Abstract
Because termites (Reticulitermes speratus) are very small, it is difficult to conduct experiments involving pathogen injection and hemocyte collection. Therefore, to observe hemocyte-mediated immune responses against foreign substances, in vitro hemocyte culture is essential. After collecting about 3 μl of hemolymph, hemocytes were cultured for 7 d, during which the cells maintained full function. Four types of hemocyte were identified, namely, granulocytes, plasmatocytes, oenocytoids, and prohemocytes, among which granulocytes are the main immune hemocytes that fight invasion by foreign substances. Most hemocytes were alive and/or functioning after 7 d of culture, but then either died or lost function.
Keywords: termite, hemocyte, culture, immune
Insect immune responses to foreign substances are largely divided into two categories: humoral and cellular. Cell-mediated defense is provided by immune-activated hemocytes. Immune-activated hemocytes fight foreign pathogens such as bacteria, fungi, and parasites through the processes of phagocytosis, encapsulation, and nodulation (Salt 1970, Kwon et al. 2014, Lee et al. 2016). Therefore, to examine cellular immune responses in insects, it is important to identify the types and characteristics of hemocytes. To investigate cellular immune activation, immune reactants (foreign substances or pathogens) are injected directly into the hemocoel, and, after a certain period of time, immunologically activated hemocytes are collected,and their morphology and characteristics are examined (Kwon et al. 2014).
In vivo experiments based on repeat pathogen injections and hemocyte sampling suggest that immune responses may be accompanied by other responses that activate cells. In addition, it is impossible to observe precise cellular immune responses because various experimental steps are performed between hemocyte collection and observation, which again may activate cells. Furthermore, in the case of small insects, in vivo experiments are impossible because it is difficult to inject foreign materials and to collect hemocytes. Therefore, it is essential to develop an in vitro culture system to overcome these problems and observe immune responses induced by hemocytes (Sohi 1979).
All cell lines currently used by the scientific community are primary cell lines isolated from tissues; these cell lines undergo somatic cell division (growth) at least 10 times over several culture periods (Mandrioli et al. 2015). The stable cell line is then frozen and preserved in a state that can be reused at any time (Mandrioli et al. 2015). The first insect cell line was cultured by Grace in 1962; since then, more than 500 cell lines derived from over 100 insect species have been reported (Grace 1962, Hink and Hall 1989, Lynn 2002). However, many cell lines used by individual scientists are commercially or scientifically unproven. In addition, many important insect species have not had cell lines developed because of difficulties in culturing (Mandrioli et al. 2015). To date, insect cell lines, such as sf9 and sf21 (ovarian tissues of Spodoptera frugiperda) and Tn-368 (Trichoplusia ni), are commonly used in the fields of animal cell medicine, molecular biology, and entomology (Vaughn et al. 1977, Drugmand et al. 2012). However, the above-mentioned insect cell lines, which were derived from tissues, cannot be used to examine cellular immune response in insects. Therefore, studies on cell-mediated immune responses in insects must be conducted using hemocytes; moreover, studies of cellular immune responses in small insects must be examined in in vitro hemocyte cultures.
The hemolymph of insects contains at least three to seven different types of hemocyte (Gupta 1979). It is impossible to separate these cells and cultivate them individually. In addition, prohemocytes often differentiate into different kinds of hemocytes in response to different signaling pathways. Also, it is impossible to cultivate different hemocyte populations in vitro because each requires specific substances, such as insect hormones (Duressa and Huybrechts 2016). To date, no insect hemocyte cell lines have been frozen beyond the primitive cell line stage and/or established through more than 10 cell divisions. Therefore, studies of insect hemocyte-mediated cellular immunity using in vitro cell culture have been based on primitive hemocytes. For example, Duressa and Huybrechts (2016) developed a method of culturing hemocytes from Locusta migratoria. The desert locust (Schistocerca gregaria) and observed viable hemocytes for 7 d (Huxham and Lackie 1988, Liang et al. 2012). At the same time, hemocyte cell culture success was judged to be immunologically active. Recently, hemocytes derived from Rhodnius prolixus were isolated and cultured for 5 d, and the antimicrobial peptide, Jaburetox, was expressed in the culture medium (Fruttero et al. 2016). As mentioned above, there are no established insect hemocyte lines; most in vitro studies are based on primitive cells.
