Abstract
Phthalates are endocrine-disrupting chemicals that can cross the placenta and affect the fetal epigenome. Among various epigenetic regulators of gene expression, long noncoding RNAs (lncRNAs) are important players that may also be involved in the manifestation of endocrine-disrupting chemical toxicity. We sought to explore the association between maternal urinary phthalate metabolite concentrations and lncRNA expression in human placenta to better understand potential mechanisms through which lncRNAs participate in mediating phthalate toxicity. Ten patients with uncomplicated dichorionic diamniotic twin pregnancies at term were included in this study. Urinary (n = 10) and placenta samples (n = 20) were collected for all participants. Urinary samples were analyzed for 15 phthalate metabolites and 2 phthalate alternative metabolites. Real-time PCR arrays were used to identify and quantify 87 lncRNAs from the placental samples. We tested the Spearman correlation matrix to compare prenatal phthalate measures against placental lncRNA levels. lncRNA levels showed large variations across samples, with no significant differences in lncRNA expression within twin pairs. Mono-(carboxynonyl) phthalate demonstrated consistently strong correlations with most lncRNAs. The strongest correlation was observed between mono-hydroxyisobutyl phthalate and LOC91450 (Rspearman = 0.88, p < .001). This correlation remained significant after Bonferroni adjustment. Other strong correlations were observed between mono-isobutyl phthalate, DPP10 and HOTTIP (Rspearman = −0.91, p < .001). AIRN, DACT3.AS1, DLX6, DPP10, HOTTIP, LOC143666, and LOC91450 were strongly correlated with the greatest number of phthalate metabolites. Further studies are needed to validate these results and understand if the altered expression of lncRNAs in human placenta has clinical significance.
Keywords: phthalates, lncRNAs, placenta, imprinting genes, twins
Phthalates are class of environmental chemicals extensively used in a variety of products including personal care products (perfumes, lotions, soaps, shampoos, hair sprays, nail polishes, and cosmetics) paints, textiles, children’s toys, solvents, adhesives, glues, electronics, agricultural adjuvants, and medical devices (Diamanti-Kandarakis et al., 2009; Hauser and Calafat, 2005; Heudorf et al., 2007; Latini, 2005; Schettler, 2006). Multiple phthalate metabolites have been detected in the urine of pregnant women, underscoring the pervasiveness of exposure (Berman et al., 2009; Wolff et al., 2008; Woodruff et al., 2011a,b). This widespread exposure to phthalates may have health consequences to both women and their offspring exposed in utero.
Indeed, phthalate metabolites can cross the placenta, passing from the maternal bloodstream to the fetus (Diamanti-Kandarakis et al., 2009; Mose et al., 2007). The placenta is an endocrine organ that ensures hormonal, nutritional, and oxygen support to the developing fetus. As such, it is also a model organ in considering the impact of environmental chemicals on the developing fetus. Epidemiological studies linked intrauterine phthalate exposure and abnormal neurocognitive development, allergies, asthma, obesity, insulin resistance, and thyroid dysfunction later in childhood (Bajkin et al., 2014). However, the mechanism(s) underlying the adverse effects of phthalate exposure on fetal development remain largely unexplored.
Recent studies have suggested that exposure to environmental toxins like bisphenol A, benzo(a)pyrene, cigarette smoking, and phthalates produces alterations in the expression of long noncoding RNAs (lncRNAs) that may be associated with a variety of human diseases (Geisler and Coller, 2013). lncRNAs are a heterogeneous class of nonprotein coding transcripts longer than 200 nucleotides. Although only a minority of lncRNAs has been described in detail, it is well understood that lncRNAs regulate multiple key biological processes that impact development, differentiation, and metabolism (Bernstein and Allis, 2005; Fatica and Bozzoni, 2014; Wang and Chang, 2011; Whitehead et al., 2009). lncRNAs have been linked to gene-regulatory roles, such as imprinting, epigenetic regulation, control of cell cycle, transcription, translation and splicing (Wapinski and Chang, 2011). Increasing evidence implicates lncRNAs in regulating gene expression both during normal development and under pathological conditions (Costa, 2010; Mercer et al., 2009; Troy and Sharpless, 2012).
Although recent studies point to the involvement of lncRNAs in numerous toxicological responses (Bai et al., 2014; Dempsey and Cui, 2017; Liu et al., 2016), their exact role in various toxicological responses remains largely unknown, necessitating the search for candidate lncRNAs that may help to elucidate their mechanism of action in response to environmental chemical exposure (Geisler and Coller, 2013). To fill this gap, we investigated possible correlations between the expression of 87 placental lncRNAs and maternal urinary levels of phthalate metabolites and phthalate alternative metabolites before delivery.
MATERIALS AND METHODS
This study was approved by our local institutional Ethics Committees (1717-14). All participants signed an informed consent document outlining the details of the study.
