Abstract
Background
Directly observed therapy (DOT) remains an integral component of treatment support and adherence monitoring in tuberculosis care. In-person DOT is resource intensive and often burdensome for patients. Video DOT (vDOT) has been proposed as an alternative to increase treatment flexibility and better meet patient-specific needs.
Methods
We conducted a pragmatic, prospective pilot implementation of vDOT at 3 TB clinics in Maryland. A mixed-methods approach was implemented to assess (1) effectiveness, (2) acceptability, and (3) cost. Medication adherence on vDOT was compared with that of in-person DOT. Interviews and surveys were conducted with patients and providers before and after implementation, with framework analysis utilized to extract salient themes. Last, a cost analysis assessed the economic impacts of vDOT implementation across heterogeneous clinic structures.
Results
Medication adherence on vDOT was comparable to that of in-person DOT (94% vs 98%, P = .17), with a higher percentage of total treatment doses (inclusive of weekend/holiday self-administration) ultimately observed during the vDOT period (72% vs 66%, P = .03). Video DOT was well received by staff and patients alike, who cited increased treatment flexibility, convenience, and patient privacy. Our cost analysis estimated a savings with vDOT of $1391 per patient for a standard 6-month treatment course.
Conclusions
Video DOT is an acceptable and important option for measurement of TB treatment adherence and may allow a higher proportion of prescribed treatment doses to be observed, compared with in-person DOT. Video DOT may be cost-saving and should be considered as a component of individualized, patient-centered case management plans.
Keywords: mHealth, medication adherence, telemedicine, tuberculosis, video DOT
Tuberculosis (TB) remains a global pandemic responsible for nearly 2 million deaths annually [1]. In the United States, previously reported declines in incident disease have stagnated in recent years [2, 3].
A central challenge in the fight against TB is overcoming the barriers presented by TB therapy itself. Side effects are common, and treatment courses are long, extending well beyond a year in some cases of drug-resistant disease [4, 5]. Poor treatment adherence has been linked to microbiologic failure, disease relapse, and the emergence of drug resistance [6, 7].
In response, and in an effort to promote treatment completion, the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have advocated for directly observed therapy (DOT), wherein the ingestion of each dose is directly monitored [4, 8, 9]. Programmatic uptake of DOT has been widespread. Within the United States, DOT is now the standard of care, and is even codified into law in many states [10].
Despite broad policy support, more recent studies looking at the effectiveness of DOT on treatment outcomes have been mixed, likely owing to heterogeneous approaches to implementation [11–13]. Nonetheless, current treatment guidelines, including that from the CDC, continue to underscore the importance of DOT, but now emphasize its role as just 1 component of a multifaceted approach to case management and treatment support [4, 14]. Further, “To be consistent with the principles of patient-centered care, decisions regarding the use of DOT must be made in concert with the patient” [4]. As such, DOT implementation must account for patient-specific needs, and should ideally couple observation of pill ingestion with strategies for adherence support.
Employing DOT in a patient-centered fashion can be challenging. Scheduling in-person DOT visits is logistically complicated and resource intensive (for patients and TB programs) and can increase both patient- and program-level costs. In some individuals, logistical barriers and perceived stigma related to DOT have led to feelings of humiliation, loss of control, and stress [15, 16]. In some situations, DOT requirements may therefore represent a barrier to adherence. What’s more, provisions for DOT may impact provider prescribing practices. While updated TB guidelines advocate daily therapy (ie, 7 days/week), our experiences suggest that health departments commonly dose TB medications Monday through Friday (M–F, ie, business days) only, or intermittently (ie, 3 days/week), in an effort to facilitate in-person DOT.
To overcome these barriers, video-based DOT (vDOT) has been proposed as an alternative to in-person observation [4, 14, 17]. Early in 2017, the CDC released a toolkit for the implementation of vDOT within TB programs [18]. However, given the limited experience with vDOT, the guideline cautions against its use in complex patients, including those with “adherence issues,” “language barriers,” and “multi-drug resistance,” and acknowledges the need for operational research. This approach, however, may restrict usage in those with complex treatment factors who could potentially benefit most from the added flexibility provided by vDOT.
As such, we designed a pilot implementation study to address several gaps in our current understanding of vDOT implementation [19–27]. We utilize a mixed-methods approach to evaluate (1) feasibility, (2) accessibility, and (3) costs when implemented under real-world conditions. First, we sought to understand feasibility and acceptability in broad patient populations, including those with previously poor adherence and drug-resistant disease. Second, we sought to assess effectiveness for observation of therapy and costs. Finally, we sought to describe implementation challenges and successes, patient selection for vDOT, and the impact of heterogeneities in clinic structure.
METHODS
Overview
We conducted a pragmatic, prospective pilot implementation study. Our objective was to assess the feasibility, acceptability, and cost of vDOT utilizing a Health Insurance Portability and Accountability Act (HIPAA)-compliant mobile app, miDOT (emocha Mobile Health Inc.), for TB treatment monitoring, adherence support, and case management (Supplementary Figure 1). The study was carried out within 3 public health TB clinics in Maryland that service a mixed urban/suburban population. Protocols were approved by the ethics committees at Johns Hopkins University, the Baltimore City Health Department, and the Maryland Department of Health.
