Murley and Rowe review the neurochemical changes arising from frontotemporal lobar degeneration, including the syndromes frontotemporal dementia, progressive supranuclear palsy and corticobasal degeneration. The evidence base from in vivo and post-mortem human studies, and preclinical models, suggests new strategies to facilitate the development of symptomatic pharmacological treatments, in stratified populations.
Keywords: frontotemporal dementia, progressive supranuclear palsy, corticobasal degeneration, neurotransmitters, dementia
Abstract
Frontotemporal lobar degeneration causes a spectrum of complex degenerative disorders including frontotemporal dementia, progressive supranuclear palsy and corticobasal syndrome, each of which is associated with changes in the principal neurotransmitter systems. We review the evidence for these neurochemical changes and propose that they contribute to symptomatology of frontotemporal lobar degeneration, over and above neuronal loss and atrophy. Despite the development of disease-modifying therapies, aiming to slow neuropathological progression, it remains important to advance symptomatic treatments to reduce the disease burden and improve patients’ and carers’ quality of life. We propose that targeting the selective deficiencies in neurotransmitter systems, including dopamine, noradrenaline, serotonin, acetylcholine, glutamate and gamma-aminobutyric acid is an important strategy towards this goal. We summarize the current evidence-base for pharmacological treatments and suggest strategies to improve the development of new, effective pharmacological treatments.
Introduction
Frontotemporal lobar degeneration (FTLD) causes diverse clinical syndromes, including frontotemporal dementia (FTD), progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) (MacKenzie et al., 2010; Riedl et al., 2014). In recent years there has been marked progress in defining these syndromes in terms of their clinical diagnostic criteria (Gorno-Tempini et al., 2011; Rascovsky et al., 2011; Armstrong et al., 2013; Höglinger et al., 2017), genetic association (Seelaar et al., 2011; Baizabal-Carvallo and Jankovic, 2016), pathology (MacKenzie et al., 2010), and clinical and imaging biomarkers (Whitwell et al., 2005; Hughes et al., 2013; Skillback et al., 2014; Rohrer et al., 2015b; Ranasinghe et al., 2016). These advances have supported the development of candidate disease-modifying therapeutics (Boxer and Boeve, 2007; Tsai and Boxer, 2014; Stamelou and Höglinger, 2016). However, treatments that slow or halt disease progression after symptoms begin must be accompanied by more effective treatment of symptoms to reduce the overall burden of disease. One strategy is to reverse neurotransmitter deficits, similar to dopaminergic therapy of Parkinson’s disease or cholinergic therapy for Alzheimer’s disease. Novel symptomatic drug treatment would improve patients’ and their families’ quality of life.
Recent changes in the clinical and pathological characterization of the major clinical syndromes caused by FTLD give anatomical and pharmacological insights that call for a reappraisal of the neurotransmitter literature. We adopt the clinical labels as set out in current consensus diagnostic criteria for the behavioural variant FTD (bvFTD) (Rascovsky et al., 2011), semantic variant of primary progressive aphasia (svPPA) (Gorno-Tempini et al., 2011), logopenic variant of PPA (lvPPA) (Gorno-Tempini et al., 2011), non-fluent agrammatic variant PPA (nfvPPA) (Gorno-Tempini et al., 2011), CBS (Armstrong et al., 2013) and PSP (Höglinger et al., 2017). However, older studies may have used different terms or overlooked the evolution of phenotype that obscures the boundaries between groups as the disease progresses (Coyle-Gilchrist et al., 2016). Where these changes are relevant to the interpretation of neurotransmitter effects, we make variations from the current standard classification explicit, but otherwise consider semantic dementia as semantic variant PPA and progressive non-fluent aphasia as non-fluent agrammatic variant PPA.
Here we review the pharmacological abnormalities associated with FTLD in terms of regional changes in neurotransmitter synthesis, release, reuptake, catabolism, and synaptic binding. We focus on the major neurotransmitter systems, dopamine, noradrenaline, serotonin, acetylcholine, glutamate and gamma aminobutyric acid (GABA) both individually (including their receptor subtypes) and the interactions between them. Table 1 provides a summary of the available evidence, with full information on references by disease and by neurotransmitter in Supplementary Table 1.
Table 1.
Summary of neurotransmitter deficits in FTLD
| Neurotransmitter pathway | FTD | PSP | CBS |
|---|---|---|---|
| Dopamine | |||
| Dopaminergic neurons | ↓↓ | ↓↓ | ↓↓ |
| Dopamine receptors | ↓ | ↓↓a | ↔ |
| Noradrenaline | |||
| Noradrenergic neurons | ↔ | ↓↓ | na |
| Noradrenergic receptors | na | na | na |
| Serotonin | |||
| Serotonergic neurons | ↓↓ | ↓ | ↓ |
| Serotonergic receptors | ↓↓ | ↑ | na |
| Acetylcholine | |||
| Cholinergic neurons | ↔b | ↓↓ | ↓↓ |
| Cholinergic receptors | ↔/↓ | ↔/↓ | na |
| Glutamate | |||
| Glutamatergic neurons | ↓↓ | ↓↓ | na |
| Glutamatergic receptors | ↓↓ | ↔ | na |
| GABA | na | ||
| GABAergic neurons | ↓ | ↓↓ | na |
| GABA receptors | na | ↓ | na |
A more detailed table, including references, is included as Supplementary Fig. 1.
↓↓ = moderate/severe deficit; ↓ = mild deficit; ↑/↔/↓ = conflicting or inconsistent results; ↔ = no significant change; ↑ = mild increase; na = no available evidence.
aIn PSP D2 receptors are reduced in the striatum and basal ganglia but D1 receptors appear to be preserved.
bCholinergic neurons are reduced in the nucleus basalis but are preserved in the cerebral cortex in bvFTD. In nfvPPA there is greater evidence of a cholinergic deficit with atrophy of basal forebrain cholinergic nuclei.
Dopamine
Dopaminergic deficits are widely associated with Parkinson’s disease but are also a common feature of FTLD. The majority of dopaminergic neurons originate in the ventral midbrain and form nigrostriatal, mesolimbic and mesocortical projections (Fig. 1A). Nigrostriatal neurons from the substantia nigra pars compacta terminate in the striatum, regulating cortico-striato-thalamo-cortical loops for motor, oculomotor and cognitive control (Rowe and Rittman, 2016). The motor circuit regulates movement, both in facilitating (via the direct pathway) and inhibiting (via the indirect pathway) actions. Loss of dopaminergic neurons in the nigrostriatal pathway causes parkinsonism in Parkinson’s disease, but also in FTLD. Additional mesolimbic and mesocortical dopaminergic neurons from the ventral tegmental area regulate reward, learning and motivation-related behaviour (Wise, 2004). The mesolimbic tract projects principally to the nucleus accumbens in the striatum and to the amygdala and hippocampus, affecting motivation, hedonia and reward (incentive salience). Changes to the mesolimbic tract may also exacerbate compulsion and impulsivity. The mesocortical tract (which projects to the prefrontal, cingulate and perirhinal cortices) regulates motivation, emotion, reward and desire, including learning of the value of goal-directed actions. Dopamine binds to five types of G protein coupled receptors; D1-class (D1 and D5) and D2-class (D2, D3 and D4), which differ in their response to dopamine agonists and antagonists (Beaulieu and Gainetdinov, 2011; Southan et al., 2016). The different receptor subtypes have distinct distribution densities across brain regions and are associated with different, although overlapping, effects on cognition and movement (Beaulieu and Gainetdinov, 2011), and may be differentially affected by FTLD.