Participants and sample collection
This study was a subanalysis of a larger study investigating the effects of maternal exposure to endocrine-disrupting chemicals (EDCs) and epigenetic modifications in twin pregnancies. In this sub-analysis, we included 10 women with uncomplicated dichorionic diamniotic (DC/DA) twin pregnancies at term in a Tertiary University Affiliated Medical Center during July 2015. We excluded monochorionic diamniotic pregnancies or monochorionic mono-amniotic pregnancies as well as pregnancies complicated by gestational diabetes (GDMA1), preeclampsia, or preterm labor.
Urine samples were collected from intended mothers on the day before or on the day of delivery/elective cesarean section. To avoid contamination, patients were advised not to use any wipes before urine sample collection and samples were obtained before any intravenous line was used and only sterile polypropylene collection cups, known not to contain phthalates, were used. Urine samples were aliquoted to 1-ml tubes and frozen at −80°C for storage before shipping for phthalate metabolite quantification. At delivery, placenta samples were collected from each twin. For each placenta, 1.0-cm3 samples were collected at the chorionic plate (fetal side) near the insertion of the umbilical cord. Each sample was cut into small pieces, trimmed of any pieces of membrane, and placed in a tube containing RNAlater Tissue Protect reagent (Ambion, Austin, Texas, USA) to stabilize RNA. Samples were incubated in the refrigerator overnight at 2°C–8°C. Placental tissues were removed the following day from the reagent into new micro centrifuge tubes and stored in −80°C until RNA extraction.
Quantification of phthalate metabolites
Urine samples were shipped to the Centers for Disease Control and Prevention (CDC) for chemical analysis. We quantified the concentrations of 15 metabolites: Mono-isobutyl phthalate (MiBP), mono-3-carboxypropyl phthalate (MCPP), mono(carboxy-isononyl) phthalate (MCNP), mono(carboxy-isooctyl) phthalate (MCOP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MnBP), mono-hydroxybutyl phthalate (MHBP), mono-hydroxyisobutyl phthalate (MHiBP), Mono-isobutyl phthalate MiBP, Mono-2-ethylhexyl phthalate (MEHP), mono-isononyl phthalate (MNP) Mono-methyl phthalate (MMP), and monoethyl phthalate (MEP) and 2 metabolites of the phthalate alternative di(isononyl)cyclohexane-1, 2-dicarboxylate; cyclohexane-1 2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH) and cyclohexane-1 2-dicarboxylic acid mono-carboxyisoctyl ester (MCOCH). These metabolites were chosen because the CDC has a validated analytical method to measure them.
Analysis was performed using solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry, following standard quality assurance/quality control procedures discussed in detail before (Silva et al., 2013, 2017). Limits of detection (LODs) were 0.2 µg/l for MiBP, MCPP, MCNP, MCOP, MECPP, MEHHP, and MEOHP; 0.3 µg/l for MBzP; 0.4 µg/l for MnBP, MHBP, MHiBP, and MHiNCH; 0.5 µg/l for MEHP, MNP, MMP, and MCOCH; and 0.6 µg/l for MEP. Concentrations below the LOD were given a value of LOD/√2, an approach used by the CDC for the analysis of National Health and Nutrition Examination Survey data (Hornung and Reed, 1990).
Placental lncRNA isolation and analysis
Placental RNA extraction was performed using miCURY RNA Isolation Kits (Exiqon, Denmark) according to the manufacturer’s protocol. To evaluate the purity and quantity of the total RNA isolated from the placenta samples, all samples were analyzed using a Pearl Nano-photometer (Implen, Germany). The A260/A280 and A260/A230 ratios were between 1.8-2.0 and 1.9-2.2, respectively. The RNA was first normalized to 250 ng/µl, and 8 µl (2 µg) was converted to cDNA using the RT2 First Strand kit (Qiagen, Valencia, California, USA). Real-time PCR arrays were used to identify and quantify 87 lncRNAs from the placenta samples. Each preprinted array contained 87 probes for specific lncRNAs, 5 probes for housekeeping genes (ACTB, beta 2-microglobulin [B2M], RPLP0, RN7SK, and SNORA73A), 2 probes for spike-in RNAs for reverse transcriptional control, 1 positive PCR control, and 1 DNA contamination control. The panel, consisting of 87 lncRNAs, was based on the human cell development and differentiation RT2 lncRNA PCR array (Cat. no. LAHS-003Z; Qiagen, Valencia, California, USA) that includes 84 differentially expressed lncRNAs during cellular differentiation and organism development, with 3 additional developmentally important lncRNAs: THRIL, PINT, and SRA1 (Karlsson et al., 2016). A STARlet liquid handling robot (Hamilton, Reno, Nevada) was used to transfer cDNA to the RT2 lncRNA arrays, which then were analyzed in a CFX384 Real-Time PCR Detection System (BioRad, Hercules, California, USA). Among the 5 endogenous housekeeping genes (Supplementary Table 1), B2M was selected for normalizing the threshold cycle values (Ct) because it was previously used as a housekeeping gene in other placenta studies (Drewlo et al., 2012).
Statistical analysis
Urinary metabolite concentrations were adjusted for urinary dilution by multiplying the metabolite concentration by ([1.015–1]/[SG-1]), where 1.015 is the mean specific gravity (SG) level for all study urine samples, and SG is the specific gravity of the participant’s urine sample (Boeniger et al., 1993).