Study Population
All adult patients receiving active TB treatment or short-course isoniazid/rifapentine-based latent TB (LTBI) therapy were eligible for participation. Inclusion required that patients be ≥18 years of age and have ≥2 months of therapy remaining. All patients initiated TB therapy with in-person DOT, though they could transition to vDOT at any point during their treatment course. The decisions to offer vDOT were made by nonconflicted health department clinicians, without explicit exclusion of non-English speakers or those with multidrug-resistant disease or poor prior adherence. Patients interested in utilizing vDOT provided written informed consent, and those without access to a smartphone were provided one by the study.
TB Treatment
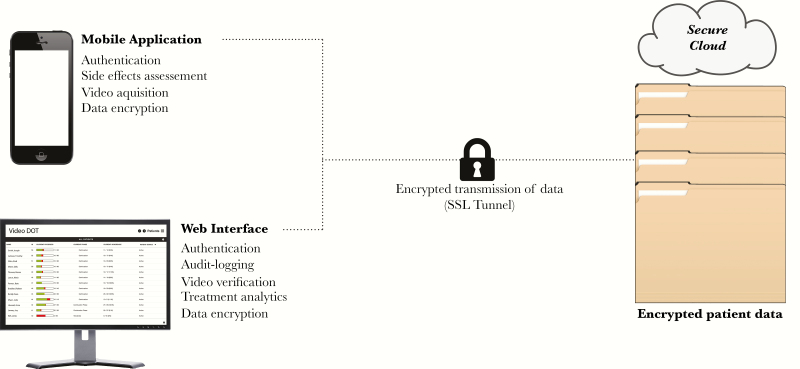
Treatment decisions were clinic-directed according to Maryland state and CDC guidelines, regardless of DOT modality [4, 28]. Under these guidelines, drug regimens generally rely on either daily or intermittent (3 days/week) dosing. While studies have not compared the efficacy of 5 vs 7 doses per week, under DOT, both regimens were referred to as “daily” [4]. Each clinic defined treatment completion and success based on ingesting a set number of target doses. Any missed doses were added to the end of therapy, extending treatment duration. At baseline, for daily dosing, TB clinics combined in-person DOT 5 days/week (M–F) with weekend (and holiday) self-administration, the latter not contributing to the overall dose target. While on vDOT, dosing frequency (ie, 5 days/week vs 7 days/week) and whether to observe and count weekend doses toward an overall dose target were left to clinic discretion. Patients were sent twice-daily SMS reminders in the absence of submitted videos and were prompted to document side effects prior to each submission (see the Supplementary Data for more on miDOT specifics). All patient data, servers, and transmissions were encrypted to protect patient privacy, and the app automatically deleted videos from the smartphone upon transmission (Figure 1).
Figure 1.
Schematic of data acquisition and transmission on miDOT.
Feasibility and Effectiveness
We assessed 2 primary outcomes, acknowledging a lack of consensus definition on measurement of adherence and differences in programmatic practices related to “expected” doses. The first was treatment adherence, or the proportion of “expected” DOT (in-person or video) that was successfully completed, in which the “expected” dose was defined by the TB clinic (usually omitting weekend and holiday self-administered doses) (Supplementary Figure 2). Given that the goal of DOT is to observe all prescribed doses, as a secondary measure, we calculated the observable fraction, or the proportion of total doses (inclusive of weekends, holiday, or other “self-administered” doses) completed under observation (either in-person or by video). All patients received case management per routine at each TB clinic site irrespective of DOT modality; this generally included case management phone calls or visits following missed doses or reported side effects.
Differences pre/post–vDOT implementation were evaluated using paired t tests, though our study was not powered, nor specifically intended, to detect between-group differences. All analyses were conducted in STATA 14.
Acceptability
Qualitative research methodology was employed to explore participant and staff perceptions of in-person and vDOT. All clinic staff (DOT workers, case managers, clinicians) and enrolled patients were approached to complete surveys and in-depth interviews pre/post–vDOT implementation; a separate informed consent was used, and patients could enroll in the study without participation in the qualitative component. All interviews were digitally recorded and transcribed verbatim. Each transcription was reviewed by 2 study members, and an iterative, open-coding strategy with framework analysis was employed to identify salient themes [29].
Cost
A cost analysis was conducted using time motion studies and an ingredients-based approach in which unit costs for labor, equipment, and consumables were multiplied by quantities required for in-person DOT and vDOT (Supplementary Table 5). To allow equal comparisons, final calculations were standardized to a 6-month treatment course (daily therapy) for drug-sensitive TB; based on clinic practices, primary analysis was standardized to a M–F dosing strategy, with a secondary analysis comparing 7-day/week therapy.
In base case analysis, we incorporated costs for a licensed practical nurse (LPN) utilizing a Department of Health (DOH)–owned vehicle for community-based, in-person DOT. For vDOT, the base case scenario incorporated costs of a program-provided smartphone (and associated data costs) and an estimated commercial software cost of $50 per patient per month (personal communication, emocha).
