Abstract
Plant cells produce reactive oxygen species (ROS) in response to many stimuli. However, the mechanism of ROS biosynthesis remains unclear. We have explored the hypothesis that the superoxide burst in plants mechanistically resembles the oxidative burst in neutrophils. First we have confirmed that ROS production, which occurs in suspension-cultured soybean (Glycine max) cells in response to hypo-osmotic shock, is inhibited by diphenylene iodonium, an inhibitor of the flavin-dependent oxidase of neutrophils. Because a Rac family G protein is an essential regulator of this NADPH oxidase, and because many plant homologs of Rac have been cloned, we next examined whether Rac-like proteins might be involved in the oxidative burst in the soybean cells. We identified a Rac-like 21-kD soybean protein that cross-reacts with antibodies to human Rac and garden pea Rop and also binds [γ-35S] GTP, a diagnostic trait of small G proteins. This Rac-related protein translocated from the cytosol to microsomes during the oxidative burst. Moreover, soybean cells transiently transformed with either a dominant negative (RacN17) or a dominant positive (RacV12) form of Rac1 showed the anticipated altered responses to three different stimuli: hypo-osmotic shock, oligo-GalUA, and harpin. In response to these stimuli, cells transformed with RacN17 produced less ROS and cells transformed with RacV12 generated more ROS than control cells. These results strongly suggest that a Rac-related protein participates in the regulation of ROS production in soybean cells, possibly via activation of an enzyme complex similar to the NADPH oxidase of phagocytes in animal systems.
Reactiveoxygen species (ROS) are produced in plant cells in response to a broad range of biological and physical stimuli, including elicitors, pathogen infections, osmotic shock, and wounding (Doke, 1983; Apostol et al., 1989; Atkinson et al., 1990; Chandra and Low, 1997; Stennis et al., 1998). The transient burst of ROS production known as the oxidative burst has a direct antimicrobial effect and is involved in inducing many other defense responses (Keppler et al., 1989; Peng and Kuc, 1992). Despite intensive investigation, the mechanism of ROS production in plant cells has yet to be clearly defined. Enzymes that may be responsible for ROS production in plants include peroxidase and NADPH oxidase, as well as several other oxidases (Auh and Murphy, 1995; Desikan et al., 1996; Dwyer et al., 1996; Kieffer et al., 1997; Xing et al., 1997; Bestwick et al., 1998; Bolwell et al., 1998). That NADPH oxidase may play a role in ROS production in plant cells is indicated by two lines of evidence. First, NADPH oxidase is responsible for ROS formation in neutrophils, and the biochemical characteristics of ROS production including maximum rate, rapid activation kinetics, desensitization, and signal transduction pathways that trigger the oxidative burst are similar in plant cells and neutrophils (Dwyer et al., 1996). Second, there exist plant proteins that cross-react with antibodies that recognize the subunits of NADPH oxidase from animal cells (Desikan et al., 1996; Dwyer et al., 1996; Kieffer et al., 1997; Xing et al., 1997). Together, these observations suggest that NADPH oxidase may be involved in a mechanism of ROS production common to the defense systems of both plant and animal cells.
In neutrophils, the low Mr G protein, Rac, plays an essential role as a regulator of the NADPH oxidase (Bokoch, 1994; Irani and Goldschmidt-Clermont, 1998). Translocation of Rac to the plasma membrane is required for assembly and activation of the NADPH oxidase complex (Han et al., 1998). Recent reports suggest that Rac is also involved in ROS production in some non-phagocytic cells in which the enzyme(s) responsible for ROS production remain unknown (Sundaresan et al., 1996; Irani et al., 1997; Kheradmand et al., 1998; Yeh et al., 1999).
Rac proteins belong to the conserved RHO family of small GTPases. In animals, RHO is divided into several subfamilies, including Rho, Cdc42, and Rac. Although orthologs of these RHO GTPases have not been identified in plants, plants possess a unique subfamily of Rho GTPases, termed Rop, that is most closely related to the Rac (Yang and Watson, 1993; Delmer et al., 1995; Xia et al., 1996; Winge et al., 1997; Li et al., 1998). Rop1 and its close relative Rop5/Arac1 play a role in the regulation of polarized growth of pollen tubes in pea and Arabidopsis (Lin and Yang, 1997; Li et al., 1998, 1999; Kost et al., 1999). In addition, there is emerging evidence that Rop plays important roles in the regulation of ROS production. Using an anti-Rac2 polyclonal antibody, Xing et al. (1997) showed that a 21-kD Rac2-related protein from tomato is translocated to the plasma membrane in response to race-specific elicitors. Rac13 may also be involved as a regulator of H2O2 production in secondary cell wall differentiation during cotton fiber development (Potikha et al., 1999). In rice, OsRac1 is involved in ROS production and cell death (Kawasaki et al., 1999). These results, together with the indirect evidence described above for the involvement of NADPH oxidase in ROS production in plant cells, suggest that a plant Rac homolog may be involved in the regulation of a plant NADPH oxidase. However, it is not known whether the Rac/Rop-like proteins of plants are true functional equivalents of Rac in the NADPH oxidase complex in animal cells.
