradation of the main components in ECMs.
Furthermore, tryptase can cleave the third component of collagen and activate latent collagenase that can participate in tissue destruction in periodontitis.
A change from gingivitis to periodontitis involves a shift from predominantly T-cell lesion to a B-cell/plasma cell lesion. MCs seem to be able to present antigens to T-cells. The resultant T-cell activation would activate MCs, leading to both degranulation and cytokine release.
In summary, periodontitis is not unidirectional, but rather it is interactive; the same cells that produce the destructive pro-inflammatory cytokines can also produce mediators that activate the healing process.[40]
CONCLUSION
MCs play a critical role in the development of inflammation at the levels of the dental pulp and periodontium, both during the early stages and during the transition from acute to chronic inflammation. MCs in these inflammatory lesions are associated with increased vascular permeability, angiogenic response, collagen synthesis, regulation of inflammation, bone resorption and ECM destruction. Based on the concept that MCs play an important role in the chronicity of inflammation, it may be possible to use drugs therapeutically in order to influence MC secretion and thereby thwart inflammation. In the future, it may be possible to develop novel approaches that influence the release of pro-inflammatory molecules or neuropeptides to ameliorate MC-driven inflammation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: A Multi-functional master cell. Front Immunol. 2015;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaje PN, Amalia Ceausu R, Jitariu A, Stratul SI, Rusu LC, Popovici RA, et al. Mast cells: Key players in the shadow in oral inflammation and in squamous cell carcinoma of the oral cavity. Biomed Res Int. 2016;2016:9235080. doi: 10.1155/2016/9235080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weller CL, Collington SJ, Williams T, Lamb JR. Mast cells in health and disease. Clin Sci (Lond) 2011;120:473–84. doi: 10.1042/CS20100459. [DOI] [PubMed] [Google Scholar]
- 4.Sheethal HS, Uma K, Rao K, Priya NS, Umadevi HS, Smitha T, et al. A quantitative analysis of mast cells in inflammatory periapical and gingival lesions. J Contemp Dent Pract. 2014;15:300–5. doi: 10.5005/jp-journals-10024-1532. [DOI] [PubMed] [Google Scholar]
- 5.Prakash S, Devnath KR, Abid S. Study of mast cells in periodontal diseases. J Oral Maxillofac Pathol. 2006;10:64–8. [Google Scholar]
- 6.Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003;14:188–98. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Ishizuka T, Okayama Y. Human mast cells and basophils as sources of cytokines. Clin Exp Allergy. 2000;30:1205–12. doi: 10.1046/j.1365-2222.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 8.Escribano L, Akin C, Castells M, Schwartz LB. Current options in the treatment of mast cell mediator-related symptoms in mastocytosis. Inflamm Allergy Drug Targets. 2006;5:61–77. doi: 10.2174/187152806775269303. [DOI] [PubMed] [Google Scholar]
- 9.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 10.Nica L, Marius R. Normal and inflammatory human dental pulp: A morphohistochemical approach. TMJ. 2004;1:70–3. [Google Scholar]
- 11.Miller GS, Sternberg RN, Piliero SJ, Rosenberg PA. Histologic identification of mast cells in human dental pulp. Oral Surg Oral Med Oral Pathol. 1978;46:559–66. doi: 10.1016/0030-4220(78)90386-9. [DOI] [PubMed] [Google Scholar]
- 12.Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M, et al. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch Med Res. 2002;33:482–4. doi: 10.1016/s0188-4409(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 13.DelBalso AM, Nishimura RS, Setterstrom JA. The effects of thermal and electrical injury on pulpal histamine levels. Oral Surg Oral Med Oral Pathol. 1976;41:110–3. doi: 10.1016/0030-4220(76)90259-0. [DOI] [PubMed] [Google Scholar]
- 14.Janiszewski J, Bienenstock J, Blennerhassett MG. Picomolar doses of substance P trigger electrical responses in mast cells without degranulation. Am J Physiol. 1994;267:C138–45. doi: 10.1152/ajpcell.1994.267.1.C138. [DOI] [PubMed] [Google Scholar]
- 15.Karapanou V, Kempuraj D, Theoharides TC. Oral neuroimmune network and mast cells. Eur J Inflamm. 2009;7:1–8. [Google Scholar]
- 16.Marçal JR, Samuel RO, Fernandes D, de Araujo MS, Napimoga MH, Pereira SA, et al. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010;36:995–9. doi: 10.1016/j.joen.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa S. Pathologic study of periapical lesions 1. Periapical granulomas: Clinical, histopathologic and immunohistopathologic studies. J Oral Pathol. 1980;9:288–300. doi: 10.1111/j.1600-0714.1980.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira Rodini C, Batista AC, Lara VS. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: Possible role of mast cells in the course of human periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:59–63. doi: 10.1016/s1079-2104(03)00378-0. [DOI] [PubMed] [Google Scholar]