The model of DC/DA twins in which each fetus has its own placenta and gestational sac, was chosen to compare placentae from 2 fetuses that were exposed to the same intrauterine conditions and thus overcome possible confounders of biologic variability. By design, we planned to achieve this by identifying within-pair differences in lcRNAs and by using such difference to identify factors that made an individual fetus more susceptible to phthalate exposure. However, because we found no significant differences in lncRNA expression within twin pairs, we analyzed the results from each placenta individually, while accounting for within-pair correlation in the analysis.
We used a Spearman correlation matrix to compare prenatal phthalate metabolite measures against placental lncRNA levels. Only phthalates or phthalate alternative metabolites that were detected in ≥70% of the samples were included in this analysis. Analyses were performed using R statistical computing software (R Foundation for Statistical Computing). A p < .05 was considered statistically significant. Bonferroni adjustments were also made to account for multiple comparisons for the correlation results. In addition, mixed effects models with random intercepts were used to compare lncRNA expression between individuals, accounting for the correlation within the same twin set. Our analysis adjusted for IVF status and the sex of the fetus.
RESULTS
Study Population
Ten Caucasian patients with uncomplicated term DC/DA twin pregnancies (37–38 weeks of gestation) were included in this study. Patients’ characteristics are shown in Table 1. Urine samples (n = 10) and placental samples (n = 20) were collected for all participants. Mean participant age ± SD was 32.9 ± 6.9 years. Four participants conceived using IVF. In total 4 of the pregnancies were of discordant sex twins, and 6 were concordant sex twins (3 male/male and 3 female/female).
Table 1.
Patients’ Characteristics (n = 10)
| Patient Number | Age | Gravida | Parity | Pregnancy Type | GA | Sex First Twin | BW First Twin (g) | Sex Second Twin | BW Second Twin (g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 2 | 1 | Spontaneous | 37 | Female | 2385 | Female | 1940 |
| 2 | 33 | 1 | 0 | Spontaneous | 37 | Female | 1867 | Female | 2100 |
| 3 | 30 | 1 | 0 | IVF | 37 | Female | 2635 | Female | 2435 |
| 4 | 26 | 2 | 1 | Spontaneous | 38 | Male | 2705 | Male | 2770 |
| 5 | 46a | 1 | 0 | IVF egg donation | 37 | Male | 2345 | Male | 1810 |
| 6 | 35 | 4 | 3 | Spontaneous | 37 | Male | 2655 | Male | 2130 |
| 7 | 28 | 1 | 0 | Spontaneous | 38 | Male | 3240 | Female | 3670 |
| 8 | 21 | 1 | 0 | Clomiphen citrate | 38 | Male | 2355 | Female | 2225 |
| 9 | 40 | 1 | 0 | IVF | 37 | Male | 2415 | Female | 2500 |
| 10 | 38 | 2 | 1 | IVF | 38 | Male | 2960 | Female | 2690 |
GA, gestational age at delivery; BW, birth weight.
Egg donor <35 years old.
lncRNA Expression in Placenta Tissue
We had a 100% measurement rate (87/87 developmentally relevant lncRNAs detected) in 10 of the 20 placenta samples, a 95%–99% measurement rate (83–86 lncRNAs) in 4 of the samples, and a 76%–94% measurement rate (74–82 lncRNAs) in the remaining 6 placentas. Among the lncRNAs, ANKRD20A5P, GAS5, H19, LINC01057, LOC101060553, MEG3, NEAT1, RP11_127I20_7, SOCS2-AS1, TUG1, and ZFAS1 were detected in all placentas. HOTAIR and IPW were detected in 19/20 placentas (95%), EGOT was detected in 16/20 (80%), DACT3 in 14/20 (70%), AIRN, DLX6, DPP10 IN 13/20 (65%) and LOC143666 in 12/20 (60%) of the placentas (Supplementary Table 2).
Detection Rate of Urinary Phthalate Metabolites
The average detection rate of 15 urinary phthalates metabolites and 2 phthalate alternative metabolites was 79.4% (range: 10%–100%). MCOP, MEOHP, MEHHP, MECPP, MHiBP, MiBP, and MEP were detected in all participants’ samples. MEP was the metabolite with the highest average concentration across participants, which ranged from 5.8 to 935 (ng/ml) with a mean of 158.7 (ng/ml) (Table 2).
Table 2.