In this report we show that a 21-kD GTP-binding protein, which cross-reacts with Rop-specific and with animal Rac1- and Rac2-specific antibodies, translocates from the cytosol to the membrane during the oxidative burst in suspension-cultured soybean (Glycine max) cells. Furthermore, transient expression of human Rac1 proteins in the soybean cells modulates ROS production in response to osmotic stress and elicitors. These results provide evidence for the existence of a soybean Rop-like GTPase, which functions as a regulator of the plant NADPH oxidase, possibly analogous to Rac in the NADPH oxidase complex in animal cells.
RESULTS
Hypo-Osmotic Shock-Induced Superoxide Anion (O2−) Formation
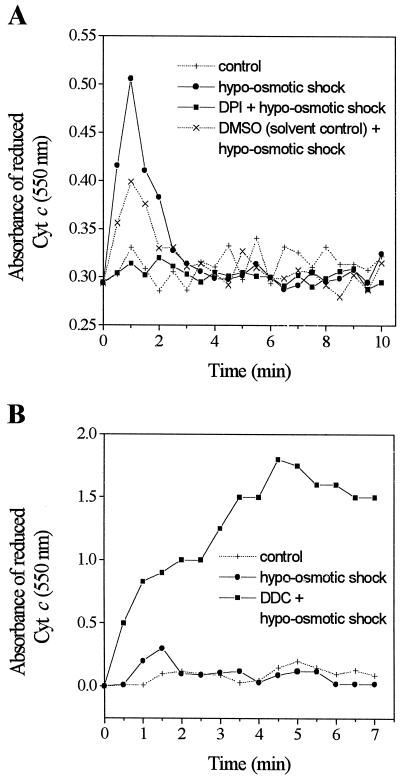
To test the hypothesis that NADPH oxidase is involved in ROS generation in soybean cells, we used a stimulus that reliably induces O2− production, hypo-osmotic shock, and a flavin-containing oxidase inhibitor, diphenylene iodonium (DPI; O'Donnell et al., 1993; Dwyer et al., 1996; Murphy and Auh, 1996). Production of O2−, measured by monitoring the formation of reduced Cyt c, was induced when suspension-cultured soybean cells were subjected to hypo-osmotic shock (dilution of the medium with water in 1:1 ratio), and the induction was almost completely inhibited in the presence of 30 μm DPI (Fig. 1A), supporting our hypothesis. In addition, the low amplitude of the response and the transient peak suggested that superoxide produced by NADPH oxidase was rapidly converted to H2O2 by endogenous SOD. Indeed, we observed that in the presence of DDC, an inhibitor of SOD, the reduced Cyt c continued to accumulate (Fig. 1B). DDC alone without osmotic shock did not induce oxidative burst (data not shown). These results strongly suggest that hypo-osmotic shock activates NADPH oxidase in soybean cells.
Figure 1.
Production of superoxide anion (O2−) in response to hypo-osmotic shock. A, Inhibition by DPI of O2− production. The cells were pretreated with or without 30 μm DPI for 30 min, then stimulated by hypo-osmotic shock. Control cells were not subjected to the stimulus. Dimethyl sulfoxide alone was added to the solvent control of DPI, which was also stimulated by hypo-osmotic shock. B, Accumulation of O2− in the presence of a superoxide dismutase (SOD) inhibitor diethyldithiocarbamate (DDC). The cells were pretreated with or without 1 mm DDC for 10 min, then stimulated by hypo-osmotic shock. Control cells were not subjected to the stimulus. Results shown are representatives from three (A) and two (B) similar independent experiments.
Identification of a Rac-Related Protein in Suspension-Cultured Soybean Cells
To test whether a Rac- or Rop-like protein might exist in soybean cells, proteins extracted from suspension-cultured soybean cells were tested for GTP binding and cross-reaction with antibodies raised against Rac1, Rac2, and Rac1(C189S) from human, and Rop1Ps from garden pea. Rac1(C189S) is an isoprenylation-deficient mutant of Rac1, and our polyclonal antibody raised against the entire protein cross-reacted with both Rac1 and Rac2 proteins (data not shown). Figure 2 shows that a soybean protein with an apparent molecular mass of 21 kD, about the size of Rac and Rop, bound [γ-35S] GTP as well as cross-reacted with all the antibodies tested. This result suggests that a Rac/Rop-like GTP-binding protein exists in soybean cells. This protein was expressed at all growth stages of the cultured cells (data not shown).
Figure 2.