The main objective of the present study was to examine the different types of Reticulitermes speratus hemocyte and their immune responses. Termites are very small, making in vivo experiments impossible. Here, we cultured termite hemocytes successfully and examined their morphology and characteristics. At the same time, we cultured hemocytes with foreign substances and identified specific cell types responsible for cellular immune response. These procedures can be applied to different types of small insect.
Materials and Methods
Insect
Three species of termites are found in Korea. R. speratus kyushuensis Morimoto, and Coptotermes formosanus Shiraki species are mainly found in the southern part of Korea. R. speratus Kolbe is known to be distributed in the northern part of Korea (Lee and Jeong 2004). In this study, R. speratus was used and collected from dead trees near Kangwon National University. Approximately, 200 adults were maintained in a cage 80 × 60 × 90 cm at 22 ± 1°C,16:8 L:D, and 30 ± 10%.
Hemolymph and Hemocyte Culture
Hemolymph was collected from adults using a micro-glass pasteur pipette by puncturing the cuticle dorsally and collecting the resulting material by capillary action. Schneider’s medium (Schneider’s Insect medium: 2 mg/ml tryptose phosphate, 10% inactivated fetal bovine serum, 0.52 mg/ml glucose, 30 mg/ml antimicrobiotics mixture, pH 7.0) was evaluated as the most suitable hemocyte culture medium (Grace 1962, Hink and Hall 1989, Lynn 2002). Cell cultures were prepared by mixing ~3 μl hemolymph and ~3 ml cell culture medium in petri dishes (16-well, 96-well flat bottom plates) and cultured at 37°C for 2 h. The media was then changed every 48 h and cultured for 14 d.
Latex Beads and Hemocyte Culture
Hemocytes and latex beads were cultured together to confirm that the cultured cells were functionally maintained. After 6 h of hemocyte culture, cellular immune activation of hemocytes was checked.
Microscopy and Staining
Cultured hemocytes were observed using a Leica microscope (Leica DM2500 upright and Leica DMI 3000B inverted fluorescence microscopes) and an Olympus FV1000 confocal microscope. Hemocytes were fixed with paraformaldehyde (~3%) in PBS pH 7.0 for 10 min and stained with DAPI (4′-6-diamidino-2-phenylindole; 10 µg/ml) for 30 min and fluorescently conjugated phalloidin (F-actin cytoskeleton) (4.0 µM; molecular probes) for 1 h.
Results and Discussion
Culture of Hemocytes From Adult Termites (R. speratus)
Adult R. speratus has an average length of 0.7 ± 0.05 cm and an average width of 0.2 ± 0.01 cm. Therefore, the amount of hemolymph obtained measured less than 5 μl; however, it was impossible to measure the amount of hemolymph and the number of hemocytes accurately because the insects are so small. In addition, usual methods of identifying immune cells (e.g., injection of bacteria or latex beads followed by cell collection and culture) could not be used because injections ruptured the gut, allowing various microorganisms and protozoa to flow into the hemocoel. Even if the immune activator was successfully injected into the hemocoel, the termites often died due to stress; therefore, this approach was abandoned.