Detection Rates of Urinary Phthalate Metabolites and Phthalate Alternative Metabolites (n = 10)
| Analyte (Long Name) | % Detected >LOD | LOD | Mean | Median | Maximum | Analyte |
|---|---|---|---|---|---|---|
| Cyclohexane-1 2-dicarboxylic acid monohydroxy isononyl ester | 60 | 0.4 | 0.49 | 0.50 | 1.5 | MHiNCH |
| Mono carboxyisononyl phthalate | 90 | 0.2 | 1.35 | 0.50 | 6.0 | MCNP |
| Mono carboxyisooctyl phthalate | 100 | 0.2 | 7.83 | 4.50 | 28.1 | MCOP |
| Mono-2-ethyl-5-carboxypentyl phthalate | 100 | 0.2 | 24.55 | 8.85 | 119.0 | MECPP |
| Mono-2-ethyl-5-hydroxyhexyl phthalate | 100 | 0.2 | 14.21 | 7.75 | 45.0 | MEHHP |
| Mono-2-ethyl-5-oxohexyl phthalate | 100 | 0.2 | 12.74 | 5.70 | 54.8 | MEOHP |
| Mono-2-ethylhexyl phthalate | 70 | 0.5 | 2.43 | 1.85 | 9.1 | MEHP |
| Mono-3-carboxypropyl phthalate | 60 | 0.2 | 0.75 | 0.45 | 2.5 | MCPP |
| Mono-hydroxybutyl phthalate | 60 | 0.4 | 2.11 | 0.90 | 14.6 | MHBP |
| Mono-hydroxyisobutyl phthalate | 100 | 0.4 | 8.00 | 2.90 | 47.3 | MHiBP |
| Mono-isobutyl phthalate | 100 | 0.2 | 35.74 | 11.30 | 196.0 | MiBP |
| Mono-isononyl phthalate | 50 | 0.5 | 0.70 | <LOD | 2.2 | MNP |
| Mono-n-butyl phthalate | 90 | 0.4 | 21.88 | 10.35 | 84.0 | MBP |
| Monobenzyl phthalate | 80 | 0.3 | 2.49 | 1.85 | 7.7 | MBzP |
| Monoethyl phthalate | 100 | 0.6 | 254.20 | 81.60 | 1870.0 | MEP2 |
| Monomethyl phthalate | 80 | 0.5 | 1.75 | 1.10 | 4.7 | MMP |
| Cyclohexane-1 2-dicarboxylic acid monocarboxyisooctyl ester | 10 | 0.5 | <LOD | <LOD | 0.5 | MCOCH |
Maternal Urinary Phthalate Metabolites and lncRNA Expression
In 20 placentas collected at delivery from 10 women with twin pregnancies, most maternal urinary phthalate metabolites were correlated with higher expression levels of placental lncRNAs (Figure 1). Among all phthalate metabolites, MCNP demonstrated consistently strong correlations with most lncRNAs (Figure 1, Supplementary Table 3). The strongest correlation was observed between MHiBP and LOC91450 (Rspearman = 0.88, p < .001). This correlation remained significant also after Bonferroni adjustment. Other strong correlations were observed between MiBP, DPP10 and HOTTIP (Rspearman = −0.91, p < .001) (Supplementary Table 3). AIRN, DACT3.AS1, DLX6, DPP10, HOTTIP, LOC143666, and LOC91450 were strongly correlated with the greatest number of phthalate metabolites (Supplementary Table 3).
Figure 1.
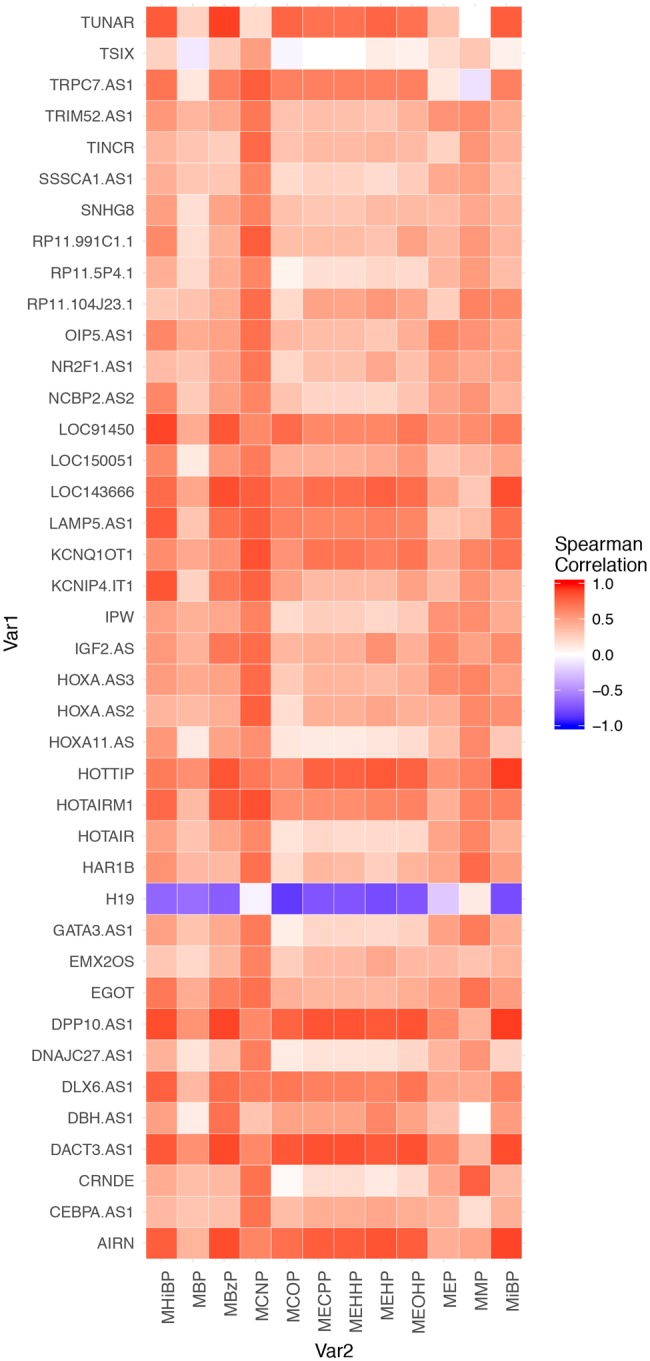
The Spearman correlation among prenatal urinary phthalates present in ≥70% of the samples and placental lncRNAs. Only lncRNAs that were highly negatively correlated (>−0.6) or highly positively correlated (>0.6) with at least 1 phthalate metabolite are present in this figure.