Sensitivity analysis was conducted to evaluate variations in consumable, labor, and equipment costs with consideration of programmatic heterogeneity in the implementation of in-person DOT (eg, type of staff conducting DOT, vehicle used, and travel distance) and vDOT (eg, range of software-associated costs from a high of $100 per patient per month to free) (Supplementary Figure 3).
RESULTS
A total of 28 patients were enrolled and treated between March 2016 and August 2017. Of these, 25 received active TB therapy and 3 received weekly rifapentine/isoniazid (INH) for LTBI (Table 1). Ninety-three percent of patients were foreign born. Only 3 patients (11%) required use of a study phone for vDOT. Thirty-nine percent had extrapulmonary disease, consistent with regional and national epidemiology [30, 31].
Table 1.
Patient Characteristics
| Variable | No. (%) (n = 28) |
|---|---|
| Age, median (IQR), y | 32 (23–49) |
| Female, No. (%) | 16 (57) |
| Foreign born, No. (%) | 26 (93) |
| Origin, No. (%) | |
| United States | 2 (7) |
| Africa | 11 (39) |
| Latin America | 8 (29) |
| South Asia | 4 (14) |
| East Asia | 2 (7) |
| Europe | 1 (4) |
| Time in United States, median (IQR),a y | 5 (3–15) |
| Limited or no English,b No. (%) | 7 (25) |
| Travel to TB endemic country within 5 y, No. (%) | 19 (67) |
| Highest level of education reached, No. (%) | |
| Grade school | 3 (12) |
| High school | 10 (38) |
| College | 9 (35) |
| Postgraduate | 4 (15) |
| Employment, No. (%) | |
| Full-time | 16 (57) |
| Part-time | 7 (25) |
| Unemployed | 5 (18) |
| Annual household income,c No. (%) | |
| <$20 000 | 8 (36) |
| $20 000–$49 999 | 9 (41) |
| $50 000–$100 000 | 4 (18) |
| >$100 000 | 1 (5) |
| Substance use,c,d No. (%) | |
| Tobacco | 1 (4) |
| Alcohol | 1 (4) |
| Illicit drugs | 1 (4) |
| Comorbidities, No. (%) | |
| HIV infected | 2 (7) |
| Hypertension | 2 (7) |
| Diabetes | 1 (4) |
| History of malignancy | 2 (7) |
| Taking daily (non-TB) medications, No. (%) | 6 (21) |
| Technology, No. (%) | |
| Regular access to smartphone | 25 (89) |
| Required study phonee | 3 (11) |
| Tuberculosis type, No. (%) | |
| Pulmonary | 9 (32) |
| Smear positive | 5 (18) |
| Smear negative | 11 (39) |
| Exclusively extrapulmonary | |
| Latent | 3 (11) |
| MDR disease,f No. (%) | 1 (4) |
Abbreviations: IQR, interquartile range; MDR, multidrug-resistant; TB, tuberculosis.
aCalculated for foreign-born individuals only, those reporting “less than 1 year” were considered to have been in the United States for 6 months for statistical purposes.
bIncluded 6 Spanish speakers and 1 Oromo.
cExcludes those for whom data were unknown.
dRepresents 3 separate patients.
eAll 3 phones were returned at study completion in good working order.
fRefers only to those treated for active TB. All LTBI patients received weekly rifapentine for 12 weeks.
Among active TB patients prescribed “daily” therapy (at any point during their treatment), a dosing strategy of DOT 5 times/week (M–F) with weekend self-administration was the most common observation strategy regardless of DOT modality, though it was more frequent during the in-person period (100% vs 76%, P = .01). Overall, intermittent thrice-weekly therapy was utilized less commonly on vDOT than during in-person (24% vs 16%, P = .32). No patients received 7 days of in-person DOT, though 2 were transitioned to this schedule while on vDOT. The mean times on therapy for in-person DOT and vDOT were 12.2 and 19.2 weeks, respectively (P = .01).
Feasibility/Effectiveness
Measured adherence was high irrespective of DOT strategy: median 98% (interquartile range [IQR], 90–100) during in-person DOT and median 94% (IQR, 88–98) while on vDOT (P = .17) (Table 2). The median observable fraction (ie, proportion of all prescribed doses observed) was statistically lower during the in-person DOT period (in-person, 66% [62%–72%]; vs vDOT, 72% [67%–92%]; P = .03). Overall, only 15% of patients had more than 80% of total prescribed doses verified through observation during in-person DOT, compared with 36% during vDOT (P = .01), a consequence of self-administered weekend and holiday doses.
Table 2.
Primary Outcomes by DOT Strategy
| Variable | In-Person DOT | vDOT | P |
|---|---|---|---|
| Adherence,a median (IQR), % | 98 (90–100) | 94 (88–98) | .17 |
| Observable fraction,b median (IQR), % | 66 (62–72) | 72 (67–92) | .03 |
| No. (%) of patients with observable fraction greater than a target 80% | 4 (15) | 10 (36) | .01 |
| DOT schedule among active TB patients (n = 25),c % | |||
| 3x/wk DOT | 6 (24) | 4 (16) | .32 |
| 5x/wk DOT | 25 (100) | 19 (76) | .01 |
| 7x/wk DOT | 0 (100) | 2 (8) | .16 |
| Treatment length, wk | .01 | ||
| Mean ± SD | 12.22 ± 6.5 | 19.2 ± 9.7 | |
| Range | 0–26 | 5–37 | |
| No. of rejected videos | |||
| Mean (SD) | 1.8 (2.4) | ||
| Range | 0–11 | ||
| Unexpected video submission | |||
| Mean (SD) | 2.7 (5.3) | ||
| Range | 0–20 | ||
| Patients reporting ≥1 side effects via mobile platform,d % | 46 | ||
| Video length, median (IQR), sec | 48 (29–63) | ||
| Video size, median (IQR), mb | 4.8 (1.4–5.8) | ||
Abbreviations: DOT, directly observed therapy; IQR, interquartile range; TB, tuberculosis; vDOT, video directly observed therapy.