Identification of a Rac/Rop-related protein in soybean cells by immunoblotting and [γ-35S] GTP-binding assay. Crude extracts from suspension-cultured soybean cells were separated by 15% SDS-PAGE, transferred to nitrocellulose membrane, and probed with antibodies raised against Rac1(C189S) (lane 1), Rop (lane 2), Rac1 (lane 3), and Rac2 (lane 4). Nitrocellulose membrane prepared in the same manner was also used in [γ-35S] GTP-binding assay, the result of which was visualized by autoradiography (lane 5). Representatives from two independent experiments with similar results are shown.
Translocation of the Endogenous Rac-Related Protein during the Oxidative Burst
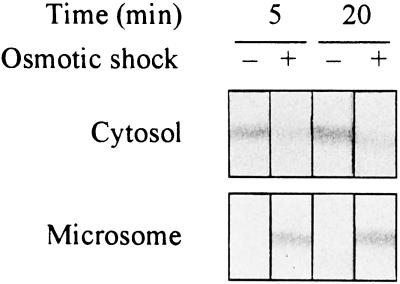
In neutrophils, Rac activation of NADPH oxidase in response to stimulation with chemo-attractant or phorbol ester is accompanied by Rac translocation from the cytosol to the membrane (Quinn et al., 1993; Nisimoto et al., 1997). If the Rac-related protein in soybean cells has a role analogous to that of Rac of neutrophils, similar membrane translocation of this protein might be expected during the oxidative burst. Therefore we examined the location of the Rac-related soybean protein using anti-Rac1(C189S) antibody, since this antiserum exhibited the highest cross-reactivity with this protein among the four antisera tested. Examination of microsomal and cytosolic fractions of soybean cells prepared before and after hypo-osmotic shock showed that most of the Rac-related protein was present in the cytosol before the shock treatment, and that a considerable portion was also found in the microsomal fraction at 5 min after shock treatment (Fig. 3) when H2O2 production was maximal. The Rac-related protein was present in the microsome even after the oxidative burst ended at 20 min after the shock (Fig. 3). There may be other factors that inactivate NADPH oxidase before Rac proteins return to the cytosol (Sathyamoorthy et al., 1997).
Figure 3.
Translocation of the Rac-related protein of soybean cells from the cytosol to the microsome. Microsomal and cytosolic fractions were prepared from suspension-cultured soybean cells before and 5 and 20 min after hypo-osmotic shock treatment. The endogenous Rac-related protein was detected by immunoblotting with antibodies raised against Rac1(C189S). Seventy micrograms of protein was loaded in each lane. Results shown are representatives from two similar independent experiments.
Altered Rates of Oxidative Burst in Mutant Rac1-Expressing Cells
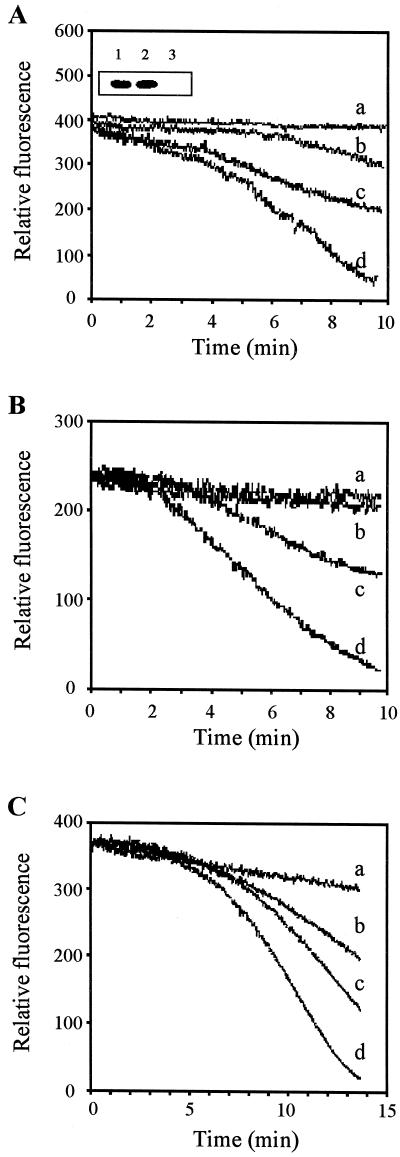
Since translocation of the endogenous Rac-related protein suggested that soybean cells may have a ROS-generating mechanism similar to that of animal cells, we then examined whether Rac of animal origin could modulate ROS generation by soybean cells. Mutant human Rac1 genes were transiently expressed in suspension-cultured soybean cells and the oxidative burst of the cells in response to mechanical stress (osmotic shock) and elicitors (oligo-GalUA [OGA] and harpin) that induce defense responses were analyzed. OGA is a plant cell wall component released during pathogen attack or wounding, and harpin is a proteinaceous bacterial elicitor from Erwinia amylovora (Chandra and Low, 1997). These three stimuli induce the oxidative burst via distinct signal transduction pathways, although the identities of the intermediates in these pathways remain largely unknown (Low and Merida, 1996). As seen in Figure 4, osmotic shock and OGA induced an oxidative burst within 2 to 3 min in cells transformed with β-glucuronidase (GUS) only and a similar response was stimulated by harpin about 5 min after elicitation. To learn whether mammalian Rac might alter this response, RacV12, a dominant positive Rac1 mutant with constitutive activity (defective in its intrinsic GTPase activity; Diekmann et al., 1991) was transformed into the soybean cells. For a similar purpose, RacN17, a dominant negative Rac1 mutant that is locked into its inactive state by its preferential affinity for GDP (Farnsworth and Feig, 1991) was also tested for its influence on the burst. Because RacN17 also competes for Rac's guanine nucleotide exchange factor (Farnsworth and Feig, 1991; Jung et al., 1994), it might also be expected to reduce activation of any endogenous plant Rac isoforms. As shown in the inset to Figure 4A, expression of both mutant Rac1 genes could be demonstrated in the soybean cells by immunoblotting with anti-myc antibody. More importantly, none of the soybean cells transformed with RacN17, RacV12 together with GUS, or GUS alone produced H2O2 in the absence of external stimuli in normal culture medium. However, in response to each of the above three stimuli, cells transformed with RacN17 produced less H2O2, whereas cells transformed with RacV12 produced more H2O2 than control cells that were transformed with the GUS construct only (Fig. 4). These data suggest that Rac-related proteins can regulate the oxidative burst in soybean cells.