We then tested possible associations between phthalate metabolites and lncRNA expression after controlling for mode of conception (IVF vs non IVF) and fetal sex. MBzP and MEHP were positively associated with large number of lncRNAs. di(2-ethylhexyl) phthalate (DEHP) metabolites MEHP, MEHHP, MECPP, and MEOHP were positively correlated with lncRNA IGF2.AS (p < .05) and lncRNA DACT3.AS1 was positively associated with MBzP, MCOP, MHiBP, and MiBP (Table 3). Although many significant associations were observed, none of the associations met the Bonferroni significance due to the small sample size of this pilot study.
Table 3.
Significant Associations (p < .05) Between Phthalate Metabolites and lncRNAs
| Phthalate Metabolite | lncRNA | Association Type | p Value |
|---|---|---|---|
| MBzP | DACT3_AS1 | Positive | .022 |
| MBzP | DBH_AS1 | Positive | .042 |
| MBzP | LOC91450 | Positive | .042 |
| MBzP | MALAT1 | Positive | .041 |
| MBzP | MEG9 | Positive | .043 |
| MBzP | RP11_127I20_7 | Positive | .046 |
| MBzP | ZFAS1 | Positive | .022 |
| MCNP | BACE1_AS | Positive | .043 |
| MCNP | LOC101060553 | Positive | .045 |
| MCNP | TUG1 | Positive | .030 |
| MCOP | AIRN | Positive | .046 |
| MCOP | DACT3_AS1 | Positive | .044 |
| MCOP | LOC91450 | Positive | .011 |
| MCOP | SOCS2_AS1 | Positive | .048 |
| MECPP | IGF2_AS | Positive | .047 |
| MEHHP | IGF2_AS | Positive | .019 |
| MEHHP | SOCS2_AS1 | Positive | .030 |
| MEHHP | TUG1 | Positive | .040 |
| MEHP | BACE1_AS | Positive | .029 |
| MEHP | IGF2_AS | Positive | .038 |
| MEHP | LINC01057 | Positive | .030 |
| MEHP | LINC01159 | Positive | .050 |
| MEHP | LOC101060553 | Positive | .039 |
| MEHP | NCBP2_AS2 | Positive | .025 |
| MEHP | OIP5_AS1 | Positive | .033 |
| MEHP | SOCS2_AS1 | Positive | .025 |
| MEHP | SSSCA1_AS1 | Positive | .019 |
| MEHP | TUG1 | Positive | .020 |
| MEOHP | IGF2_AS | Positive | .032 |
| MHiBP | DACT3_AS1 | Positive | .049 |
| MHiBP | LINC01057 | Positive | .024 |
| MHiBP | SOCS2_AS1 | Positive | .006 |
| MiBP | DACT3_AS1 | Positive | .046 |
| MMP | NEAT1 | Negative | .020 |
| MMP | TINCR | Positive | .041 |
Models are adjusted for IVF status and the sex of the fetus.
DISCUSSION
In this pilot study, we show ubiquitous expression of a high number of the analyzed lncRNAs (out of 87 tested) in placental tissues collected from uncomplicated DC/DA twin pregnancies. Most lncRNAs demonstrated similar response patterns to maternal urinary phthalate metabolites, with the most substantial upregulations seen with MCNP. MEHP, MEHHP, MECPP, and MEOHP were positively correlated with lncRNA H19 and with lncRNA IGF2-AS after adjustment for IVF and sex.
lncRNAs play a critical task shaping the epigenome and regulating genomic imprinting (Fatima et al., 2015). Genomic Imprinting is a phenomenon of epigenetic silencing of an allele inherited from one of the parents (Wood and Oakey, 2006). By determining genomic imprinting of target genes, lncRNAs may play an essential role in important biological functions as placental and embryonic growth, as well as cell differentiation (Kanduri, 2016). Imprinting disorders have been linked Alzheimer disease, autism, psychiatric diseases, diabetes, obesity, Angelman and Prader-Willi Syndromes, and various types of cancers (Falls et al., 1999). Imprinted genes are prone to be altered by environmental factors (Butler, 2009) and in utero exposure to antibiotic or maternal under nutrition during pregnancy can be associated with altered expression of imprinted genes in the offspring (Lee, 2015; Vidal et al., 2013). Because they are coded within and regulate imprinting loci, lncRNA may represent a novel link between EDCs, imprinting, and fetal development.