Only participants treated for active TB included (n = 25).
aPercentage of “expected” DOT doses (in-person or video) completed, excluding self-administered doses (ie, weekends or clinic holidays). An additional, less stringent analysis was also conducted wherein “completed” vDOT was loosely defined to include both verified and rejected miDOT videos: in-person 98% (90%–100%) vs vDOT 96% (89%–100%), P = .37.
bPercentage of total planned doses (inclusive of weekend/holiday self-administered) that were observed (in-person or video). For vDOT, “observation” was loosely defined to include all forms of uploaded miDOT videos (verified, rejected, unexpected), though only 1 video was counted for a given dosing day. An additional, stricter analysis was also conducted wherein, for vDOT, “observation” referred only to verified videos: in-person 66% (62%–71%) vs vDOT 70% (63%–90%), P = .22.
cTotal number of regimens exceeds sample size (n = 25, active TB only) as some participants had >1 dosing frequency during their therapy.
dThe miDOT video system prompts patients to indicate side effects prior to video submission using checkboxes on the mobile app, with positives resulting in an automatic provider alert. The most common symptom reported was abdominal pain, followed by weakness. Other reported side effects included nausea/vomiting, rash, sores on lips/mouth, joint pain, yellowish skin or eyes, and other. Of note, some patients digitally captured side effects during the video recordings (eg, rash).
Fifty-seven percent of patients had at least 1 rejected video (mean, 1.8; range, 0–11), representing 2.1% of all submitted videos (2350). The 2 most commonly cited reasons for rejection were “Medication dose not visible” and “Poor video quality.”
A total of 4 patients traveled internationally while on miDOT, though they continued to successfully submit videos. Two patients were transferred from Health Department care after permanently leaving the United States prior to treatment completion (1 to Liberia and 1 to Ivory Coast); both had been on vDOT for >16 weeks with adherence of 72% and 87%, respectively, at the time of study exit. A single patient had vDOT discontinued prematurely after 5 weeks due to an adherence of 63% (on 7 days/week of DOT); the patient had been on in-person DOT for 17 weeks prior to vDOT, expressed an interest to return to her prior routine of in-person DOT, and successfully completed therapy with an adherence of 100%.
Acceptability
All staff and patients were approached to explore attitudes toward in-person DOT and vDOT. Twenty staff members participated before vDOT implementation, and 16 post-implimentation; 25 patients were included before vDOT, with 10 providing post-treatment feedback. vDOT adherence did not differ between patients completing and those not completing post-intervention qualitative assessment (adherence, 89% vs 90%; P = .92).
At baseline, nearly all staff members felt that in-person DOT provided beneficial social support (95%), and only a few (10%) considered self-administered therapy to be sufficient alone (Supplementary Tables 1 and 2). Both staff (95%) and patients (92%) were comfortable using smartphones from the outset. Following the intervention, all surveyed patients felt that the miDOT platform was “easy to use” and preferred it over in-person DOT.
Themes related to this preference for vDOT were common during interviews and focused on convenience and increased flexibility (Table 3; Supplementary Tables 3 and 4). Both patients and staff commented on the limitations of in-person DOT when managing complex schedules. Speaking to the impact of foreign travel, 1 staff member noted, “We try to arrange jurisdictional coverage during [travel] times, but if it’s outside the country, you really can’t.”
Table 3.