Figure 4.
Altered rates of H2O2 production by mutant Rac1-transformed soybean cells. Oxidative burst assay of suspension-cultured soybean cells transformed with RacN17 (b), RacV12 together with GUS (d), or GUS alone (c) in response to hypo-osmotic shock (A), OGA (B), and harpin (C). a, A control showing RacV12-transformed cells in the absence of any stimuli. Controls using all other transformants showed similar response. The inset of A shows western hybridization using anti-myc antibody to probe total protein extracted from soybean cells transformed with myc-tagged RacV12 (lane 1), RacN17 (lane 2), and the GUS gene without an epitope tag (lane 3). Results shown are representatives from four (osmotic shock), five (OGA), and two (harpin) similar independent experiments. Each experiment consisted of three replicates each for each sample type.
Membrane Translocation of Human Rac during the Oxidative Burst
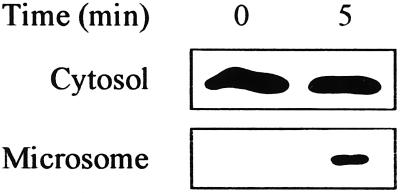
Because animal Rac modified the oxidative burst rate in soybean cells, we next examined whether human Rac expressed in soybean cells might also translocate in a manner similar to the endogenous Rac-like soybean protein. Microsomal membrane and cytosol were prepared from transformed cells before and 5 min after osmotic shock. RacV12, identified by anti-myc antibody, was found only in the cytosol in the absence of stimulus, but was also located in the microsomal fraction following osmotic shock (Fig. 5).
Figure 5.
Translocation of the RacV12 of animal origin from the cytosol to the microsome. Microsomal and cytosolic fractions were prepared from suspension-cultured soybean cells transformed with RacV12 before and 5 min after hypo-osmotic shock treatment. RacV12 protein was detected by immunoblotting with anti-myc antibody. Representatives from two independent experiments with similar results are shown.
DISCUSSION
Our studies suggest that a 21-kD GTP-binding protein, which cross-reacts with both anti-Rop and anti-Rac antibodies (Fig. 2), is associated with production of ROS in suspension-cultured soybean cells. The cross-reactivity of the 21-kD protein with the antibodies suggested that this protein is likely a member of the plant-specific Rop subfamily of RHO GTPases because Rop is most closely related to Rac, sharing 65% amino acid sequence identity with each other (Li et al., 1998). During the oxidative burst, this Rop-like protein is translocated to the microsomal membrane (Fig. 3), as is Rac, a regulatory component of the NADPH oxidase complex in neutrophil cells (Bokoch, 1994; Irani and Goldschmidt-Clermont, 1998). In a similar manner, the subcellular location of a 21-kD protein of tobacco and tomato cells changed in response to elicitor treatment (Kieffer et al., 1997; Xing et al., 1997). However, in the latter studies, anti-Rac2, but not anti-Rac1 antibody was found to recognize the cross-reacting band at 21 kD, whereas in our experiments both Rac1 and Rac2 antibodies cross-reacted with the 21-kD soybean protein. The reason for this discrepancy is not clear. Because these antibodies were raised against the C-terminal region of their respective Rac proteins, it is possible that the C-terminal region for the tomato and tobacco Rop-like proteins is quite different from that for the soybean Rop-like protein. Rop is encoded by a large gene family in Arabidopsis and probably in other plant species as well (Li et al., 1998). Further studies are obviously needed to determine which Rop-like protein is associated with the oxidative burst.