IGF2 and H19 are among the most studied imprinted genes and both are located on chromosome 11. IGF2 is a paternally expressed gene, whereas the maternally expressed lncRNA gene H19 is located downstream of IGF2; both influence placental and fetal growth (Kappil et al., 2016). In a recent study, high levels of urinary phthalate metabolites at the first trimester of pregnancy were associated with a decrease in H19 and IGF2DMR0 methylation values (LaRocca et al., 2014). Specifically, for both male and female infants, an increase in MEOHP or MEP exposure was associated with decreased methylation in IGF2DMR0 methylation. The urinary concentrations of 3 of the 4 metabolites of DEHP measured (MEHP, MECPP, and MEHHP) were significantly associated with decreased IGF2DMR0 methylation in placenta samples from female infants, but were not associated with methylation levels in placenta samples from male infant (LaRocca et al., 2014). In line with LaRocca’s study, we found that unadjusted MEHP, MEHHP, MECPP, and MEOHP levels were highly negatively correlated (>0.7) with lncRNA H19 although the correlation was significant only for MEHP (p = .04). After adjustment for sex and IVF, MEHP, MEHHP, MECPP, and MEOHP were positively associated with lncRNA IGF2 expression (p < .05 for all metabolites).
The mechanisms through which lncRNAs regulate gene expression are not yet fully understood, as only a few lncRNAs have been functionally characterized to date, and the role of lncRNAs in pregnancy remains mostly undetermined (Karlsson and Baccarelli, 2016). Studies have reported a link between the expression of several placental lncRNAs and pregnancy complications such as intrauterine growth retardation, preeclampsia, and hemolysis, elevated liver enzymes and low platelet count syndrome (Gremlich et al., 2014; He et al., 2013; Troy and Sharpless, 2012; van Dijk et al., 2012), but the nature of these correlations has yet to be established. Furthermore, 2 recent studies reported an association between lncRNA expression in chorionic villi and miscarriage (Wang et al., 2014, 2017). In vitro experiments have shown that lncRNAs are important for many trophoblast cell functions, including proliferation, invasion and migration, and cell cycle progression (McAninch et al., 2017).
Recent studies in other tissues, also suggest that altered lncRNA expression predict worse outcome in osteosarcoma patients (Yang et al., 2017) and play a role in the pathogenesis of diabetes retinopathy (Yan et al., 2014), glomerulonephritis (Sui et al., 2012) and Parkinson’s disease (Kraus et al., 2017).
There are some limitations to our study. First, the pilot study is limited to 20 placentas and therefore we did not have sufficient statistical power to test further associations between lncRNAs expression, phthalate metabolites and other parameters as gestational age, birth weight, or birth length in multivariate regression models. Second, this study included only uncomplicated twin pregnancies. To the best of our knowledge, there are no publications to date comparing lncRNA profile in placentas from singletons and twins. Thus, our results need to be further tested in uncomplicated singleton pregnancies. Third, only 1 maternal urine sample was collected to assess phthalate concentrations. Given the short half-life of some of the phthalates, a single sample may not represent maternal exposure throughout pregnancy. Assessment of placental weight or any other placental physiological outcomes was beyond the scope of the current pilot, although their examination is warranted in further larger studies.
Our pilot study also has significant strengths. To the best of our knowledge this is the first study to assess correlations between phthalate exposures and a panel of lncRNAs. In addition, assessing uncomplicated pregnancies provides a foundation on which to identify lncRNAs associated with pathologic conditions. Identification of the specific functions of lncRNAs in human placenta and understanding the effects of exposure to EDCs can help aid the understanding of the mechanisms of fetal development and provide insights into normal and pathologic conditions. Further mechanistic experiments are needed to confirm the importance of possible associations between placental lncRNAs and phthalates.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by the Environment and Health Fund, Israel (RG1502); National Institutes of Health (P30ES009089, R01ES021357, R21ES024236, and P30ES00002).
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge Antonia Calafat (CDC, Atlanta, Georgia) for her scientific advice, Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, and Tao Jia (CDC, Atlanta, Georgia) for measuring the urinary concentrations of the environmental biomarkers, and Letizia Trevisi for her help in the statistical analysis.