Subset of Themes from Qualitative Analysis
| Theme | Subtheme | Representative Quote |
|---|---|---|
| Patient | ||
| Impact of DOT on patients | sDOT can be burdensome for patients | “I’m about to start a class, and the class…doesn’t really match the time that I have to be here to take the pill.… I won’t be able to do the class, and I need the class more than I need [DOT].” |
| sDOT can cause emotional stress | “In-person DOT had an emotional impact on me; it was stressful. It made me resent [the treatment team].” | |
| DOT logistics | sDOT efficacy is limited by patient factors | “[sDOT] just doesn’t work. Like tonight, I work, I don’t get off until 7:30 am, and then I go to school.… There is no time.” |
| vDOT increases access to transient patients | “When I was in Peru for 2 months, the system worked perfectly. Sometimes I even used it outside of the city or at the beach.” | |
| vDOT increases access to those with complicated work schedules | “I have very long working hours.… It’s not possible for me to meet with a DOT nurse.… With video DOT, I could continue with my work and still take the medicine.” | |
| Confidentiality | sDOT can violate patient privacy | “When somebody has to come to your house driving that [DOH] car, coming in…the whole neighborhood’s going to look and start asking questions.” |
| vDOT is more private than sDOT | “With [vDOT], we can control [the] setting we are in.… It’s in your hand[s].… Just avoid taking videos in places where you can be viewed by others.… We have control.” | |
| Provider | ||
| Impact of DOT on staff | vDOT convenient for staff | “Especially for people who have to get up very early in the morning to go to work, [vDOT] saves us from having to...be at their house at 5:00 am.” |
| vDOT may threaten livelihood | “The only rumor that I’m hearing is that some of the DOT workers are thinking that [vDOT] is going to take their jobs.” | |
| Treatment effects of vDOT | vDOT able to shorten therapy | “For patients who aren’t [home] during our normal hours, video DOT...is much more effective.... They can dose anytime during the daytime as long as they have their phone available...and they’re still getting a counted dose.... We can actually count that dose towards their end goal as an observed dose, and their treatment is shortened by several days.” |
| vDOT allows for observed therapy 7x per week | “The ability to do 7 days a week [with vDOT], rather than 5, is really kind of uncharted territory.... We don’t actually know whether people are taking their medicines over the weekends, and a lot of programs don’t even prescribe weekend packs, which when you think about it is sort of odd.” | |
| vDOT on clinic operations | vDOT may increase clinic capacity | “I don’t have to spend 2 hours, 3 hours in the morning driving all over and around the county. It frees me up time-wise enormously. I can see more patients in my office.” |
| Decisions about DOT should be patient centered | Some with poor adherence on sDOT may actually do better on vDOT | “We [had a] patient that was highly nonadherent in standard DOT. She was missing 3 or 4 doses a week.... We were going to quarantine this individual, but [we decided to] attempt video DOT, and...for about a month or 2 [she] was nearly 100% adherent on a 7-day regimen of medicine on video DOT.” |
Abbreviations: DOT, directly observed therapy; sDOT, standard directly observed therapy (ie, in-person); vDOT, video directly observed therapy.
aOnly a subset of themes presented. For the full list, see Supplementary Tables 3 and 4.
Another prominent theme was the impression that in-person DOT threatened patient privacy. This concern appeared to be driven by the public optics of daily visits (at home/work) from DOH staff. In speaking to this concern, 1 patient stated, “Sometimes they meet me…at work…. I’m afraid I’ll be seen.” The added flexibility provided by vDOT seemed to allay these fears. As 1 nurse commented, “You can do [vDOT] in your car on the way to work. You can sit out in your driveway and do it…. It’s more private than having a nurse come to the house.” Notably, no patients or staff raised concerns regarding data security with the use of mobile phones to share private health information.
While an uncommon theme, 1 nurse manager discussed a fear of displacement among staff, stating, “Some DOT workers worry that video DOT will take their jobs.” At the same time, she went on to highlight the ability of vDOT to maximize clinic resources, noting, “[vDOT] actually helps because a lot of the time we’re short [staffed and] when you have this.… You don’t want your workers running around the streets all day.” Further, it was noted that DOT workers could take on larger patient panels and spend more individual time face to face with those remaining on in-person DOT. Additional comments and themes can be found in the Supplementary Data.
Cost Analysis
In our primary analysis (observation 5 days/week for a 6-month course), we projected that vDOT implementation would lead to an incremental cost-savings of $1391 per person compared with using in-person DOT (Table 3).
Cost for in-person DOT was driven largely by labor. In our primary analysis, labor costs totaled $1838, amounting to >90% of the overall DOT expenditure for a standard TB treatment course (Table 4). Labor costs varied markedly in sensitivity analysis based on health care worker type (eg, community health worker [CHW] vs registered nurse [RN]); overall, we estimated total in-person DOT costs at $866 to $5616.
Table 4.
Cost Analysis of vDOT Implementation
| DOT Strategy | Equipment | Consumables | Laborf | Total | Incremental | |
|---|---|---|---|---|---|---|
| DOT 5x per week | In-person DOT (range) | $175b ($0–$562) | $52d ($29–$648) | $1838 ($869–$4406) | $2065 ($898–$5616) | Ref |
| vDOT (range) | $48c ($4–$136) | $495e ($0–$900) | $131 ($62–$413) | $674 ($66–$1449) | –$1391 | |
| DOT 7x per week | In-person DOT (range) | $175b ($0–$562) | $72d ($40–$907) | $2573 ($1217–$6169) | $2820 ($1234–$7638) | Ref |
| vDOT (range) | $48c ($4–$136) | $495e ($0–$900) | $183 ($87–$578) | $726 ($91–$1614) | –$2094 |
Abbreviations: DOT, directly observed therapy; vDOT, video directly observed therapy.
aCost are per patient and calculated for a standard 6-month treatment course.
bBase case assumes a Health Department vehicle (economy class) used to treat 15 patients per year, annualized over the expected lifespan of the vehicle. In the sensitivity analysis, we varied the number of patients treated annually and calculated alternative pricing structures, including ones wherein health care workers utilized a personal vehicle and received mileage reimbursement.
cBase case assumes a program-provided smartphone and dedicated clinic computer. The sensitivity analysis incorporates the scenarios wherein a patient phone/data are used for vDOT (ie, no clinic cost incurred).