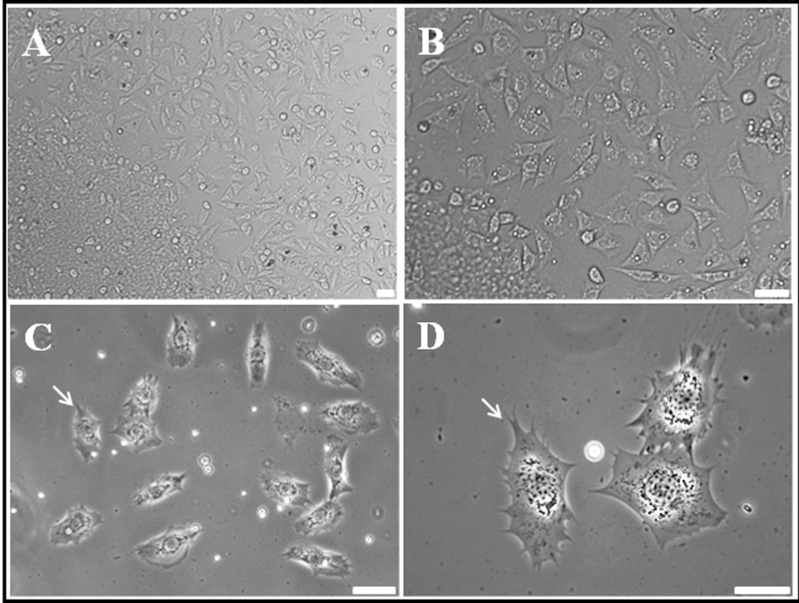
To overcome these problems and to identify immune cells, adult R. speratus hemocytes were cultured in vitro (Fig. 1). After several attempts under different conditions, we succeeded in culturing primitive hemocytes. Figure 1 shows the hemocytes obtained after 12 h of incubation (magnification: A, 20×; B, 40×; C, 60×; D, 100×). Most cultured cells were actively moving, and so culture was stable. Some cells, especially those with lobopodia-like or fan-like structures around the cell membrane, were very active (Fig. 1C and D; lobopodia-like or fan-like structures are indicated by white arrows). The types of insect hemocytes vary depending on the species. Among these, hemocytes usually responsible for immune responses in insects can be classified into two types: granulocytes and plasmatocytes (Salt 1970, Gupta 1979). The morphological characteristics of granulocytes and plasmatocytes are based on observation of lobopodia-like or fan-like structures around the cell membrane (Fig 1C and D) (Kwon et al. 2014, Hwang et al. 2015, Lee et al. 2016). Cells exhibiting pseudopods and extracellular traps around the cell membrane during culture were considered to be attached to the culture slides. Whereas, round and oval hemocytes (with no pseudopodia or filopodia on the plasma membrane), which are optically very smooth, were suspended in the culture medium.
Fig. 1.
Light micrographs showing termite (Reticulitermes speratus) primitive blood cells at 12 h of culture. (A) Overall shape and relative size of cultured hemocytes (mag. ×10). (B–D) Light micrographs showing cultured hemocytes by higher magnification (×20, ×40, and ×100). The pseudopodia-like and extracellular trap-like structures of granulocytes are indicated by white arrows. Scale bar = 10 µm.
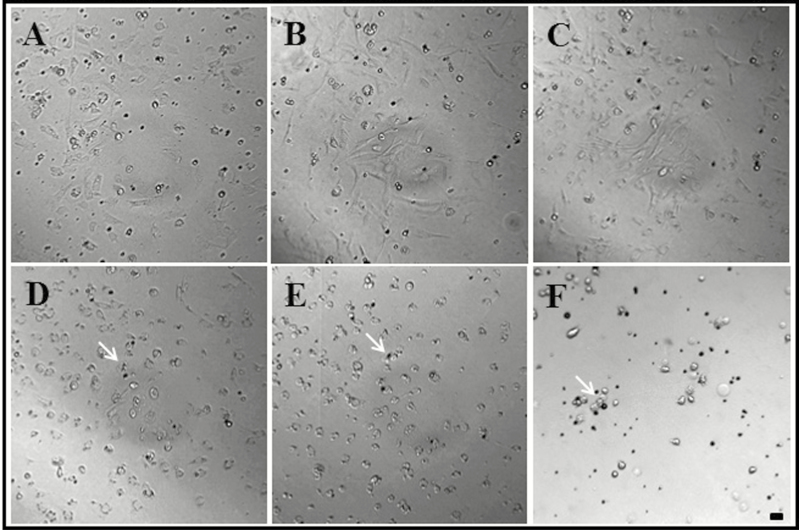