REFERENCES
- Bai W., Yang J., Yang G., Niu P., Tian L., Gao A. (2014). Long non-coding RNA NR_045623 and NR_028291 involved in benzene hematotoxicity in occupationally benzene-exposed workers. Exp. Mol. Pathol. 96, 354–360.http://dx.doi.org/10.1016/j.yexmp.2014.02.016 [DOI] [PubMed] [Google Scholar]
- Bajkin I., Bjelica A., Icin T., Dobric V., Zavisic B. K., Stojanoska M. M. (2014). Effects of phthalic acid esters on fetal health. Med. Pregl. 67, 172–175. [DOI] [PubMed] [Google Scholar]
- Berman T., Hochner-Celnikier D., Calafat A. M., Needham L. L., Amitai Y., Wormser U., Richter E. (2009). Phthalate exposure among pregnant women in Jerusalem, Israel: Results of a pilot study. Environ. Int. 35, 353–357. [DOI] [PubMed] [Google Scholar]
- Bernstein E., Allis C. D. (2005). RNA meets chromatin. Genes Dev. 19, 1635–1655.http://dx.doi.org/10.1101/gad.1324305 [DOI] [PubMed] [Google Scholar]
- Boeniger M. F., Lowry L. K., Rosenberg J. (1993). Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am. Ind. Hyg. Assoc. J. 54, 615–627.http://dx.doi.org/10.1080/15298669391355134 [DOI] [PubMed] [Google Scholar]
- Butler M. G. (2009). Genomic imprinting disorders in humans: A mini-review. J. Assist. Reprod. Genet. 26, 477–486.http://dx.doi.org/10.1007/s10815-009-9353-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F. F. (2010). Non-coding RNAs: Meet thy masters. BioEssays 32, 599–608.http://dx.doi.org/10.1002/bies.200900112 [DOI] [PubMed] [Google Scholar]
- Dempsey J. L., Cui J. Y. (2017). Long non-coding RNAs: A novel paradigm for toxicology. Toxicol. Sci. 155, 3–21.http://dx.doi.org/10.1093/toxsci/kfw203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M., Zoeller R. T., Gore A. C. (2009). Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewlo S., Levytska K., Kingdom J. (2012). Revisiting the housekeeping genes of human placental development and insufficiency syndromes. Placenta 33, 952–954.http://dx.doi.org/10.1016/j.placenta.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Falls J. G., Pulford D. J., Wylie A. A., Jirtle R. L. (1999). Genomic imprinting: Implications for human disease. Am. J. Pathol. 154, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatica A., Bozzoni I. (2014). Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 15, 7–21.http://dx.doi.org/10.1038/nrg3606 [DOI] [PubMed] [Google Scholar]
- Fatima R., Akhade V. S., Pal D., Rao S. M. (2015). Long noncoding RNAs in development and cancer: Potential biomarkers and therapeutic targets. Mol. Cell. Ther. 3, 5.http://dx.doi.org/10.1186/s40591-015-0042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S., Coller J. (2013). RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 14, 699–712.http://dx.doi.org/10.1038/nrm3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremlich S., Damnon F., Reymondin D., Braissant O., Schittny J. C., Baud D., Gerber S., Roth-Kleiner M. (2014). The long non-coding RNA NEAT1 is increased in IUGR placentas, leading to potential new hypotheses of IUGR origin/development. Placenta 35, 44–49.http://dx.doi.org/10.1016/j.placenta.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Hauser R., Calafat A. M. (2005). Phthalates and human health. Occup. Environ. Med. 62, 806–818.http://dx.doi.org/10.1136/oem.2004.017590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., He Y., Xi B., Zheng J., Zeng X., Cai Q., Ouyang Y., Wang C., Zhou X., Huang H. et al. , (2013). LncRNAs expression in preeclampsia placenta reveals the potential role of LncRNAs contributing to preeclampsia pathogenesis. PloS One 8, e81437.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U., Mersch-Sundermann V., Angerer J. (2007). Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 210, 623–634. [DOI] [PubMed] [Google Scholar]
- Hornung R. W., Reed L. D. (1990). Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 5, 46–51.http://dx.doi.org/10.1080/1047322X.1990.10389587 [Google Scholar]
- Kanduri C. (2016). Long noncoding RNAs: Lessons from genomic imprinting. Biochim. Biophys. Acta 1859, 102–111.http://dx.doi.org/10.1016/j.bbagrm.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Kappil M. A., Li Q., Li A., Dassanayake P. S., Xia Y., Nanes J. A., Landrigan P. J., Stodgell C. J., Aagaard K. M., Schadt E. E. et al. , (2016). In utero exposures to environmental organic pollutants disrupt epigenetic marks linked to fetoplacental development. Environ. Epigenet. 2, pii: Dvv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Baccarelli A. A. (2016). Environmental health and long non-coding RNAs. Curr. Environ. Health Rep. 3, 178–187.http://dx.doi.org/10.1007/s40572-016-0092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Rodosthenous R. S., Jara C., Brennan K. J., Wright R. O., Baccarelli A. A., Wright R. J. (2016). Detection of long non-coding RNAs in human breastmilk extracellular vesicles: Implications for early child development. Epigenetics [Epub ahead of print]. doi: 10.1080/15592294.2016.1216285, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus T. F. J., Haider M., Spanner J., Steinmaurer M., Dietinger V., Kretzschmar H. A. (2017). Altered long noncoding RNA expression precedes the course of Parkinson’s disease-a preliminary report. Mol. Neurobiol. 54, 2869–2877. [DOI] [PubMed] [Google Scholar]