dMiles traveled was estimated from discussions with clinic managers, DOT workers, and through evaluation of monthly gas and mileage reimbursements logs. Range incorporates fluctuations in gas price and variability in the distance between patients.
eSoftware estimates were provided directly by emocha Mobile Health Inc., with the base case assuming a flat monthly rate of $50 per patient per month. The low-end estimate assumes free software and a patient-provided data plan, while the high-end accounts for variable data costs and a flat monthly software fee of $100 per patient. Commercial pricing may vary.
fBase case assumes an LPN conducting DOT activities. Time spent per patient was calculated as an average of that observed through time motion studies. The low range assumes a community health worker and the lowest possible estimates of time per patient. The high range assumes an RN (highest salary) and uses the highest possible estimate for time spent per patient. Note, labor cost is calculated based on the time required specifically for DOT activities.
For vDOT, we found that costs were driven by consumables, namely estimated software ($0–$100 per month) and data costs. In our base case, consumable costs totaled $495 ($0 to $900), comprising two-thirds of net treatment costs. Labor costs were low, totaling only $131 ($62–$413) and accounting for <20% of overall costs ($674). At the highest estimates of consumable costs ($900), driven by a monthly charge of $50 for data and $100 for software, vDOT was still associated with a cost-savings of roughly $1000 per treatment course, compared with in-person DOT.
DISCUSSION
In our pragmatic mixed-methods implementation of treatment monitoring strategies at 3 separate public health TB clinics in Maryland, we found broad patient and staff acceptability of vDOT, with similar adherence and an increased proportion of prescribed doses confirmed through observation. Our economic evaluation suggests potential cost-savings with vDOT, when compared with exclusive usage of in-person DOT. Our study is unique compared with prior evaluations of vDOT in its broad patient inclusion criteria, allowing for a real-world assessment and insights related to vDOT implementation. In-depth interviews with patients and staff revealed that TB programs considered vDOT a preferred option for patients in whom in-person DOT was logistically infeasible (eg, complex schedules or travel where the alternative was self-administration) or represented a barrier to care (eg, stigma). Program managers reported that associated time- and cost-savings allowed task-shifting with redistribution of limited clinic resources. Overall, our results suggest that vDOT is able to more effectively measure TB treatment adherence (including weekends and holidays), compared with in-person DOT, and can be successfully integrated into patient-centered, individualized case management plans that result in high rates of adherence and treatment success.
Our study has several important limitations. Given current TB case rates, our sample size was modest, and we were not powered to identify small changes in adherence. Nonetheless, we found improvements in the “observable fraction” of prescribed doses with vDOT, and our study is strengthened by in-depth qualitative and cost analyses that will help guide future larger-scale public health implementations. We did not assess for clinical end points, such as sputum conversion or disease relapse. While our study design allowed for within patient comparisons, these data must be interpreted with caution given the potential for time-varying confounders, such as medication adherence, which is known to decline as patients feel better and undergo treatment fatigue [32]. These factors could have reduced the observed vDOT adherence compared with in-person DOT, given vDOT initiation later in the treatment course. Lastly, our study sample was based on clinic (and patient) discretion and was not randomized; as such, our conclusions may not apply to all patients indiscriminately. Nonetheless, we included a range of TB patients, from the latently infected to those with extrapulmonary disease, and did not exclude patients based on prior adherence. Furthermore, it is important for TB programs to consider that while observation of pill ingestion may facilitate measurement of adherence, it is not the sole determinant of one’s adherence; reported adherence and treatment outcomes may therefore differ according to how DOT services are integrated into broader case management strategies. At our study sites, all patients continued to receive dedicated case management and other adherence support interventions per routine, irrespective of DOT modality (particularly after missed doses or reported side effects). As such, our quantitative and qualitative results provide support for the promotion of individualized case management plans and argue against a “one size fits all” strategy for providing treatment support and treatment monitoring.
Overall, our study provides needed insights on key aspects of vDOT usage related to patient selection, implementation, effectiveness, and costs. We found that many patients were ultimately enrolled because of social factors thought to preclude, or at least impact, the ability to conduct in-person DOT. For example, several patients were able to have treatment observation and adherence measurements using vDOT while traveling outside of the United States. Such examples have practical implications. In most public health TB programs, prior to vDOT implementation, such doses (taken under self-administration) would not have “counted” toward treatment progress (ie, would need to be made up), ultimately prolonging treatment.
Beyond facilitating early recognition of poor adherence or side effects, DOT also has other critical roles in promoting successful TB control. In the absence of a biological marker for disease cure, TB programs base treatment completion on a prespecified number of treatment doses [4]. When applied consistently, DOT therefore serves as a key method to measure adherence and represents a mechanism to track treatment progress. In this regard, our study highlights an important consideration in adherence measurement and dosing frequencies. Current treatment guidelines have placed increasing emphasis on daily (7 days/week) therapy, though they still accept a 5-day/week alternative “daily” schedule (for drug-sensitive disease), acknowledging that “there are no studies that compare 5 to 7 daily doses” [4]. Given logistical constraints, many TB programs in the United States utilize a hybrid treatment schedule, wherein a regimen of 5 days of DOT (M–F) is coupled with self-administered weekend doses; some programs omit weekend doses altogether. Self-administered weekend (or holiday) doses are generally not applied to the overall treatment dose count or adherence calculations (ie, they are not “expected” and are not “made up” if missed). In effect, with current practices, “in-person DOT” is only able to measure 5 of 7 (71%) prescribed weekly doses.