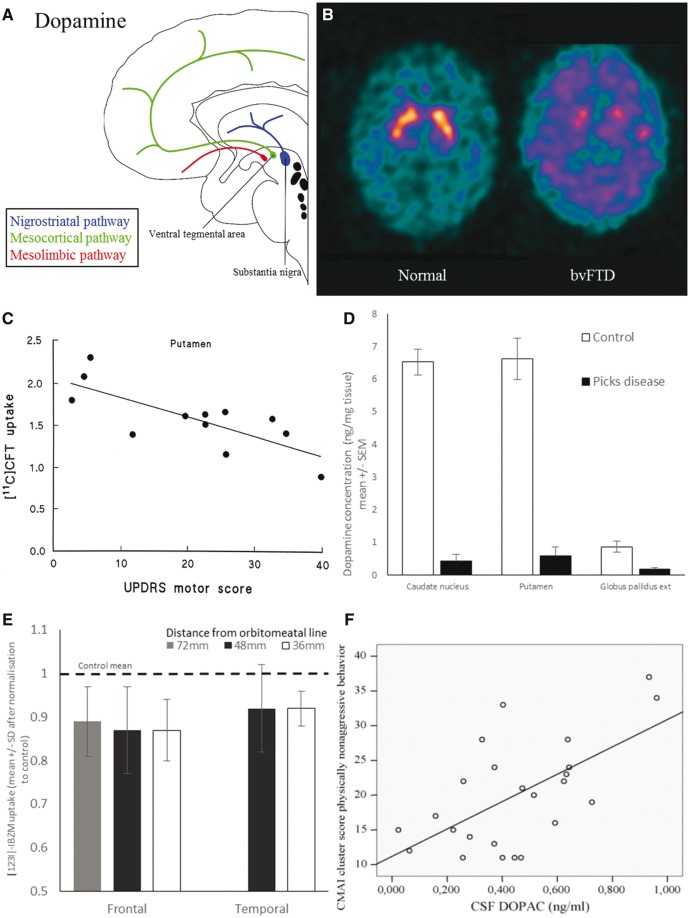
Figure 1.
Dopamine deficits in FTD. (A) Schematic illustration of dopaminergic pathways. (B) Ioflupane SPECT scan showing loss of pre-synaptic dopaminergic neurons in the striatum of FTD compared with normal scan. (C) Loss of dopaminergic neurons in the putamen (measured by 11C-CFT-PET) correlates with severity of extra-pyramidal motor symptoms (Unified Parkinson’s Disease Rating Scale motor score). From Rinne et al. (2002). Reprinted with permission from Wolter Kluwer. (D) Dopamine levels are reduced in the caudate, putamen and globus pallidus. Graph of data from Kanazawa et al. (1988). Reprinted with permission from Elsevier. (E) There is loss of D2 dopamine receptors in the frontal lobes (as measured by 123I-IBZM-PET). Graph of data from Frisoni et al. (1994). Reprinted with permission from Elsevier. (F) CSF DOPAC levels (3,4-dihydroxyphenylacetic acid, a dopamine metabolite) correlate with behavioural disturbance. From Engelborghs et al. (2008). Reprinted with permission from Elsevier.
Frontotemporal dementia
There is clinical and experimental evidence of a nigrostriatal deficit in many cases of FTD, with loss of pre-synaptic dopaminergic neurons, reduced dopamine levels, reduced dopamine transporter binding, and abnormal dopamine receptor binding. Extrapyramidal symptoms of bradykinesia, rigidity and gait dysfunction are seen in up to 70% of patients at some stage during the disease course (Rinne et al., 2002; Padovani et al., 2007; Kertesz et al., 2011; Gil-Navarro et al., 2013). In vivo imaging reveals that dopamine transporter levels (a marker of presynaptic neuron integrity in the striatum) are reduced in the caudate and putamen (Fig. 1B) (Rinne et al., 2002; Sedaghat et al., 2007). The degree of this loss correlates with extra-pyramidal symptom severity (Fig. 1C) (Rinne et al., 2002; Sedaghat et al., 2007).
In bvFTD there are low levels of dopamine, measured by high performance liquid chromatography, in the putamen, caudate and substantia nigra (Kanazawa et al., 1988; Nagaoka et al., 1995) (Fig. 1D). Parkinsonism is commonly seen in bvFTD, especially when caused by certain genetic mutations (Baizabal-Carvallo and Jankovic, 2016). Mutations on chromosome 17, including in the MAPT (Hutton et al., 1998) and PGRN (Baker et al., 2006) genes, are associated with rigidity, akinesia and neuronal loss in the substantia nigra, although symptom onset and severity vary with each specific mutation (Foster et al., 1997; Pickering-Brown et al., 2002; Le Ber et al., 2008; Siuda et al., 2014; Baizabal-Carvallo and Jankovic, 2016). For example, an early PET study in three patients with FTD associated with a chromosome 17 mutation found severe reduction in presynaptic dopaminergic neurons with normal D2 receptor levels in the striatum (Pal et al., 2001). The hexanucleatide expansion in the C9orf72 gene on chromosome 9 is most typically associated with FTD with amyotrophic lateral sclerosis (Rohrer et al., 2015a), but up to half of patients have parkinsonism, with decreased dopamine transporter levels in the basal ganglia (Boeve et al., 2012; O’Dowd et al., 2012). Extra-pyramidal symptoms are also seen with mutations in CHMP2B, FUS, TARDBP, TREM2 and VCP (Siuda et al., 2014; Baizabal-Carvallo and Jankovic, 2016). In non-fluent agrammatic variant PPA, there is frequent loss of dopaminergic neurons in the striatum (Gil-Navarro et al., 2013), which underlies the frequent progression of motor symptoms in this disorder, and its clinical overlap with CBS and PSP (Rohrer et al., 2010). Parkinsonism in bvFTD and non-fluent agrammatic variant PPA appears to occur with all types of underlying pathology; tau (Hutton et al., 1998), TDP-43 (Boeve et al., 2012) and FUS pathology (Deng et al., 2014) are all associated with motor symptoms (Baizabal-Carvallo and Jankovic, 2016).
In addition to extrapyramidal motor features, degeneration of dopaminergic tracts, especially the mesocortical pathway, could contribute to behavioural symptoms of FTD. For example, D2 dopamine receptors are reduced in the frontal lobes of patients with FTD (Frisoni et al., 1994) (Fig. 1E), while CSF levels of dopamine and its metabolites are reduced in some (Sjogren et al., 1998) but not all studies (Vermeiren et al., 2013). CSF levels of dopamine correlate with agitation and caregiver burden in FTD (Fig. 1F) (Engelborghs et al., 2008). However, these findings contrast with a study that found higher dopamine levels in the prefrontal cortex at post-mortem (Vermeiren et al., 2016). Such inconsistencies may result from technological or methodological differences in tissue preparation or analysis, but they may also reflect true heterogeneity in the FTD population, especially in small post-mortem analyses.
Aggression, agitation and psychosis are distressing and burdensome aspects of FTD. Antipsychotic medications with dopaminergic receptor affinity are often used to treat them but patients can be extremely sensitive to the extra-pyramidal side effects due to pretreatment nigrostriatal deficits (Pijnenburg et al., 2003). Atypical antipsychotics such as quetiapine, olanzapine or clozapine cause fewer extrapyramidal side effects (Moretti et al., 2003b) while noting that there is less evidence for their efficacy in dementia. In an open label, non-randomized study, olanzapine improved behavioural fluctuations, wandering and irritability (Moretti et al., 2003b). An alternative strategy using methylphenidate, a noradrenaline and dopamine reuptake inhibitor, reduced risk-taking behaviour in a small double-blind, placebo-controlled study, but without effects on a wide range of cognitive tasks (Rahman et al., 2006). There is a case report of improved behaviour with methylphenidate and bupropion (another noradrenaline and dopamine reuptake inhibitor) in one patient with FTD (Goforth et al., 2004). In addition to the uncertainty over dopaminergic strategies to treat cognitive and behavioural symptoms in FTD, systematic evidence is lacking of the efficacy of levodopa or dopamine agonists to ameliorate parkinsonism in FTD, with only case reports of benefit in some patients (Chow, 2002; Tsai and Boxer, 2014).