Next, we examined whether these primary cell lines could be cultured for several days with periodic changes of culture medium (Fig. 2). We have confirmed that the cells are actively living after 1 d of incubation (Fig. 2A). Cells cultured between 3 d (Fig. 2B) and 1 wk continued to show vigorous movement (Fig. 2C). However, after 10 d of cell culture, the hemocytes clustered together and became circular, and the color of cells changed to brown (Fig. 2D). After 12 and 14 d, cells did not move and were no longer considered to be alive (Fig. 2E and F). Generally, cell lines widely used by the scientific community are separated from primary tissue (i.e., they are primary cell lines) and undergo at least 10 times of growth/somatic division during several changes of cell culture medium. The established cell lines are then used frozen and stored (Mandrioli et al. 2015). Therefore, we performed real-time imaging to determine whether somatic cell division proceeded normally. However, we were able to observe intermittent cell division only in some small, round cells at the beginning of culture. This suggests that adult R. speratus hemocytes can be cultured only as primitive cell lines. It is difficult to establish insect hemocyte cell lines from primitive hemocytes because insect hemolymph contains seven types of these cells, all of which grow alongside each other and communicate via various signals (Lebel et al. 1996, Sampson et al. 2013). In addition, hemocyte growth in vitro requires several growth factors (such as epidermal growth factors or ecdysone) expressed by insect organs (Lebel et al. 1996, Drugmand et al. 2012, Sampson et al. 2013). In the case of the silkworm (Bombyx mori), e.g., growth and differentiation of hemocytes are dependent upon growth factors that originate from hematopoietic organs (Nakahara et al. 2010). Many studies of Drosophila melanogaster show that the hemocytes originate from hematopoietic tissues (Wood and Jacinto 2007). Therefore, hematopoietic cell tissues are essential for hemocyte somatic cell division, and hemocytes separated from hematopoietic cells will not grow in vitro (Duressa and Huybrechts 2016).