We therefore a priori chose to report a related metric, the observable fraction, to quantify the true percentage of prescribed doses, inclusive of weekend self-administration, that could be measured through observation (in-person or video). Prior to the study, we assumed that clinics would move away from intermittent dosing regimens, in favor of 7-day/week therapy upon transition to vDOT. Ultimately, we did see a significant 8% increase in the observable fraction upon transition to vDOT; however, the absolute fraction was only 76% (vs 68% with in-person DOT). This result stemmed from the fact that only 2 participants had their monitoring frequencies increased to 7 days/week on vDOT, likely a result of entrenched provider practices. For example, some clinics explicitly instructed patients not to submit weekend videos, while others actively rejected any such submissions. Our study demonstrates the need to adapt clinic workflows to this new monitoring approach, as vDOT ultimately enables the expansion of treatment monitoring to 7 days/week and eliminates the need for self-administered doses. This increase in the number of observable doses is likely to reduce overall treatment duration by eliminating the need to make up extra doses related to self-administered or unobserved doses (under the assumption that programs only count observed doses toward treatment progress).
Finally, our study provided the first in-depth cost analysis of asynchronous vDOT. We found marked heterogeneity across health departments, both in terms of staffing and the operational implementation of in-person DOT. Despite this diversity, we estimated vDOT to save programs at least $1000 per patient if implemented for a standard 6-month treatment course (vs 5 days per week in-person DOT). When considering TB clinic costs and staffing overall, it is important to acknowledge that DOT represents 1 of several TB treatment– and case management–related activities. During our in-depth interviews, a single CHW expressed concerns about being displaced by this new technology; such considerations need acknowledgment during implementation. However, several staff members also presented alternative perspectives noting that vDOT allowed for increased time and attention to be directed toward other required activities (eg, contact investigations, patient counseling, and social support). In an era of increasing responsibilities and limited funds, maximizing staff potential is often a necessity.
Overall, our study contributes to the growing literature on usage of alternative modalities for TB treatment monitoring and expands on prior efforts by demonstrating the feasibility, acceptability, and cost-savings in a previously unstudied environment and among a broader patient population [20–23]. By using a rigorous mixed-methods implementation science approach, our results identified and highlighted several important considerations related to patient selection, treatment frequency, and measurement of adherence that will guide policy makers and TB programs considering vDOT implementation. Importantly, our findings suggest the need for flexible, individualized case management plans that consider patient needs while achieving public health goals.
Supplementary Material
Acknowledgments
We would like to thank those who provided clinical care at each of our 3 study sites for their hard work. We would also like the thank the leadership and development team at emocha Mobile Health Inc., including Sebastian Seiguer, JD, MBA, Katrina Rios, and Gorkem Sevic, MSE.
Author contributions. Study concept and design: M.S. Acquisition of data: S.B.H. Statistical analysis: S.B.H. Qualitative coding: S.B.H, A.Z. Data interpretation: S.B.H, M.S. Drafting of initial manuscript: S.B.H. Clinical revision of manuscript: All authors.
Financial support. This work was supported by the Small Business Innovation Research (SBIR) program at the National Institutes of Health (NIH) awarded to emocha Mobile Health Inc. (grant number R43 MD010521). Additional National Institutes of Health support was provided through a Postdoctoral Training Grant (grant number T32 AI007291-25 to S.B.H.).
Conflicts of interest. Dr. Shah is one of the inventors of the miDOT technology. Under a license agreement between emocha Mobile Health Inc. and the Johns Hopkins University, Dr. Shah and the university are entitled to royalties on the technology described in this article. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. To mitigate any potential conflicts of interest, all clinical decision-making regarding the use of miDOT or enrollment in the study was made by nonconflicted Department of Health clinicians and staff; M.S. recused himself from all data analysis but assisted with interpretation of results.
References
- 1. World Health Organization. Fact sheet 2017. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed April 2017.
- 2. Centers for Disease Control. Fact sheet 2016. Available at: https://www.cdc.gov/tb/publications/factsheets/statistics/tbtrends.htm. Accessed January 2018.