Progressive supranuclear palsy
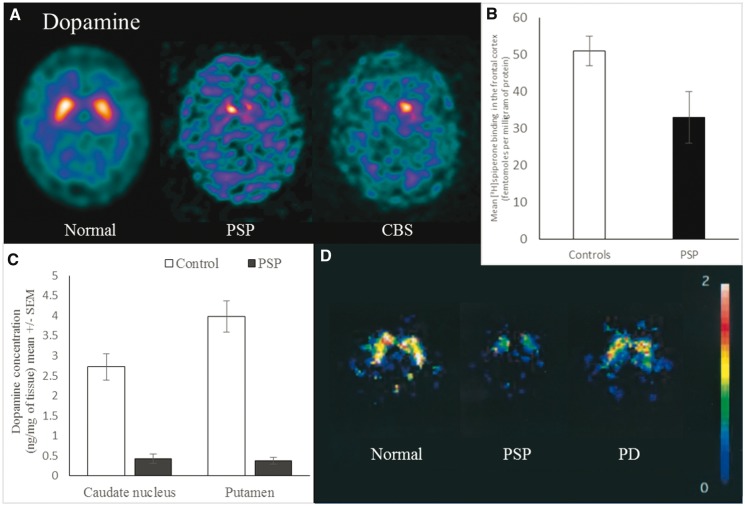
In vivo and post-mortem studies show that the extrapyramidal features of PSP are associated with a severe loss of dopaminergic neurons and changes in dopamine receptors, particularly D2 receptors. Pathological tau aggregates, including neuronal tangles and glial inclusions, develop in areas with a high density of dopaminergic neurons including the substantia nigra and striatum (Litvan et al., 1996; Hardman et al., 1997). There is marked loss of pigmented dopaminergic neurons in the substantia nigra pars compacta on examination post-mortem (Hardman et al., 1997; Oyanagi et al., 2001). There is also loss of both dopaminergic neurons and dopamine receptors in the striatum (Baron et al., 1986; Kim et al., 2002; Oyanagi, 2002; Im et al., 2006; Oh et al., 2012). Dopamine transporter binding is reduced in the caudate, putamen and globus pallidus at post-mortem (Warren et al., 2007b) and in vivo (Fig. 2A) (Seppi et al., 2006). Dopamine levels are reduced in the putamen, caudate nucleus, substantia nigra and globus pallidus at post-mortem (Fig. 2C) (Ruberg et al., 1985; Hornykiewicz and Shannak, 1994). In vivo PET and single photon emission computed tomography (SPECT) studies indicate reduced levels of D2 receptors in the basal ganglia (Fig. 2D) (Brooks et al., 1992; Arnold et al., 2002; Oyanagi, 2002) while post-mortem studies show corresponding loss of D2 receptors in the putamen, caudate and substantia innominata (Ruberg et al., 1985; Pierot et al., 1988; Pascual et al., 1992; Landwehrmeyer and Palacios, 1994). One study reported higher D2 receptor binding in the striatum compared with controls (Warren et al., 2007a), which might represent receptor upregulation in response to loss of presynaptic dopaminergic neurons. In contrast D1 receptors appear relatively well preserved (Pierot et al., 1988). There is also evidence that the mesocortical pathway is impaired in PSP, with degeneration of dopaminergic neurons in the ventral tegmental area (Murphy et al., 2008) and loss of dopamine receptors in the frontal cortex, measured post-mortem with 3H-spiperone (Fig. 2B) (Ruberg et al., 1985). This is especially relevant to the often profound change in motivation and apathy in PSP.
Figure 2.
Dopamine deficits in PSP and CBS. (A) Ioflupane SPECT scan showing reduced pre-synaptic dopaminergic neurons in the striatum of PSP and CBS compared to a normal scan. (B) Post-mortem dopamine receptor levels (measured by spiperone binding) are reduced in the frontal cortex in PSP. Graph of data from Ruberg et al. (1985). Reprinted with permission from Wiley. (C) Dopamine levels are reduced in the caudate nucleus and putamen in PSP. Graph of data from Ruberg et al. (1985). (D) D2 dopamine receptor levels (measured by 123I-iodobenzofuran SPECT) are reduced in the striatum of PSP when compared with healthy controls and Parkinson’s disease. From Oyanagi (2002). Reprinted with permission from Wiley.
In contrast to Parkinson’s disease, motor symptoms in typical clinical presentations of PSP (increasingly known as progressive supranuclear palsy-Richardson’s syndrome, or PSP-RS, to distinguish it from other phenotypes of PSP pathology) (Höglinger et al., 2017) typically do not respond well to dopaminergic therapy. This may be because in PSP there is loss of both dopaminergic neurons and receptors in the basal ganglia and cerebral cortex. This contrasts with Parkinson’s disease, in which predominant loss of presynaptic nigrostriatal dopaminergic neurons is greater than the relative preservation, or even upregulation, of post-synaptic dopamine receptor densities (Olanow, 2004).
Corticobasal syndrome
CBS is caused by corticobasal degeneration (CBD) pathology in about 60% of cases, the remainder being due to PSP, FTD, Alzheimer’s disease and other pathology (Boeve et al., 1999; Alexander et al., 2014). Patients with CBD have pathological neuroglial tau deposits, severe neuronal loss and gliosis in the substantia nigra and striatum, typically with a history of extrapyramidal signs (Oyanagi et al., 2001; Armstrong et al., 2013; Coyle-Gilchrist et al., 2016). Despite this, the in vivo imaging evidence of dopaminergic deficits is inconsistent. Fluorodopa PET indicates presynaptic dopaminergic reductions in the caudate, putamen and frontal cortex (Sawle et al., 1991; Nagasawa et al., 1996; Laureys et al., 1999; Klaffke et al., 2006; Pirker et al., 2015), but with wide variation and surprisingly no correlation with disease duration or severity (Cilia et al., 2011). Indeed some patients with autopsy-confirmed CBD have had a normal dopamine transporter SPECT scan despite prominent parkinsonian features (Chaal and Rowe, 2013; Kaasinen et al., 2013), and D2 receptor levels can be unchanged (Klaffke et al., 2006; Pirker et al., 2013). These conflicting results may partly reflect the poor clinicopathological correlation of CBS with CBD (Boeve et al., 1999; Cilia et al., 2011; Alexander et al., 2014). This is arguably a greater problem in the older literature, which often used CBD when referring to CBS, and therefore may include a high proportion of Alzheimer’s disease in their cases. We suggest that future studies of CBS need corollary pathological or biomarker evidence to distinguish CBD and non-CBD causes of CBS. The current evidence suggests a complex and inconsistent relationship between nigrostriatal dopamine deficiency and symptoms in patients with CBS, but evidence is scarce in comparison to other disorders.
Noradrenaline
The locus coeruleus in the pons is the principle site of noradrenaline synthesis in the brain and contains the soma of noradrenergic neurons that project to the forebrain (Fig. 3A). Different subpopulations of neurons within the locus coeruleus project to the orbitofrontal, medial prefrontal, anterior cingulate and motor cortices (Chandler et al., 2014). These noradrenergic pathways have an important role in regulating the function of the prefrontal cortex (McGaughy et al., 2008; Chandler et al., 2014), while in contrast to dopamine, there is minimal noradrenergic innervation of the striatum. Noradrenaline acts via α and β G protein coupled receptor families, each of which comprise subtypes that have different responses to ligand binding (Sara, 2009). The effect of noradrenaline depends on the relative densities of these receptors (Aston-Jones and Cohen, 2005). For example, noradrenergic input to the basal forebrain can promote arousal by activating cholinergic neurons through α1 and β1 receptors and inhibiting GABAergic neurons through α2 receptors (Schwarz and Luo, 2015), while presynaptic auto-inhibitory α2 receptors may paradoxically enhance noradrenergic transmission in response to antagonists (Invernizzi and Garattini, 2004). Noradrenaline is involved in regulating a range of behaviours including wakefulness, attention, memory and decision-making (Rowe et al., 1996; Sara, 2009; Dalley et al., 2011; Aston-Jones and Waterhouse, 2016). In comparative models, for example rats, limiting noradrenergic transmission results in impaired executive function (Newman et al., 2008; Chandler et al., 2014) and increasing noradrenaline levels reduces impulsivity (Robinson et al., 2008). Computational and neurophysiological models suggest noradrenergic pathways mediate salience and shift in attention (Aston-Jones and Cohen, 2005).