Fig. 2.
Termite (Reticulitermes speratus) primitive hemocyte culture after (A) 1 d, (B) 3 d, (C) 7 d, (D) 10 d, (E) 12 d, and (F) 14 d. The hemocytes clustered together and became circular, and the color of cells changed to brown (indicated by white arrows). Scale bar = 10 µm.
Characterization of Hemocytes Derived From R. speratus
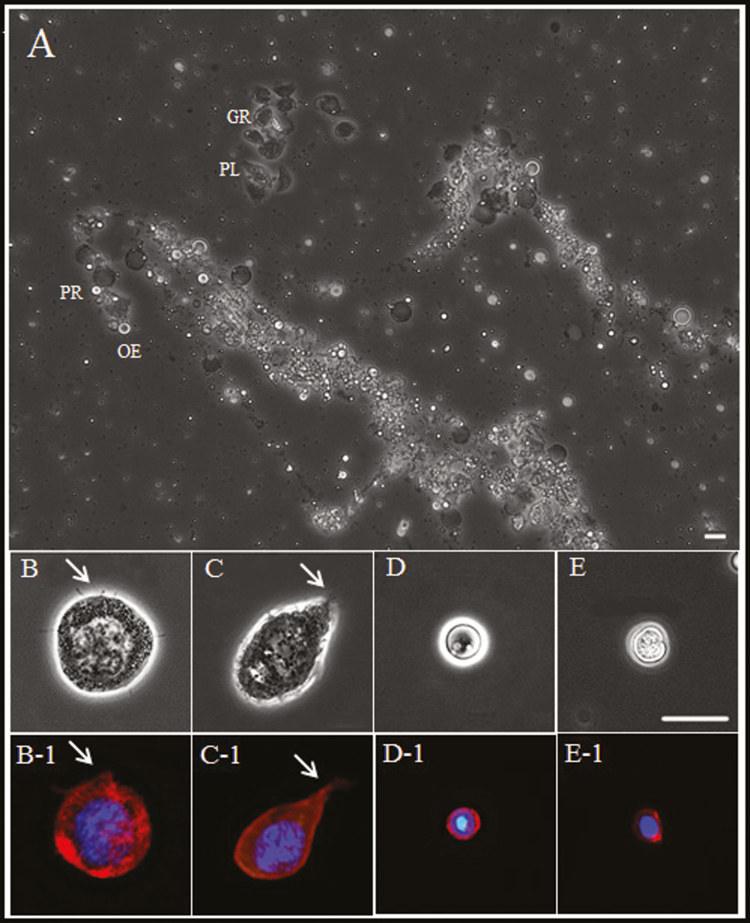
We observed hemocytes at high magnification to identify morphological features and characteristics (Fig. 3). The relative size and shape of four types of hemocyte are shown in Fig. 3A (granulocytes, GR; plasmatocytes, PL; prohemocytes, PR; and oenocytoids, OE). Figure 3B to E also shows hemocytes stained with DAPI (nuclei) and for F-actin (cell membrane). Figure 3B and B-1 shows typical granulocytes harboring various types of granule in the cytoplasm and lobopodia-like or fan-like structures around the cell membrane. The shape and function of insect granulocytes have been studied extensively; these cells are recognized as one of the most important immune cells in insects (Gupta 1979, Kwon et al. 2014, Cho 2016, Lee et al. 2016). Plasmatocytes exhibited a typical spindle shape with lobopodia-like or fan-like structures around the cell membrane (Fig. 3C and C-1). Plasmatocytes are another major immune cell in insects. In particular, plasmatocytes from D. melanogaster mediate various immune responses, including phagocytosis and encapsulation (Vlisidou and Wood 2015). Oenocytoids were generally round, with no visible structures on the cell membrane; the cytoplasm was thick and slightly opaque (Fig. 3D and D-1) (Kwon et al. 2014, Hwang et al. 2015). Oenocytoids are the largest hemocyte in insects but not in R. speratus. Prohemocytes were not easily distinguishable from oenocytoids (both were similar in terms of size and shape) but contained a more transparent cytoplasm and had nuclear predominating in the cytoplasm (Fig. 3E and E-1). These hemocytes (prohemocytes) underwent intermittent somatic cell division at the early stage of cell culture.