We have shown that the constitutively active and dominant negative forms of human Rac increased and decreased, respectively, the rate of ROS production induced in soybean cells by various stimuli (Fig. 4), similar to their effects in neutrophils (Irani et al., 1997; Kheradmand et al., 1998). This result suggests that a Rop-like protein may promote the oxidative burst in soybean cells, as suggested for OsRac1 in rice and Rac13 in cotton fiber cells (Kawasaki et al., 1999; Potikha et al., 1999). O2− generation by soybean cells exposed to hypo-osmotic shock and its inhibition by DPI also suggest that NADPH oxidase is likely responsible for at least some part of the oxidative burst of soybean cells (Fig. 1). Moreover, three different stimuli, which act through different signaling cascades, induced the same changes in the rates of ROS production in Rac-transformed soybean cells, suggesting that the Rac protein performs a common function in diverse oxidative burst signaling pathways. Finally, the soybean Rac-related protein and the heterologously expressed mammalian Rac both translocated to the membrane from the cytosol during ROS production (Figs. 3 and 5), as does Rac in the NADPH oxidase complex of neutrophils (Bokoch, 1994; Irani and Goldschmidt-Clermont, 1998). Taken together, these results indicate a conserved role for a Rac-like G protein in regulation of ROS in soybean cells and they suggest that soybean cells employ a Rac/Rop-like protein to modulate an enzymatic system similar to the NADPH oxidase of neutrophils.
We have also shown that the constitutively active Rac mutant does not constitutively activate the oxidative burst in soybean cells; instead, induction of the oxidative burst by the dominant positive Rac mutant was stimulus-dependent (Fig. 4). Stimulus-dependence was also found in its translocation to the microsomes (Fig. 5). These data suggest that the stimulus-mediated activation of NADPH oxidase in soybean cells involves at least two steps: one that activates Rac and another that is independent of Rac activation. The second step is most likely required for the translocation of Rac to the plasma membrane, as suggested by stimulus-activated Rac translocation to membranes. The stimulus-dependent promotion of the oxidative burst by activated human Rac in soybean cells is different from the action of activated Rop in rice or cotton fiber cells where constitutively active Rop causes constitutive activation of H2O2 production (Kawasaki et al., 1999; Potikha et al., 1999). Rop in these systems could have a function distinct from that of the Rop-like protein in soybean. On the other hand, the difference may be due to different functions of plant Rop and heterologous human Rac in plant cells; Rops may function both as a regulatory component of NADPH oxidase and as an upstream regulator of the signaling cascade that eventually activates NADPH oxidase, whereas the heterologous mammalian Rac may not have the latter function in plant cells. Identification of the soybean Rop protein involved in the regulation of NADPH oxidase will help to address this problem.
As a final point, we should point out that although we presented evidence for possible similarity between the ROS producing mechanisms of soybean cells and neutrophils, our data do not exclude the possibility that the Rac/Rop-like protein of soybean or heterologously expressed Rac modulate oxidative burst at a site different from the NADPH oxidase. ROS has been recently shown to be produced in some animal cells where existence of the components of the NADPH oxidase is questionable, and there activated Rac also enhances the rate of ROS production (Sundaresan et al., 1996; Irani et al., 1997; Kheradmand et al., 1998; Yeh et al., 1999). Further studies are necessary to understand the exact mechanism of modulation of ROS production by Rac/Rop-like protein in soybean cells.
MATERIALS AND METHODS
Plant Cell Culture
Suspension-cultured soybean (Glycine max) cells were maintained in Murashige and Skoog medium (Murashige and Skoog, 1962) containing 0.1 mg kinetin, 3 mg 2,4-dichlorophenoxyacetic acid, 1 g casein, and 0.5 g MES [2-(N-morpholino)ethanesulfonic acid] per liter. Two milliliters of cells was transferred to 25 mL of fresh Murashige and Skoog medium every 7 d. The cells were grown in an incubator at 23°C, with shaking at 80 to 90 rpm and 18-h light/6-h dark cycles.
Rac Constructs
The plasmids including pEXV-RacV12 and pEXV-RacN17 were gifts from Dr. Jae-Hong Kim (Kim and Kim, 1997; Kim et al., 1997). For particle bombardment, Rac constructs were prepared by inserting the Rac1 coding region into EcoRI site of the binary vector pGA748 (provided by Dr. Gynheung An) under 35S promoter, and all constructs were tagged with an N-terminal 9E10 epitope.
Detection of H2O2 Production by Fluorescence Quenching
H2O2 production in suspension-cultured soybean cells was detected by monitoring the oxidative quenching of the fluorescent dye, pyranine (8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt: λex 405 nm, λem 512 nm, Molecular Probes, Eugene, OR) using a spectrofluorimeter (RF 5000, Shimadzu, Kyoto, Japan) as previously described (Dwyer et al., 1996). Hypo-osmotic shock treatment was performed by diluting the cell suspension with an equal volume of distilled water. Elicitation with OGA and harpin (kind gifts of Drs. Philip S. Low and Steven Beer, respectively; Chandra and Low, 1997) was carried out by the addition of 10 μg of OGA or 60 μg of harpin to 1.5 mL of cell suspension. The cells were stirred gently in a quartz cuvette during fluorescence measurements.