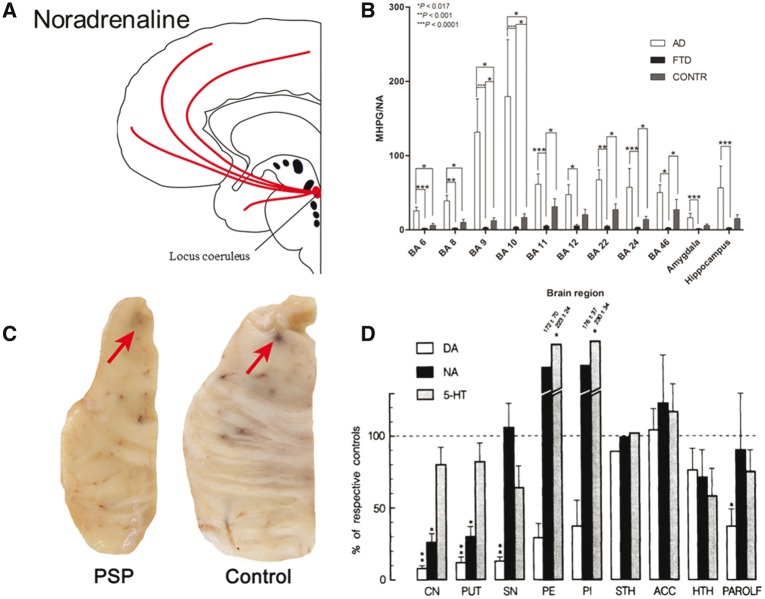
Figure 3.
Noradrenergic deficits in FTD and PSP. (A) Schematic illustration of noradrenergic pathways. (B) MHPG/noradrenaline ratios, indicative of catabolic noradrenergic turnover, are reduced in Brodmann areas 11, 22, 24 and 46 in FTD. From Vermeiren et al. (2016). Reprinted with permission from the authors and IOS Press. The publication is available at IOS Press through http://dx.doi.org/10.3233/JAD-160320. (C) Post-mortem brainstem tissue from control and PSP brains. There is a paler locus coeruleus suggesting loss of melatonin-containing noradrenergic neurons. Courtesy of Kieran Allison, Cambridge Brain Bank. (D) Noradrenaline levels are reduced in the caudate (CN), putamen (PUT), hippocampus (HTH) and parolfactory cortex (PAROLF). Serotonin levels are reduced in those areas as well as in the subthalamic nucleus (SN). Dopamine levels are reduced in those areas as well as the globus pallidus externa (GPe) and interna (GPi). From Hornykiewicz and Shannak (1994). Reprinted with permission from Springer.
Frontotemporal dementia
There is limited evidence for noradrenergic changes in FTD but in many respects, the noradrenergic pathways appear to be normal or near normal, relative to the marked deficits seen in other neurotransmitter pathways. For example, neuropathological studies of FTD suggest the preservation of cell density in the locus coeruleus, and noradrenaline levels are normal or even elevated in the frontal lobe (Vermeiren et al., 2016), despite the presence of pathological tau inclusions (Nagaoka et al., 1995; Yang and Schmitt, 2001; Brunnström et al., 2011; Irwin et al., 2016). However, there may be reduced noradrenaline catabolism and turnover. For example, one study found low 3-methoxy-4-hydroxyphenylglycol (MHPG) to noradrenaline ratios, a proposed marker of noradrenergic turnover, in the frontal and temporal lobes, anterior cingulate, amygdala and hippocampus (Fig. 3B) (Vermeiren et al., 2016). In contrast, several studies show normal levels of noradrenaline and MHPG in CSF (Sjogren et al., 1998; Engelborghs et al., 2008; Vermeiren et al., 2013). However, in one of these studies there was a correlation between CSF levels of noradrenaline and disease severity, even though overall levels were unchanged (Engelborghs et al., 2008). The enzyme monoamine oxidase, which metabolizes noradrenaline, is reduced in some areas of the brain (including the temporal lobe) although levels are unchanged in the frontal lobe (Sparks et al., 1991). This anatomical heterogeneity may be one reason for the inconsistent reports of MHPG/noradrenaline levels. However, an alternative explanation is that the locus coeruleus receives inhibitory serotoninergic innervation from the upper raphe nuclei (Yang and Schmitt, 2001) such that the major loss of serotonergic projections in FTD (see below) serves indirectly to increase noradrenaline signalling to the frontal lobe.
Idazoxan is an α2 adrenoceptor antagonist that increases synaptic noradrenaline levels by antagonism of inhibitory autoreceptors on noradrenergic neurons. Idazoxan improved attention, planning and problem-solving in a small group of patients with FTD (Sahakian et al., 1994; Coull et al., 1996). Looking ahead to candidate symptomatic therapies, selective noradrenergic reuptake inhibitors such as atomoxetine and reboxetine, or combined serotonin and noradrenaline reuptake inhibitors like venlafaxine and duloxetine, may provide better tolerated augmentation of noradrenergic neurotransmission in FTD building on the evidence of their safety and efficacy in other disorders (Wang et al., 2011; Cubillo et al., 2014; Kehagia et al., 2014; Ye et al., 2015; Rae et al., 2016).
Progressive supranuclear palsy and corticobasal syndrome
Evidence is emerging of an early noradrenergic deficit in PSP, with loss of noradrenergic neurons and low noradrenaline levels in the basal ganglia. There is significant pathology in the locus coeruleus with both tau deposition (Dickson, 1999; Arnold et al., 2013), and neuronal loss (Fig. 3C) (Hauw et al., 1994; Mori et al., 2002; Dickson et al., 2010). A single post-mortem study also found reduced levels of noradrenaline in the caudate and putamen (Hornykiewicz and Shannak, 1994) (Fig. 3D), although noradrenergic receptor density is normally low in the striatum compared to cortex. These early and sometimes severe noradrenergic changes may be directly linked to cognitive and behavioural manifestations of PSP, such as rigidity and impulsivity, analogous to the treatable noradrenergic deficit underlying aspects of impulsivity in Parkinson’s disease (Kehagia et al., 2014; Rae et al., 2016). In keeping with this, a double-blind cross-over study of the α2 antagonist idazoxan showed improvement in motor function in PSP (Ghika et al., 1991). However a larger study with a more potent α2 antagonist (efaroxan) found no effect (Rascol et al., 1998). Atomoxetine has been shown to reduce impulsivity and executive deficits in Parkinson’s disease (Marsh et al., 2009; Kehagia et al., 2014; Ye et al., 2015), but evidence is lacking in PSP. Evidence is also lacking for noradrenergic changes in CBS, although tau pathology is present in the locus coeruleus (Dickson, 1999).
Serotonin
Serotonin (5-HT) is synthesized mainly by two groups of neurons in the raphe nuclei in the brainstem, which project widely (Fig. 4A) (Charnay and Léger, 2010). The rostral group, comprising 85% of serotonergic neuron cell bodies, project to the cerebral cortex, thalamus, hypothalamus and basal ganglia (Hornung, 2003). The caudal group project mainly to the brainstem and spinal cord (Hornung, 2003). With these widespread projections, serotonin regulates many higher brain functions related to cognitive control, learning, and affect (Harvey, 2003; Ciranna, 2006; Canli and Lesch, 2007; Artigas, 2013). There are seven different serotonin receptor families (5-HT1–7), which are neuromodulatory G protein coupled receptors except for the 5-HT3 receptor family, which includes ligand-gated ion channels (Barnes and Sharp, 1999; Southan et al., 2016). To add to this complexity, genetic polymorphisms within a receptor subtype (Barnes and Sharp, 1999) and presynaptic transporter (Porcelli et al., 2012), influence serotonergic function.