Fig. 3.
Confocal microscope images of cultured termite (Reticulitermes speratus) hemolymph. (A) Overall shape and relative size of cultured hemocytes. (B) Hemocytes were classified as granulocytes (GR), (C) plasmatocytes (PL), (D) prohemocytes (PR), and (E) oenocytoids (OE). Confocal images of hemocytes stained with DAPI (blue) for nuclei and with filamentous actin (F-actin; red) for cytoskeleton visualization (B-1 through E-1). The pseudopodia-like and extracellular trap-like structures of granulocytes and plasmatocytes are indicated by white arrows. Scale bar = 10 µm.
Cellular Immune Responses Mediated by Hemocytes From R. speratus
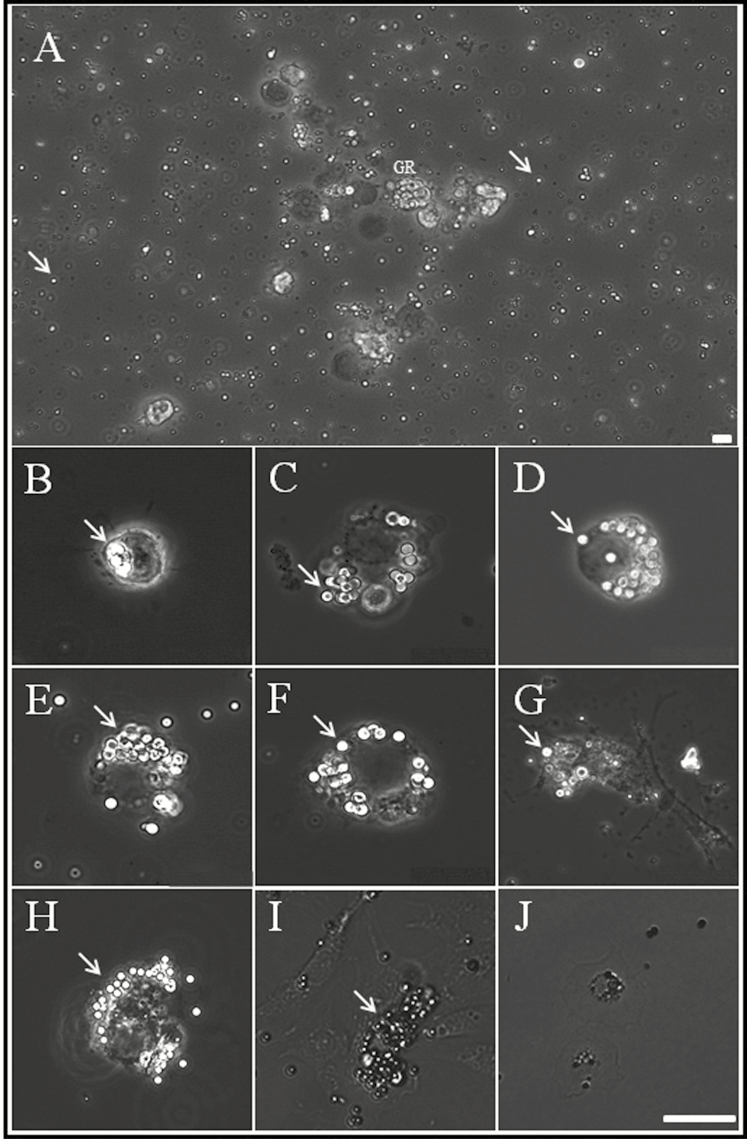
To determine which of the four cell types were involved in cellular immunity, we cultured hemocytes with beads (Fig. 4). Bacteria and fungi could not be used as immune trigger agents because the culture medium contained antibiotics. Instead, we used latex beads containing bacterial cell membrane components as immunoinducers and observed time-dependent activation of cells. Figure 4A shows a low magnification microscope image taken after 12 h of cell culture; the shiny small spheres (arrows) are latex beads. We observed morphological differences between the four types of hemocyte when cultured in the presence of latex beads. Among the four hemocyte types, granulocytes moved and transformed into considerably larger cells with lobopodia-like or fan-like structures. In addition, we observed development and enlargement of different-sized vacuoles in the cytoplasm. These morphological changes were observed from 6 h until 14 d (Fig. 4; 6 h–14 d). Early granulocytes were observed mainly engulfing one or two latex beads (Fig. 4; 6 h); after 9 h, the granulocytes engulfed a large number of beads, and at the same time, many beads were trapped in the fan-like structures around the cell membrane. From 12 h to 1 d, more and more beads accumulated in the cytoplasm. After 2 d, most granulocytes became irregular in shape, and beads were still observed in the cytoplasm. Plasmatocytes did not undergo rapid morphological changes, but occasionally, three to four latex beads were captured in the cytoplasm. However, no changes were observed in prohemocytes or oenocytoids. This suggests that the main immune cell in R. speratus is the granulocyte and that its function is maintained even after 2 d of culture.
Fig. 4.
Light micrographs showing blood cells cultured with carboxylate-modified polystyrene latex beads. (A) Overall shape of cultured hemocytes and beads (indicated by white arrows). (B–J) Cultured granulocytes at 6 h, 9 h, 12 h, 18 h, 1 day, 2 days, 7 days, 10 days, and 14 days. Scale bar = 10 µm. By 7 d of culture, most granulocytes showed polymorphic glittering vacuoles (beads), which were closely packed within the cytoplasm (indicated by white arrows).
Next, hemocytes were cultured for 7–14 d to examine whether they maintained function continuously. Granulocytes were activated, even after 7 d. However, granulocytes had differentiated into an irregular form (10 d). Also, many beads accumulated in the cytoplasm. Hemocytes cultured for 14 d showed no further movement, and granulocytes did not seem to function anymore. Thus, culture of functional primitive R. speratus hemocytes can be maintained for up to 7 d; after that, cultured hemocytes gradually lose viability.
Worldwide studies on termites have mainly focused on evolutionary relationships of symbiotic microbes/protozoa in the intestine and development of termite control methods (Verma et al. 2009, Peterson and Scharf 2016). However, this study is the first to describe in vitro culture of hemocytes that allows identification of different cell types and their function. In particular, this culture method can be used to culture hemocytes obtained from small insects in which it is not possible to observe morphological characteristics and immune responses in vivo.
Acknowledgments
This work was supported by the Korea Research Foundation (Project Number: NRF-2017R1D1A3A03000529).