Superoxide Anion Determination by Cyt c Reduction Assay
The amount of O2− accumulated in the soybean cell suspension during the oxidative burst was measured by monitoring the reduction of Cyt c, which displays a change in absorbance when it accepts an electron from the superoxide anion. At time zero, Cyt c (type VI; horse heart, Sigma, St. Louis) was added to 50 μL of cell suspension at a final concentration of 50 μm, and the cells were subjected to osmotic shock by the addition of an equal volume of distilled water. Reduced Cyt c was quantified by monitoring A550 in a bio-kinetics reader (EL 312e, BIO-TEK Instruments, Denkendorf, Germany; Curnutte et al., 1989). To assess whether the O2− accumulation involves NADPH oxidase, DPI, an inhibitor of flavin oxidase, was dissolved in dimethyl sulfoxide and added at a final concentration of 30 μm 30 min before the start of the Cyt c reduction assay. To confirm the identity of the reducing agent of Cyt c as superoxide, we used an inhibitor of SOD, DDC (Sigma). DDC was added to 0.5 mL of cells at a final concentration of 1 mm, 10 min before the start of the assay. The Cyt c reduction assay was performed in the same manner as described above, except in this case the reduced Cyt c was quantified with a spectrophotometer (UV-160A, Shimadzu).
Preparation of Microsomal and Cytosolic Fractions
Cells were collected by filtration and homogenized in grinding medium {100 mm KCl, 3 mm NaCl, 1 mm ATP, 3.5 mm MgCl2, 5 mm Suc, 1 mm phenylmethylsulfonyl fluoride, 2 mm dithiothreitol, and 10 mm PIPES [piperazine-N,N′-bis-(2-ethanesulfonic acid)], pH 7.3} using a mortar and a pestle. The homogenate was centrifuged at 8,000g for 15 min. The supernatant was collected and centrifuged again at 100,000g for 1 h. The resulting pellet was resuspended in grinding medium and used as the microsomal fraction. The cytosolic fraction was prepared by concentrating the supernatant from the second centrifugation step using Centricon filters (10-kD cut-off value; Millipore, Bedfold, MA), giving a final protein concentration of 3 to 4 mg mL−1. Protein concentrations were measured according to the Bradford method (Bradford, 1976) using bovine serum albumin as a standard.
Electrophoresis and Protein Immunoblotting
Protein extracts (100 μg) were subjected to 15% SDS-PAGE and then electrophoretically transferred to a 0.22-μm nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The membrane was blocked for 1 h in TTBS buffer {20 mm Tris [Tris(hydroxymethyl)aminomethane], 130 mm NaCl, 0.05% [w/v] NaN3, and 0.05% [v/v] Tween 20, pH 7.4} supplemented with 5% (w/v) non-fat milk powder. Primary antibodies were then added at 1:500 dilution in TTBS. Anti-Rac1(C189S) antiserum was prepared by immunizing rabbits with purified Rac1(C189S) protein (Kreck et al., 1994) according to standard protocols as previously described (Han et al., 1998). Anti-Rop antiserum was prepared as previously described (Lin et al., 1996). Anti-myc (Invitrogen, Carlsbad, CA), anti-Rac1, and anti-Rac2 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were purchased from commercial suppliers as indicated. Horseradish peroxidase-conjugated anti-mouse IgG antibody (Amersham, Buckinghamshire, UK) was used for detection of anti-myc antibody, and alkaline phosphatase-conjugated anti-rabbit IgG antibody (Promega, Madison, WI) was used for detection of anti-Rac1(C189S), anti-Rac1, anti-Rac2, and anti-Rop antibodies. Secondary antibodies were diluted 1:2,000 in TTBS. All primary antibodies except anti-myc antibody were polyclonal. Anti-Rac1(C189S) and anti-Rop antibodies were raised against whole proteins, whereas Rac1- and Rac2-specific antibodies were raised against the C-terminal 11 amino acids of the respective proteins.
[γ-35S] GTP-Binding Assay
Soybean proteins were transferred onto a nitrocellulose membrane as described above. The membrane was washed with 100 mL of renaturation buffer (50 mm Tris-HCl, pH 7.5, 0.1% [w/v] bovine serum albumin, 5 mm MgCl2, 2 mm dithiothreitol, and 0.1% [v/v] Triton X-100) for 90 min and then incubated in 10 mL of fresh renaturation buffer containing 2.7 nm [γ-35S] GTP with gentle agitation for a further 90 min. During the next 90 min, the membrane was washed six times with renaturation buffer. All reactions were performed at room temperature. The membrane was air-dried and radioactive bands were visualized by autoradiography.