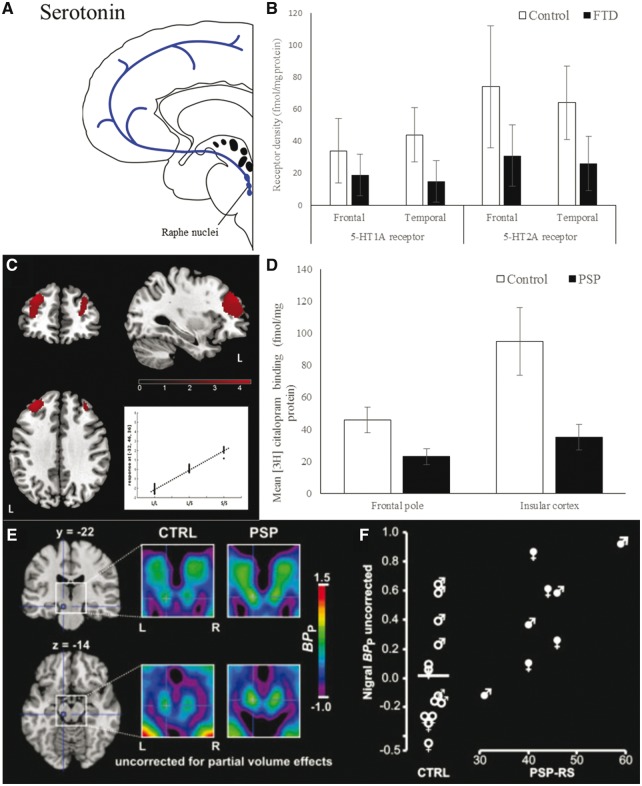
Figure 4.
Serotonergic deficits in FTD and PSP. (A) Schematic illustration of serotonin pathways. (B) 5-HT1 and 2A receptor density is reduced in the frontal and temporal lobe in FTD. Graph of data from Bowen et al. (2008). Reprinted with permission of the authors and Springer. (C) Effect of 5-HTTLPR genotype on brain perfusion in FTD patients. Comparison of long (L/L) versus short (S/S) carriers at the same disease stage showing reduced perfusion of some areas of the frontal lobe in L/L carriers. From Premi et al. (2015). Reprinted with permission from Elsevier. (D) Presynaptic serotonergic neurons (measured by citalopram binding to post-mortem tissue) are reduced in the frontal and insular cortices in PSP. Graph of data from Chinaclia and Landwehrmeyer (1993). Reprinted with permission from Elsevier. (E) 5-HT2A receptor PET binding is increased bilaterally in the striatum and substantia nigra compared with controls. In the same study (F) disease severity positively correlated with 5-HT2A binding potential in the striatum. From Stamelou et al. (2009). Reprinted with permission from Wiley.
Serotonin receptors are among the most complex and varied of neurotransmitter receptors, and while there is clear evidence of serotonergic deficits in FTLD, studies to date mainly lack a detailed breakdown of receptor subtypes, or focus on 1A and 2A receptors. Serotonin has important roles in synaptic plasticity and as a neuromodulator of the direct effects of other neurotransmitters (Celada et al., 2013). For example, serotonin inhibits dopamine release and modulates glutamate and GABA transmission (Ciranna, 2006). In the hippocampus serotonin receptors reduce glutamate and stimulate GABA from inhibitory interneurons, reducing long term potentiation (Ciranna, 2006). In the frontal cortex glutamate release is inhibited by serotonin whereas in the prefrontal cortex serotonin enhances glutamate transmission (Dawson et al., 2001; Ciranna, 2006). This suggests that FTLD-induced serotonin deficiency could cause widespread cognitive, motor and affective symptoms, directly and through the disruption of its modulation of other systems.
Frontotemporal dementia
Serotonin dysfunction is a significant contributor to the behavioural and cognitive symptoms seen in bvFTD (Huey et al., 2006; Hughes et al., 2015). Reductions in serotonin transmission or postsynaptic receptor densities are associated with several symptoms seen in FTD including aggression, impulsivity, increased appetite and depression (Huey et al., 2006). At post-mortem examination, 5HT1A and 2A receptors are reduced in the frontal and temporal lobes and the hypothalamus (Fig. 4B) (Sparks and Markesbery, 1991; Francis et al., 1993; Procter et al., 1999; Bowen et al., 2008). In vivo PET studies corroborate the post-mortem findings with the 5-HT2A receptor binding potential reduced in the midbrain and medial frontal cortex (Franceschi et al., 2005) and the 5-HT1A binding potential reduced across all cortical areas (Lanctôt et al., 2007).
Evidence for actual serotonergic neuronal cell loss is less conclusive. One post-mortem study found loss of neurons in the raphe nucleus and their projections to the cerebral cortex, which correlated with disease duration (Yang and Schmitt, 2001). There is also pathological tau deposition in the raphe nuclei (Irwin et al., 2016). This contrasts with other studies that report no change in imipramine binding, proposed as a measure of presynaptic serotonergic terminals (Sparks and Markesbery, 1991), while post-mortem biochemical assays of serotonin are normal or elevated in FTD (Bowen et al., 2008; Vermeiren et al., 2016) and CSF measures of serotonin and its metabolites are unchanged (Engelborghs et al., 2008). Nonetheless, CSF homovanillic acid/5-hydroxyindoleacetic acid (HVA/5-HIAA) levels (a proposed marker of the serotonergic modulation of dopaminergic neurotransmission) correlate with aggressive behaviour in FTD (Engelborghs et al., 2004, 2008). 5-HIAA/5-HT ratios (a proposed marker of serotonergic turnover) are also lower in FTD compared to controls in the frontal and temporal lobes (Vermeiren et al., 2016). It is possible that these apparent inconsistencies between biochemical assays and receptor or neuronal markers result from different stages of serotonergic cell loss and downstream functional compensation. To test this hypothesis would require the comparison of methods within the same pathological cohort, preferably one that includes patients with a wide range of neurocognitive severity.
There appears to be an association between FTD and length polymorphism in the gene promotor S(5-HTTLPR) of the serotonin transporter gene (SLC6A4) which suggests serotonin may be involved in the pathogenesis of FTD. A short allele (5-HTTLPR-s) was associated with a greater susceptibility to FTD in one study (Albani et al., 2008) although this was not replicated (Yokoyama et al., 2015). The 5-HTTLPR variant also affects brain atrophy in FTD. Patients with a long 5-HTTLPR allele have correspondingly greater atrophy and lower perfusion at equivalent disease stages (Fig. 4C) (Premi et al., 2015) while the short allele is associated with more atrophy in the left inferior frontal gyrus and less in the right temporal lobe (Yokoyama et al., 2015). The long allele may have a protective effect on cognitive presentation but is not associated with better prognosis (Borroni et al., 2010).
In bvFTD there are reduced neurophysiological markers of inhibitory control and prefrontal cortical function, which are restored with the selective serotonin reuptake inhibitor citalopram in a placebo-controlled double-blind assessment (Hughes et al., 2015). Several open label studies without placebo-control have shown improvement in behavioural symptoms with serotonergic drugs. For example, citalopram reduced disinhibition, irritability and depression (Herrmann et al., 2012) and improved Frontal Assessment Battery test scores (Herrmann et al., 2012) and inappropriate sexual behaviour (Anneser et al., 2007). Paroxetine improved behavioural symptoms in an open label study (Moretti et al., 2003a) but this was not supported by a subsequent placebo-controlled blinded study (Deakin et al., 2004). Trazodone may improve behavioural symptoms in bvFTD based on a randomized control cross-over study (Lebert et al., 2004). Interestingly, trazodone differs from selective serotonin reuptake inhibitors (SSRIs): it is an antagonist of a range of serotonin receptors apart from 5HT1A where it is an agonist, and it inhibits the serotonin transporter. A meta-analysis of antidepressants in FTD showed a combined mean reduction of 15 points on the Neuropsychiatric Inventory, noting, however, that the evidence was mainly from small, non-placebo controlled trials (Huey et al., 2006).