References Cited
- Cho S. 2016. Ultrastructure characterization of hemocytes in larva of Protaetia brevitarsis seulensis. Korean J. Appl. Entomol. 55: 215–221. [Google Scholar]
- Drugmand J. C., Schneider Y. J., and Agathos S. N.. 2012. Insect cells as factories for biomanufacturing. Biotechnol. Adv. 30: 1140–1157. [DOI] [PubMed] [Google Scholar]
- Duressa T. F., and Huybrechts R.. 2016. Development of primary cell cultures using hemocytes and phagocytic tissue cells of Locusta migratoria: an application for locust immunity studies. In Vitro Cell. Dev. Biol. Anim. 52: 100–106. [DOI] [PubMed] [Google Scholar]
- Fruttero L. L., Moyetta N. R., Uberti A. F., Grahl M. V., Lopes F. C., Broll V., Feder D., and Carlini C. R.. 2016. Humoral and cellular immune responses induced by the urease-derived peptide Jaburetox in the model organism Rhodnius prolixus. Parasit. Vectors 9: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace T. D. C. 1962. Establishment of four strains of cells from insect tissue grown in vitro. Nature 195: 788–789. [DOI] [PubMed] [Google Scholar]
- Gupta A. P. 1979. Insect Hemocytes Development, Forms, Functions and Techniques. Cambridge University Press, New York, NY. [Google Scholar]
- Hink W. F., and Hall R. L.. 1989. Recently Established Invertebrate Cell Lines. CRC Press Inc, Florida, FL. [Google Scholar]
- Huxham I. M., and Lackie A. M.. 1988. Behavior in vitro of separated fractions of hemocytes of the locust Schistocerca gregaria. Cell Tissue Res. 251: 677–684. [Google Scholar]
- Hwang S., Bang K., Lee J., and Cho S.. 2015. Circulating hemocytes from larvae of the Japanese rhinoceros beetle Allomyrina dichotoma (Linnaeus) (Coleoptera: Scarabaeidae) and the cellular immune response to microorganisms. PLoS One 10: e0128519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Bang K., and Cho S.. 2014. Characterization of the hemocytes in Larvae of Protaetia brevitarsis seulensis: involvement of granulocyte-mediated phagocytosis. PLoS One 9: e103620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel J. M., Giard W., Favrel P., and Boucaud-Camou E.. 1996. Effects of different vertebrate growth factors on primary cultures of hemocytes from the gastropod mollusc, Haliotis tuberculata. Biol. Cell. 86: 67–72. [PubMed] [Google Scholar]
- Lee G., and Jeong S.. 2004. Ecological characteristics of termite (Reticulitermes speratus kyushuensis) for preservation of wooden cultural heritage. Cult. Prop. 37: 327–348. [Google Scholar]
- Lee J., Hwang S., and Cho S.. 2016. Immune tolerance to an intestine-adapted bacteria, Chryseobacterium sp., injected into the hemocoel of Protaetia brevitarsis seulensis. Sci. Rep. 6: 31722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T., Ji H., Du J., Ou J., Li W., Wu T., Meng Q., Gu W., and Wang W.. 2012. Primary culture of hemocytes from Eriocheir sinensis and their immune effects to the novel crustacean pathogen Spiroplasma eriocheiris. Mol. Biol. Rep. 39: 9747–9754. [DOI] [PubMed] [Google Scholar]
- Lynn D. E. 2002. Methods for maintaining insect cell cultures. J. Insect Sci. 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrioli M., Monti M., and Tedeschi R.. 2015. A practical guide to insect cell cultures: establishment and maintenance of primary cell cultures. Halteres 6: 132–141. [Google Scholar]
- Nakahara Y., Kanamori Y., Kiuchi M., and Kamimura M.. 2010. Two hemocyte lineages exist in silkworm larval hematopoietic organ. PLoS One 5: e11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. F., and Scharf M. E.. 2016. Metatranscriptome analysis reveals bacterial symbiont contributions to lower termite physiology and potential immune functions. BMC Genomics 17: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt G. 1970. The Cellular Defense Reactions of Insects. Cambridge University Press, New York, NY. [Google Scholar]
- Sampson C. J., Amin U., and Couso J. P.. 2013. Activation of Drosophila hemocyte motility by the ecdysone hormone. Biol. Open. 2: 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohi S. 1979. Hemocyte Cultures and Insect Hemocytology. Cambridge University Press, New York, NY. [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., and McCawley P.. 1977. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 13: 213–217. [DOI] [PubMed] [Google Scholar]
- Verma M., Sharma S., and Prasad R.. 2009. Biological alternatives for termite control: a review. Int. Biodeterior. Biodegradation 63: 1–14. [Google Scholar]
- Vlisidou I., and Wood W.. 2015. Drosophila blood cells and their role in immune responses. FEBS J. 282: 1368–1382. [DOI] [PubMed] [Google Scholar]
- Wood W., and Jacinto A.. 2007. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat. Rev. Mol. Cell Biol. 8: 542–551. [DOI] [PubMed] [Google Scholar]