Microprojectile Bombardment of Suspension-Cultured Soybean Cells with Mutant Rac Genes
One milliliter of soybean cell suspension culture was harvested 7 d after subculture. Cells were spread in a thin layer over a filter paper moistened with a small amount of growth medium. M-10 tungsten particles (Bio-Rad, Hercules, CA) were coated with 30 μg of plasmid DNA (containing equal amount of Rac and GUS constructs) and used as the microprojectiles in the transfections. After microprojectile bombardment (Biolistic PDS-1000/He System, Bio-Rad) according to the manufacturer's instructions, the cells were scraped from the filter paper and transferred into 5 mL of fresh growth medium. The cells were grown in a shaking incubator as described above for 24 h before measurement of the oxidative burst. The efficiency of transformation was estimated by determining the percentage of cells expressing GUS. GUS activity was assayed by staining the cells with a substrate solution (100 mm sodium phosphate, pH 7.0, 1 mm EDTA, 5 mm potassium ferrocyanide, 5 mm potassium ferricyanide, 1% [v/v] Triton X-100, and 1 mg mL−1 5-bromo-4-chloro-3-indoyl-β-d-GlcUA cyclohexylamine salt from Rose Scientific, Edmonton, AB, Canada; Citovsky et al., 1994). Transformation efficiency normally reached 30% to 60%. Expression of Rac proteins in the soybean cells was confirmed by western analysis using anti-myc antibody.
To optimize transformation efficiency, we sometimes performed the osmotic treatment described by Vain et al. (1993) by placing the filtered soybean cells onto an osmoticum-containing solid medium for 4 h before and 16 h after bombardment. The osmoticum consisted of an equimolar mixture of mannitol and sorbitol to give a final concentration of 0.25 m.
ACKNOWLEDGMENTS
We thank Drs. Philip S. Low and Sung Ho Ryu for useful discussions and critical reading of the manuscript. We also thank Drs. Jae-Hong Kim, Gynheung An, Philip S. Low, Steven Beer, and J. David Lambeth for providing us with the mutant Rac gene constructs, binary vectors, OGA, harpin, and anti-Rac1(C189S) antibody, respectively, and Won-Yong Song for technical assistance.
Footnotes
This work was supported by grants from the Korea Science and Engineering Foundation (to Y.L.) and from the National Science Foundation (no. MCD–9724047 to Z.Y.).
LITERATURE CITED
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MM, Keppler LD, Orlandi EW, Baker CJ, Mischke CF. Involvement of plasma membrane calcium influx in bacterial induction of the K+/H+ exchange and hypersensitive responses in tobacco. Plant Physiol. 1990;92:215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auh CK, Murphy TM. Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Mansfield JW. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol. 1998;118:1067–1078. doi: 10.1104/pp.118.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Regulation of the human neutrophil NADPH oxidase by the Rac GTP-binding proteins. Curr Opin Cell Biol. 1994;6:212–218. doi: 10.1016/0955-0674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chandra S, Low PS. Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem. 1997;272:28274–28280. doi: 10.1074/jbc.272.45.28274. [DOI] [PubMed] [Google Scholar]
- Citovsky V, Warnick D, Zambryski P. Nuclear import of Agrobacterium VirD2 and VirE2 proteins in maize and tobacco. Proc Natl Acad Sci USA. 1994;91:3210–3214. doi: 10.1073/pnas.91.8.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte JT, Scott PJ, Mayo LA. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci USA. 1989;86:825–829. doi: 10.1073/pnas.86.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer DP, Pear JR, Andrawis A, Stalker DM. Genes encoding small GTP-binding proteins analogous to mammalian rac are preferentially expressed in developing cotton fibers. Mol Gen Genet. 1995;248:43–51. doi: 10.1007/BF02456612. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Coffey MJ, Neill SJ. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 1996;382:213–217. doi: 10.1016/0014-5793(96)00177-9. [DOI] [PubMed] [Google Scholar]
- Diekmann D, Brill S, Garrett MD, Totty N, Hsuan J, Monfries C, Hall C, Lim L, Hall A. Bcr encodes a GTPase activating protein for p21rac. Nature. 1991;351:400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- Doke N. Involvement of superoxide anion generation in hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans. Physiol Plant Pathol. 1983;23:345–347. [Google Scholar]
- Dwyer SC, Legendre L, Low PS, Leto TL. Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim Biophys Acta. 1996;1289:231–237. doi: 10.1016/0304-4165(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Farnsworth CA, Feig LA. Dominant inhibitory mutations in the Mg2+-binding site of RasH prevent its activation by GTP. Mol Cell Biol. 1991;11:4822–4829. doi: 10.1128/mcb.11.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CH, Freeman JLR, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase: identification of an activation domain in p67phox. J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- Irani K, Goldschmidt-Clermont PJ. Ras, superoxide and signal transduction. Biochem Pharmacol. 1998;55:1339–1346. doi: 10.1016/s0006-2952(97)00616-3. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Jung V, Wei W, Ballester R, Camonis J, Mi S, Van Aelst L, Wigler M, Broek D. Two types of RAS mutants that dominantly interfere with activators of RAS. Mol Cell Biol. 1994;14:3707–3718. doi: 10.1128/mcb.14.6.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimamoto K. The small GTP-binding protein Rac is a regulator of cell death in plants. Proc Natl Acad Sci USA. 1999;96:10922–10926. doi: 10.1073/pnas.96.19.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ, Atkinson MM. Activated oxygen production during a bacteria-induced hypersensitive reaction in tobacco suspension cells. Phytopathology. 1989;79:974–978. [Google Scholar]
- Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- Kieffer F, Simon-Plas F, Maume BF, Blein JP. Tobacco cells contain a protein, immunologically related to the neutrophil small G protein Rac2 and involved in elicitor-induced oxidative burst. FEBS Lett. 1997;403:149–153. doi: 10.1016/s0014-5793(97)00038-0. [DOI] [PubMed] [Google Scholar]
- Kim BC, Kim JH. Nuclear signalling by Rac GTPase: essential role of phospholipase A2. Biochem J. 1997;326:333–337. doi: 10.1042/bj3260333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kwack HJ, Choi SE, Kim BC, Kim YS, Kang IJ, Kumar CC. Essential role of Rac GTPase in hydrogen peroxide-induced activation of c-fos serum response element. FEBS Lett. 1997;406:93–96. doi: 10.1016/s0014-5793(97)00249-4. [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreck ML, Uhlinger DJ, Tyagi SR, Inge KL, Lambeth JD. Participation of the small molecular weight GTP-binding protein Rac1 in cell-free activation and assembly of the respiratory burst oxidase. J Biol Chem. 1994;269:4161–4168. [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a Rop GTPases-dependent pathway that leads to tip-localized calcium influx. Plant Cell. 1999;11:1731–1741. doi: 10.1105/tpc.11.9.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu G, Ware D, Davis KR, Yang Z. Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol. 1998;118:407–417. doi: 10.1104/pp.118.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang Y, Zhu J, Yang Z. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Yang Z. Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggest a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell. 1997;9:1647–1659. doi: 10.1105/tpc.9.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense: function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Murphy TM, Auh CK. The superoxide synthases of plasma membrane preparations from cultured rose cells. Plant Physiol. 1996;110:621–629. doi: 10.1104/pp.110.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisimoto Y, Freeman JLR, Motalebi SA, Hirshberg M, Lambeth JD. Rac binding to p67phox: structural basis for interactions of the Rac1 effector region and insert region with components of the respiratory burst oxidase. J Biol Chem. 1997;272:18834–18841. doi: 10.1074/jbc.272.30.18834. [DOI] [PubMed] [Google Scholar]
- O'Donnell VB, Tew DG, Jones OTG, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Kuc J. Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disk. Phytopathology. 1992;82:696–699. [Google Scholar]
- Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999;119:849–858. doi: 10.1104/pp.119.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation. J Biol Chem. 1993;268:20983–20987. [PubMed] [Google Scholar]
- Sathyamoorthy M, Mendez ID, Adams AG, Leto TL. p40phox down-regulates NADPH oxidase activity through interactions with its SH3 domain. J Biol Chem. 1997;272:9141–9146. doi: 10.1074/jbc.272.14.9141. [DOI] [PubMed] [Google Scholar]
- Stennis MJ, Chandra S, Ryan CA, Low PS. Systemin potentiates the oxidative burst in cultured tomato cells. Plant Physiol. 1998;117:1031–1036. doi: 10.1104/pp.117.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vain P, McMullen MD, Finer JJ. Osmotic treatment enhances particle bombardment-mediated transient and stable transformation of maize. Plant Cell Reports. 1993;12:84–88. doi: 10.1007/BF00241940. [DOI] [PubMed] [Google Scholar]
- Winge P, Brembu T, Bones AM. Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol Biol. 1997;35:483–495. doi: 10.1023/a:1005804508902. [DOI] [PubMed] [Google Scholar]
- Xia G, Ramachandran S, Hong Y, Chan YS, Simanis V, Chua NH. Identification of plant cytoskeletal, cell cycle-related and polarity-related proteins using Schizosaccharomyces pombe. Plant J. 1996;10:761–769. doi: 10.1046/j.1365-313x.1996.10040761.x. [DOI] [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E. Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell. 1997;9:249–259. doi: 10.1105/tpc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Watson JC. Molecular cloning and characterization of rho, a ras-related small GTP-binding protein from the garden pea. Proc Natl Acad Sci USA. 1993;90:8732–8736. doi: 10.1073/pnas.90.18.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh LH, Park YJ, Hansalia RJ, Ahmed IS, Deshpande SS, Goldschmidt-Clermont PJ, Irani K, Alevriadou BR. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol. 1999;276:C838–C847. doi: 10.1152/ajpcell.1999.276.4.C838. [DOI] [PubMed] [Google Scholar]