Progressive supranuclear palsy and corticobasal syndrome
Pathological tau inclusions are found post-mortem in the raphe nuclei with PSP (Revesz et al., 1996) while presynaptic serotonergic neurons are reduced in the caudate nucleus, frontal and temporal cortex (Fig. 4D) (Chinaclia and Landwehrmeyer, 1993). Serotonin levels were not significantly reduced in one post-mortem study (Hornykiewicz and Shannak, 1994). PET and post-mortem studies have both shown upregulation of 5-HT1B and 2A receptors in the substantia nigra and striatum (Fig. 4E) (Castro et al., 1998; Stamelou et al., 2009), which might represent compensation for loss of presynaptic serotonergic neurons. This upregulation correlated with severity of motor impairment (Fig. 4F) (Stamelou et al., 2009), but information on the correlation with cognitive, affective or associative functions is also needed.
There have been case reports of patients with PSP showing some improvements in motor function with an SSRI (Miyaoka et al., 2002), and anecdotal reports of serotonergic reuptake inhibition as an effective treatment for emotional lability (Rittman et al., 2016). Overall there is not strong evidence for the efficacy of serotonergic drugs in PSP (Stamelou and Höglinger, 2016). This lack of evidence may be because studies have focussed on depression and anxiety as outcomes of treatment, rather than impulsivity, disinhibition or cognitive change (Rittman et al., 2016).
There is neuronal loss and gliosis in the raphe nucleus in CBD (Gibb et al., 1989). However in vivo data are lacking on the serotonergic pathways and receptor density in CBS, and there are no systematic trials of serotonin reuptake inhibitors.
Acetylcholine
Acetylcholine is neuromodulatory on many areas of the forebrain (Everitt and Robbins, 1997), and influences a wide range of cognitive functions including attention, memory and emotion, but also motor control, through cortical and subcortical transmission in the cortico-striato-thalamocortical circuits (Picciotto et al., 2012). The major cholinergic inputs to the cerebral cortex originate in the nucleus basalis of Meynert and adjacent nuclei in the basal forebrain (Fig. 5A) (Selden, 1998). Two other cholinergic nuclei in the brainstem, the pedunculopontine and lateral dorsal tegmental nuclei, project to the thalamus. Acetylcholine acts on two main receptor classes in the brain; muscarinic G protein coupled receptors (M1–5) and nicotinic ligand-gated ion channels (Picciotto et al., 2012). Cholinergic receptors can have excitatory or inhibitory effects depending on their subtype and pre- versus postsynaptic location (Picciotto et al., 2012).
Figure 5.
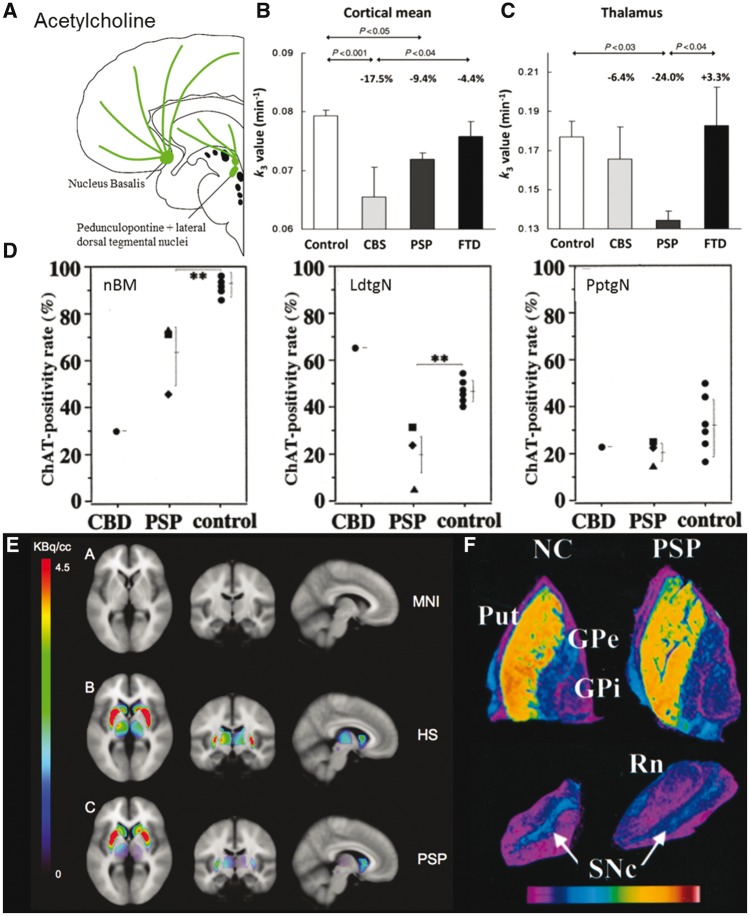
Cholinergic deficits in FTD, PSP and CBS. (A) Schematic illustration of cholinergic pathways. (B and C) 11C-MP4A PET, a measure of acetylcholinesterase activity, in healthy controls, CBS, PSP and FTD. Cortical k3 (a measure of PET ligand binding) is reduced in CBS and PSP but not FTD. Thalamic mean k3 is reduced in PSP but not CBS or FTD. From Hirano et al. (2010). Reprinted with permission from Oxford University Press. (D) Quantitative estimation of choline acetyltransferase (ChAT) positivity rate (%) in the nucleus basalis of Meynert (nBM), laterodorsal tegmental (LdtgN) and pedunculopontine tegmental (PptgN) nuclei. From Kasashima and Oda (2003). Reprinted with permission of Springer. (E) SPECT of acetylcholine transporter. MNI = MRI template; HS = healthy subject. Specific binding in the striatum, thalamus and pedunculopontine nucleus extracted by subtracting reference from region of interest binding. Binding is lower in the thalamus and pedunculopontine nucleus. From Mazere et al. (2012). Reproduced with permission from the Radiological Society of North America. (F) Autoradiogram of brain tissue from a healthy control (NC) and PSP. 3H-vesamicol binding to acetylcholine transporter (VAChT). There is reduction in binding in the putamen (Put) and substantia nigra pars compacta (SNc). Rn = red nucleus. Image intensity converted to pseudocolour representation according to key. From Suzuki et al. (2002). Reproduced with permission from Wolters Kluwer.
Cholinergic drugs are in widespread use clinically, although not specifically in FTLD. For example, anti-cholinergic drugs reduce tremor and dystonia in movement disorders (Rifkin et al., 1978), although they can cause impairments in learning and memory (Everitt and Robbins, 1997). The loss of cholinergic neurons and reduced choline acetyltransferase in Alzheimer’s disease (Francis et al., 1999) lies behind the widespread use of cholinesterase inhibitors to enhance cholinergic transmission and thereby alleviate cognitive symptoms in Alzheimer’s disease (Rogers et al., 1998). This cholinergic hypothesis has led to research into the role of cholinergic therapies in other dementias, including syndromes arising from FTLD.
Frontotemporal dementia
Cholinergic pathways are affected in FTD but not to the same extent as in Alzheimer’s disease. While there is some loss of cholinergic neuronal markers in the nucleus basalis, overall cholinergic pathways to the cortex appear unaffected. Choline acetyltransferase, the enzyme for the synthesis of acetylcholine, can be used as a marker of presynaptic cholinergic neuron integrity. Post-mortem levels of choline acetyltransferase are reduced in the nucleus basalis of Meynert and the hypothalamus but are normal in the frontal, temporal and parietal lobes (Wood et al., 1983; Hansen et al., 1988; Sparks and Markesbery, 1991; Procter et al., 1999). Acetylcholinesterase, which catalyses the breakdown of acetylcholine, is predominantly located on the presynaptic cholinergic neurons. Levels are reduced in the nucleus basalis at post-mortem (Sparks and Markesbery, 1991) but have been normal in the thalamus and cerebral cortex when measured in vivo with 11C-MP4A PET (Fig. 5B and C) (Hirano et al., 2010) or at post-mortem (Meier-Ruge et al., 1984; Sparks and Markesbery, 1991).