- LaRocca J., Binder A. M., McElrath T. F., Michels K. B. (2014). The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ. Res. 133, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G. (2005). Monitoring phthalate exposure in humans. Clin. Chim. 361, 20–29.http://dx.doi.org/10.1016/j.cccn.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Lee H. S. (2015). Impact of maternal diet on the epigenome during in utero life and the developmental programming of diseases in childhood and adulthood. Nutrients 7, 9492–9507.http://dx.doi.org/10.3390/nu7115467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang B., Liu X., Lu L., Luo F., Lu X., Shi L., Xu W., Liu Q. (2016). Epigenetic silencing of p21 by long non-coding RNA HOTAIR is involved in the cell cycle disorder induced by cigarette smoke extract. Toxicol. Lett. 240, 60–67. [DOI] [PubMed] [Google Scholar]
- McAninch D., Roberts C. T., Bianco-Miotto T. (2017). Mechanistic insight into long noncoding RNAs and the placenta. Int. J. Mol. Sci. 18, 1371.http://dx.doi.org/10.3390/ijms18071371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T. R., Dinger M. E., Mattick J. S. (2009). Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 10, 155–159.http://dx.doi.org/10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- Mose T., Mortensen G. K., Hedegaard M., Knudsen L. E. (2007). Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod. Toxicol. 23, 83–91. [DOI] [PubMed] [Google Scholar]
- Schettler T. (2006). Human exposure to phthalates via consumer products. Int. J. Androl. 29, 134–139. discussion 181–185.http://dx.doi.org/10.1111/j.1365-2605.2005.00567.x [DOI] [PubMed] [Google Scholar]
- Silva M. J., Jia T., Samandar E., Preau J. L. Jr, Calafat A. M. (2013). Environmental exposure to the plasticizer 1, 2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000-2012). Environ. Res. 126, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J., Wong L. Y., Samandar E., Preau J. L., Calafat A. M., Ye X. (2017). Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Arch. Toxicol. 91, 3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui W., Li H., Ou M., Tang D., Dai Y. (2012). Altered long non-coding RNA expression profile in patients with IgA-negative mesangial proliferative glomerulonephritis. Int. J. Mol. Med. 30, 173–178. [DOI] [PubMed] [Google Scholar]
- Troy A., Sharpless N. E. (2012). Genetic “lnc”-age of noncoding RNAs to human disease. J. Clin. Investig. 122, 3837–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk M., Thulluru H. K., Mulders J., Michel O. J., Poutsma A., Windhorst S., Kleiverda G., Sie D., Lachmeijer A. M., Oudejans C. B. (2012). HELLP babies link a novel lincRNA to the trophoblast cell cycle. J. Clin. Investig. 122, 4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal A. C., Murphy S. K., Murtha A. P., Schildkraut J. M., Soubry A., Huang Z., Neelon S. E., Fuemmeler B., Iversen E., Wang F. et al. , (2013). Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int. J. Obes. 37, 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Cao Q., Ge J., Liu C., Ma Y., Meng Y., Wang Y., Zhao X., Liu R., Li C. et al. , (2014). LncRNA-regulated infection and inflammation pathways associated with pregnancy loss: Genome wide differential expression of lncRNAs in early spontaneous abortion. Am. J. Reprod. Immunol. 72, 359–375. [DOI] [PubMed] [Google Scholar]
- Wang K. C., Chang H. Y. (2011). Molecular mechanisms of long noncoding RNAs. Mol. Cell 43, 904–914.http://dx.doi.org/10.1016/j.molcel.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Tang H., Xiong Y., Tang L. (2017). Differential expression profile of long noncoding RNAs in human chorionic villi of early recurrent miscarriage. Clin. Chim. Acta 464, 17–23.http://dx.doi.org/10.1016/j.cca.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Wapinski O., Chang H. Y. (2011). Long noncoding RNAs and human disease. Trends Cell Biol. 21, 354–361.http://dx.doi.org/10.1016/j.tcb.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Whitehead J., Pandey G. K., Kanduri C. (2009). Regulation of the mammalian epigenome by long noncoding RNAs. Biochim. Biophys. Acta 1790, 936–947.http://dx.doi.org/10.1016/j.bbagen.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Wolff M. S., Engel S. M., Berkowitz G. S., Ye X., Silva M. J., Zhu C., Wetmur J., Calafat A. M. (2008). Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 116, 1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. J., Oakey R. J. (2006). Genomic imprinting in mammals: Emerging themes and established theories. PLoS Genet. 2, e147..http://dx.doi.org/10.1371/journal.pgen.0020147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T. J., Zota A. R., Schwartz J. M. (2011a). Environmental chemicals in pregnant women in the United States: nHANES 2003-2004. Environ. Health Perspect. 119, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T. J., Zota A. R., Schwartz J. M. (2011b). Environmental chemicals in pregnant women in the US: nHANES 2003-2004. Environ. Health Perspect. 119, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Tao Z. F., Li X. M., Zhang H., Yao J., Jiang Q. (2014). Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 55, 941–951.http://dx.doi.org/10.1167/iovs.13-13221 [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang S., Li T. (2017). Altered long non-coding RNAs predict worse outcome in osteosarcoma patients: Evidence from a meta-analysis. Oncotarget 8, 35234–35243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