- 19.Walsh LJ, Davis MF, Xu LJ, Savage NW. Relationship between mast cell degranulation and inflammation in the oral cavity. J Oral Pathol Med. 1995;24:266–72. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z, Mackenzie IC, Rittman BR, Korszun AK, Williams DM, Cruchley AT, et al. Immunocytochemical examination of immune cells in periapical granulomata and odontogenic cysts. J Oral Pathol. 1988;17:84–90. doi: 10.1111/j.1600-0714.1988.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 21.Ankle MR, Kale AD, Nayak R. Mast cells are increased in leukoplakia, oral submucous fibrosis, oral lichen planus, and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2007;11:18–22. [Google Scholar]
- 22.Smith G, Smith AJ, Basu MK. Mast cells in human odontogenic cysts. J Oral Pathol Med. 1989;18:274–8. doi: 10.1111/j.1600-0714.1989.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 23.Teronen O, Hietanen J, Lindqvist C, Salo T, Sorsa T, Eklund KK, et al. Mast cell-derived tryptase in odontogenic cysts. J Oral Pathol Med. 1996;25:376–81. doi: 10.1111/j.1600-0714.1996.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith G, Smith AJ, Browne RM. Histochemical studies on glycosaminoglycans of odontogenic cysts. J Oral Pathol. 1988;17:55–9. doi: 10.1111/j.1600-0714.1988.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 25.Ledesma-Montes C, Garcés-Ortíz M, Rosales-García G, Hernández-Guerrero JC. Importance of mast cells in human periapical inflammatory lesions. J Endod. 2004;30:855–9. doi: 10.1097/01.don.0000134207.67360.fc. [DOI] [PubMed] [Google Scholar]
- 26.Tiecke RA, Stuteville OH, Calandra JC. Pathologic Physiology of Oral Disease. St. Louis: Mosby Publications; 1959. p. 283. [Google Scholar]
- 27.Subramani T, Rathnavelu V, Yeap SK, Alitheen NB. Influence of mast cells in drug-induced gingival overgrowth. Mediators Inflamm. 2013;2013:275172. doi: 10.1155/2013/275172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zachrisson BU. Mast cells of the human gingival. J Periodontal Res. 2006;1:46–55. doi: 10.1111/j.1600-0765.1969.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 29.Riekki R, Harvima IT, Jukkola A, Risteli J, Oikarinen A. The production of collagen and the activity of mast-cell chymase increase in human skin after irradiation therapy. Exp Dermatol. 2004;13:364–71. doi: 10.1111/j.0906-6705.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 30.Jordana M, Befus AD, Newhouse MT, Bienenstock J, Gauldie J. Effect of histamine on proliferation of normal human adult lung fibroblasts. Thorax. 1988;43:552–8. doi: 10.1136/thx.43.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatamochi A, Fujiwara K, Ueki H. Effects of histamine on collagen by cultured fibroblasts derived from guinea pig skin. Arch Oral Biol. 1984;277:60–4. doi: 10.1007/BF00406482. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt AP, Dholakia HM. Mast cell density in oral submucous fibrosis. J Indian Dent Assoc. 1977;49:187–91. [Google Scholar]
- 33.Kamel R, Dahiya P, Palaskar S, Shetty VP. Comparative analysis of mast cell count in normal oral mouse and oral pyogenic granuloma. J Clin Exp Dent. 2011;3:e1–4. [Google Scholar]
- 34.Haritanont H, Tuntaing T, Tunstool K, Yaicharearn J, Iamaroon A. Association Between Mast Cells and Oral Lesions: Pyogenic Granuloma and Fibroma. Prachinburi, Thailand: Poster Presented at 5th Thai Scientific Conference in Dental Research; 2001. [Google Scholar]
- 35.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S, et al. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 36.Hagiwara K, Khaskhely NM, Uezato H, Nonaka S. Mast cell “densities” in vascular proliferations: A preliminary study of pyogenic granuloma, portwine stain, cavernous hemangioma, cherry angioma, Kaposi's sarcoma, and malignant hemangioendothelioma. J Dermatol. 1999;26:577–86. doi: 10.1111/j.1346-8138.1999.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 37.Lagdive SS, Lagdive SB, Mani A, Anarthe R, Pendyala G, Pawar B, et al. Correlation of mast cells in periodontal diseases. J Indian Soc Periodontol. 2013;17:63–7. doi: 10.4103/0972-124X.107500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carranza FA, Jr, Cabrini RL. Mast cells in human gingival. Oral Surg Oral Med Oral Pathol. 1955;8:1093–9. doi: 10.1016/0030-4220(55)90061-x. [DOI] [PubMed] [Google Scholar]
- 39.Aeschlimann CR, Kaminski EJ, Robinson PJ. The effects of periodontal therapy on the mast cell population in gingival tissues. J Periodontol. 1980;51:193–8. doi: 10.1902/jop.1980.51.4.193. [DOI] [PubMed] [Google Scholar]
- 40.Naesse EP, Schreurs O, Helgeland K, Schenck K, Steinsvoll S. Matrix metalloproteinases and their inhibitors in gingival mast cells in persons with and without human immunodeficiency virus infection. J Periodontal Res. 2003;38:575–82. doi: 10.1034/j.1600-0765.2003.00687.x. [DOI] [PubMed] [Google Scholar]