Studies are inconsistent on cholinergic receptors in bvFTD. 123IQNB SPECT imaging of two patients with Pick’s disease indicated reduced muscarinic receptor density in the frontal and temporal cortex (Weinberger et al., 1991) consistent with autoradiography in a case report (Yates et al., 1980). In contrast two studies found no significant change in muscarinic receptor density post-mortem (Wood et al., 1983; Procter et al., 1999).
There is evidence of a cholinergic deficit in primary progressive aphasia. In patients with semantic dementia there was loss of muscarinic receptors in the temporal lobe (Odawara et al., 2003). Disproportionate atrophy of the basal forebrain nuclei was identified in a high resolution MRI study, most evidently in the semantic variant, and to a lesser extent the non-fluent variant (Teipel et al., 2016). This is relevant in view of the evidence that the frontotemporal language networks of a healthy brain receive significant cholinergic inputs (Amunts et al., 2010). The logopenic variant had minimal structural change, despite its strong clinicopathological correlation with Alzheimer’s disease.
Despite the possible cholinergic deficits in bvFTD and PPA, cholinesterase inhibitors do not convincingly improve cognitive function. An open label non-randomized study found that behavioural changes improved with rivastigmine, in comparison to a group that took antipsychotics and benzodiazepines (Moretti et al., 2004). In contrast bvFTD patients taking donepezil had worsening disinhibition and compulsive behaviour (Mendez et al., 2007). A randomized, double-blind trial of galantamine versus placebo found no effect on cognitive function or activities of daily living (Kertesz et al., 2008).
Progressive supranuclear palsy and corticobasal syndrome
There are marked cholinergic deficits in PSP, which may contribute not only to cognitive impairment but also postural instability via the pedunculopontine nucleus (Jellinger, 1988; Warren et al., 2005). There is loss of cholinergic neurons and their presynaptic terminals in many subcortical regions in PSP. Choline acetyltransferase is reduced in the nucleus basalis of Meynert, midbrain nuclei and pedunculopontine nucleus (Fig. 5D) (Juncos et al., 1991; Javoy-Agid, 1994; Kasashima and Oda, 2003) as well as the putamen, caudate and pallidum (Ruberg et al., 1985; Pierot et al., 1988; Javoy-Agid, 1994). Presynaptic acetylcholine transporters are reduced in the putamen and substantia nigra, while sparing the globus pallidus and cerebral cortex (Fig. 5F) (Suzuki et al., 2002). 123I-IBVM SPECT, which binds to acetylcholine transporters, reveals reduced signal in the thalamus of PSP patients (Fig. 5E) (Mazere et al., 2012) and PET studies show reduced acetylcholinesterase binding in the pons, basal ganglia and thalamus (Shinotoh et al., 1999; Gilman et al., 2010; Hirano et al., 2010).
There is loss of cholinergic projections from the brainstem (pedunculopontine and laterodorsal tegmental nuclei) to the thalamus (Hirsch et al., 1987; Jellinger, 1988; Kasashima and Oda, 2003). The pedunculopontine loss is especially relevant to the impairment of movement, gait and muscle tone in PSP (Benarroch, 2013). Deep brain stimulation of the pedunculopontine nucleus has been reported to improve PSP motor symptoms in selected cases, but definitive trials are lacking (Hazrati et al., 2012; Servello et al., 2014). One study reported that acetylcholine receptors are relatively well preserved in the striatum (Ruberg et al., 1985) while other studies report a reduction in muscarinic and nicotinic receptors in the striatum (Landwehrmeyer and Palacios, 1994; Warren et al., 2007b). With such small series, and variable methods, it is unclear if technical or phenotypic differences account for these inconsistencies.
There is also some limited evidence for cholinergic deficits in the cerebral cortex. Acetyltransferase levels are reduced in frontal cortex of PSP patients compared with controls both at post-mortem and with in vivo PET imaging (Ruberg et al., 1985; Javoy-Agid, 1994; Hirano et al., 2010). However, cortical muscarinic receptor levels appear to be unaffected in PSP, with levels similar to controls in PET studies (Ruberg et al., 1985; Asahina et al., 1998).
In clinical practice, cholinergic blockade with hyoscine is sometimes used for sialorrhoea and drooling, but it may worsen gait and memory in PSP (Litvan et al., 1994). Despite this deleterious effect of anti-cholinergic medication, the converse ‘pro-cholinergic’ treatment by cholinesterase inhibitors is typically ineffective (Stamelou and Höglinger, 2016). A case series of rivastigmine in five patients found that it improved working memory, memory and verbal fluency but worsened motor function (Liepelt et al., 2010). A randomized, placebo-controlled crossover study of donepezil showed no effect on quality of life, Progressive Supranuclear Palsy Rating Scale or global cognitive function (Litvan et al., 2001). This study did find a slight improvement in one memory task but also worsened motor activities of daily living (Litvan et al., 2001). Interestingly, the syndrome of pure akinesia and gait freezing, now recognized as a prodromal variant of PSP (Höglinger et al., 2017) has been reported to improve after cholinesterase inhibition in an open case series (Kondo, 2006). Despite this encouraging study, replication in a placebo controlled trial is awaited.
In a post-mortem study of a single case of CBD the number of cholinergic acetyltransferase positive neurons in the nucleus basalis of Meynert was reduced (Kasashima and Oda, 2003). This was replicated in vivo, with reduced acetylcholinesterase levels in the frontal, parietal and occipital cortex (Hirano et al., 2010). There is insufficient data on cholinergic treatment of patients with CBS, although it should be noted that ∼20–40% of patients with CBS have Alzheimer’s-type pathology not CBD (Boeve et al., 1999; Alexander et al., 2014). It is plausible, but not proven, that the Alzheimer pathology cases of CBS would respond better to cholinesterase inhibitors despite appearing similar to CBD cases in other clinical features. We therefore anticipate that clinical trials of CBS will stratify treatment according to biomarkers, such as amyloid PET imaging or CSF, to distinguish CBD from Alzheimer’s disease aetiology.
Glutamate
Glutamate is the principle excitatory neurotransmitter in the brain. Glutamate acts on fast, short acting ionotropic receptors and slower but longer acting metabotropic glutamate receptors (mGluR) (Meldrum, 2000). The three main ionotropic glutamate receptors are named after the selective agonists N-methyl d-aspartate (NMDA), α-amino-3-hydroxyl-5-methyl-isoxazolepropionic acid (AMPA) and kainite (Meldrum, 2000). Glutamate has an important role in learning and memory formation. For example, NMDA receptors in the hippocampus regulate long term potentiation (Morris et al., 1986; Rowland et al., 2005) while sustained activation of the dorsolateral prefrontal cortex during working memory requires NMDA stimulation (Wang et al., 2013). NMDA receptor antagonists impair attention, reaction time, processing speed and working memory in healthy humans (Malhotra et al., 1996; Newcomer et al., 2000), and may exacerbate psychotic symptoms (Gilmour et al., 2012). Glutamate signalling through NMDA receptors is required to create and maintain gamma oscillations (Carlé et al., 2011), which support many higher cognitive functions (Lange et al., 1997; Bartos et al., 2007; Williams and Boksa, 2010; Gaetz et al., 2012; Gorelova et al., 2012).
While glutamatergic transmission is essential for cognition, excessive glutamatergic transmission may also be harmful, promoting excitotoxic neuronal death (Mark et al., 2001) that contributes to neurodegeneration in models of Alzheimer’s disease (Danysz et al., 2000; Kalia et al., 2008). It is possible that FTLD is similarly affected. Functionally, continuous overactivation of NMDA receptors alters the efficacy of information processing by reducing the sensitivity of neural networks and impairing their ability to detect a relevant signal from upstream neurons (Danysz et al., 2000). Memantine is a low affinity NMDA receptor antagonist and selectively blocks pathological tonic NMDA receptor activation (associated with amyloid plaques) without preventing NMDA-mediated synaptic transmission. In addition to potential symptomatic effects on cognition (Reisberg et al., 2003), it might therefore also reduce chronic glutamatergic excitotoxicity (Danysz and Parsons, 2012).