- 3. Dieleman JL, Templin T, Sadat N, et al. . National spending on health by source for 184 countries between 2013 and 2040. Lancet 2016; 387:2521–35. [DOI] [PubMed] [Google Scholar]
- 4. Nahid P, Dorman SE, Alipanah N, et al. . Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO Treatment Guidelines for Drug-Resistant Tuberculosis: 2016 Update. Geneva, Switzerland: WHO Press; 2016. [Google Scholar]
- 6. Burman WJ, Cohn DL, Rietmeijer CA, et al. . Noncompliance with directly observed therapy for tuberculosis. Epidemiology and effect on the outcome of treatment. Chest 1997; 111:1168–73. [DOI] [PubMed] [Google Scholar]
- 7. Picon PD, Bassanesi SL, Caramori ML, et al. . Risk factors for recurrence of tuberculosis. J Bras Pneumol 2007; 33:572–8. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization. Treatment of Tuberculosis: Guidelines. 4th ed Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 9. Hopewell PC, Pai M, Maher D, et al. . International standards for tuberculosis care. Lancet Infect Dis 2006; 6:710–25. [DOI] [PubMed] [Google Scholar]
- 10. Hodge JH, Anderson E, Gayle N, Larson L. Tuberculosis Control Laws and Policies: A Handbook for Public Health and Legal Practitioners Atlanta, GA: The Centers for Disease Control and Prevention; 2009. Available at: https://www.cdc.gov/tb/programs/tblawpolicyhandbook.pdf. Accessed 10 October 2017. [Google Scholar]
- 11. Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev 2015:CD003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasipanodya JG, Gumbo T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis 2013; 57:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Ehiri J, Yang H, et al. . Impact of community-based DOT on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS One 2016; 11:e0147744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. Guidlines for Treatment of Drug-Suseptible Tuberculosis and Patient Care: 2017 Update. Geneva, Switzerland; World Health Organization; 2017. [Google Scholar]
- 15. Sagbakken M, Bjune GA, Frich JC. Humiliation or care? A qualitative study of patients’ and health professionals’ experiences with tuberculosis treatment in Norway. Scand J Caring Sci 2012; 26:313–23. [DOI] [PubMed] [Google Scholar]
- 16. Noyes J, Popay J. Directly observed therapy and tuberculosis: how can a systematic review of qualitative research contribute to improving services? A qualitative meta-synthesis. J Adv Nurs 2007; 57:227–43. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Digital Health for the End TB Strategy: An Agenda for Action. Geneva, Switzerland: WHO Press; 2015. [Google Scholar]
- 18. Implementing an Electronic Directly Observed Therapy (eDOT) Program: A Toolkit for Tuberculosis (TB) Programs. Centers for Disease Control and Prevention; 2017. Available at: https://www.cdc.gov/tb/publications/pdf/tbedottoolkit.pdf. Accessed 16 September 2017. [Google Scholar]
- 19. Hemming S, Story A, Possas L, et al. . Using virtually observed treatment (VOT) for hard to manage tuberculosis: a pilot study. Paper presented at: European Respiratory Society Annual CongressBarcelona, Spain: 7–11 September 2013. [Google Scholar]
- 20. Mirsaeidi M, Farshidpour M, Banks-Tripp D, et al. . Video directly observed therapy for treatment of tuberculosis is patient-oriented and cost-effective. Eur Respir J 2015; 46:871–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chuck C, Robinson E, Macaraig M, et al. . Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int J Tuberc Lung Dis 2016; 20:588–93. [DOI] [PubMed] [Google Scholar]
- 22. Garfein RS, Collins K, Muñoz F, et al. . Feasibility of tuberculosis treatment monitoring by video directly observed therapy: a binational pilot study. Int J Tuberc Lung Dis 2015; 19:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffman JA, Cunningham JR, Suleh AJ, et al. . Mobile direct observation treatment for tuberculosis patients: a technical feasibility pilot using mobile phones in Nairobi, Kenya. Am J Prev Med 2010; 39:78–80. [DOI] [PubMed] [Google Scholar]
- 24. DeMaio J, Schwartz L, Cooley P, Tice A. The application of telemedicine technology to a directly observed therapy program for tuberculosis: a pilot project. Clin Infect Dis 2001; 33:2082–4. [DOI] [PubMed] [Google Scholar]
- 25. Wade VA, Karnon J, Eliott JA, Hiller JE. Home videophones improve direct observation in tuberculosis treatment: a mixed methods evaluation. PLoS One 2012; 7:e50155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Au-Yeung KY, DiCarlo L. Cost comparison of wirelessly vs. directly observed therapy for adherence confirmation in anti-tuberculosis treatment. Int J Tuberc Lung Dis 2012; 16:1498–504. [DOI] [PubMed] [Google Scholar]
- 27. Krueger K, Ruby D, Cooley P, et al. . Videophone utilization as an alternative to directly observed therapy for tuberculosis. Int J Tuberc Lung Dis 2010; 14:779–81. [PubMed] [Google Scholar]
- 28. Maryland Department of Health. Maryland TB guidelines for prevention and treatment of tuberculosis 2007. Available at: http://ideha.dhmh.maryland.gov/OIDPCS/CTBCP/CTBCPDocuments/tbguidelines.pdf. Accessed May 2012.
- 29. Strauss A, Corbin J.. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks, CA: Sage Publications, Inc; 1998. [Google Scholar]
- 30. Sama JN, Chida N, Polan RM, et al. . High proportion of extrapulmonary tuberculosis in a low prevalence setting: a retrospective cohort study. Public Health 2016; 138:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peto HM, Pratt RH, Harrington TA, et al. . Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis 2009; 49:1350–7. [DOI] [PubMed] [Google Scholar]
- 32. Reyes H, Guiscafré H, Muñoz O, et al. . Antibiotic noncompliance and waste in upper respiratory infections and acute diarrhea. J Clin Epidemiol 1997; 50:1297–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.