Frontotemporal dementia
There is preclinical and clinical evidence that glutamate is important in the pathogenesis of FTD. For example, transgenic mice that express pathological human tau have repetitive and disinhibited behaviour, coupled with NMDA receptor hypofunction (Warmus et al., 2014). Treatment with an NMDA agonist restores their behaviour. Transgenic mice expressing mutations in the FTD-associated gene CHMP2B, have altered AMPA receptor composition (Gascon et al., 2014), with impaired sociability, which can be reversed if normal AMPA receptor composition is restored (Gascon et al., 2014). Mouse models expressing pathological human tau suggest glutamate mediated excitotoxicity could accelerate neuronal loss in tauopathies such as FTD (Decker et al., 2016). These preclinical studies raise the possibility that pharmacological glutamatergic treatments might reduce symptom severity and improve prognosis.
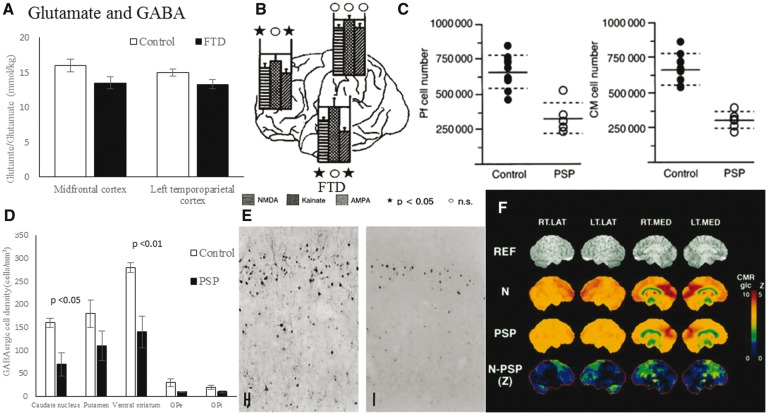
In patients, glutamatergic pyramidal neurons are reduced in the thalamus, frontal and temporal cortex (Ferrer, 1999). Magnetic resonance spectroscopy of patients with FTD has found glutamate/glutamine levels are reduced in the frontal and temporal lobes (Fig. 6A) (Ernst et al., 1997; Sarac et al., 2008). There is an inverse correlation between CSF glutamate levels and verbal agitation (Vermeiren et al., 2013).
Figure 6.
Glutamate and GABA deficits in FTD and PSP. (A) Mean metabolite concentrations using magnetic resonance spectroscopy. Glutamine–glutamate concentrations are reduced in the frontal cortex of FTD. Graph of data from Ernst et al. (1997). Reprinted with permission from the authors and the Radiological Society of North America. (B) Post-mortem glutamatergic receptor binding in FTD. Binding to NMDA and AMPA receptors is reduced in the frontal and temporal lobes. From Procter et al. (1999). Reprinted with permission from S. Karger AG. (C) Neuron number in two thalamic nuclei [parafascicular (Pf) and centromedian (CM)] that contain glutamatergic neurons is reduced in PSP compared with controls. Adapted from Henderson et al. (2000), with permission from the authors and Oxford University Press. (D) Numbers of GABAergic neurons (measured by glutamic acid decarboxylase mRNA expression) in the striatum and pallidum in controls and PSP patients. There is significant reduction in striatal GABAergic neurons in patients. Graph of data from Levy et al. (1995), reprinted with permission from the authors and Wolters Kluwer. (E) Calbindin immunohistochemistry of GABAergic cells in the frontal cortex of FTD and control brains. From Ferrer (1999). Reproduced with permission from Karger. (F) 11C-flumazenil PET binding to benzodiazepine receptors in healthy controls (N), PSP and the group difference in cortical and subcortical areas. From Foster et al. (2000). Reproduced with permission from Wolters Kluwer.
Both ionotropic and metabotropic glutamate receptors are affected in FTD. For example, AMPA and NMDA receptor densities are reduced in the frontal and temporal lobes of patients at post-mortem (Francis et al., 1993; Procter et al., 1999; Bowen et al., 2008), while AMPA receptor composition is also abnormal (Fig. 6B) (Gascon et al., 2014). Using the ligand 11C-ABP688, PET of patients with bvFTD found reduced availability of metabotropic glutamate receptors (mGluR5) in the frontal and temporal lobes, basal ganglia and thalamus (Leuzy et al., 2016). However, one study found that post-mortem levels of metabotropic glutamate receptors type 1 and 5 (mGluR1 and 5) are increased in the frontal cortex (Dalfo et al., 2005).
A phase II randomized placebo-controlled trial of memantine showed no benefit in patients with bvFTD (Boxer et al., 2013). A double-blind placebo-controlled crossover trial of memantine in PPA was also negative (Johnson et al., 2010). However, these studies were not powered to detect small treatment effects. While there may be no true benefit, it remains possible that small treatment effects exist which would be amplified if other neurotransmitter deficits were also normalized, in particular GABAergic impairments. The GABA–glutamate interaction is of particular relevance because it supports precisely tuned oscillatory dynamics of neural circuits for cognition (Bastos et al., 2012).
Progressive supranuclear palsy and corticobasal syndrome
Loss of glutamatergic neurons in the basal ganglia may partly explain why dopaminergic therapy is ineffective in PSP. Glutamate modulates dopamine release and loss of glutamatergic neurons may prevent patients compensating for dopaminergic neuron loss (Lange et al., 1997). Glutamatergic neurons from the caudal intralaminar nuclei that form the thalamostriatal pathway are reduced in PSP (Fig. 6C) (Henderson et al., 2000). However, the severity of this neuronal loss does not correlate with disease duration or severity (Henderson et al., 2000). In contrast, NMDA receptor levels are preserved in the frontal and temporal lobes and striatum (Holemans et al., 1991).
Glutamatergic over-activity is implicated in Parkinson’s disease and by analogy has been considered a candidate mechanism of accelerated neurodegeneration in PSP (Lange et al., 1997). Amantadine is an NMDA receptor antagonist that is often used to treat motor symptoms (Kompoliti et al., 1998; Stamelou and Höglinger, 2016), although there is no randomized controlled trial evidence of efficacy in PSP. Gabapentin has complex pharmacodynamics and in part acts by increasing GABA and reducing glutamate levels (Sills, 2006). A randomized blinded trial of gabapentin in 14 patients found no effect on motor function but improved outcome in anti-saccade control (Poujois et al., 2007), which is associated with frontal lobe integrity (Mirsky et al., 2011; Perneczky et al., 2011) and commonly impaired in PSP (Garbutt et al., 2008; Zhang et al., 2016). There are no reports of post-mortem or in vivo glutamate measurements in CBS.
Gamma-aminobutyric acid
GABA is the predominant inhibitory neurotransmitter in the brain, formed by glutamate decarboxylase conversion of glutamate to GABA in interneurons. There are two classes of GABA receptors: GABAA ligand-gated ion channels and GABAB G protein coupled neuromodulatory receptors. GABAergic inhibitory neurons dampen and balance excitation within neural circuits, but do more than simply counteract excitatory glutamatergic neurons. They have a key role in the regulation of oscillatory dynamics, including the generation of gamma oscillations and regulation of the magnitude and frequency of these oscillations (Owens and Kriegstein, 2002; Mann and Paulsen, 2007; Buzsáki and Wang, 2012). This is essential for coordinating information transfer and information processing in the brain (Fries, 2009; Bastos et al., 2012). Increasing synaptic GABA levels increases gamma power during cognitive control tasks (Frankle et al., 2009) whereas inhibiting GABA receptors reduces gamma oscillatory power and impairs inhibition and working memory (Hines et al., 2013). Gamma oscillations correlate with GABA concentrations (as measured by magnetic resonance spectroscopy) in the visual (Muthukumaraswamy et al., 2009), primary motor (Gaetz et al., 2011) and dorsolateral prefrontal cortex (Kujala et al., 2015) while GABAA receptor density (as measured by flumazenil-PET) correlates with gamma frequency and magnitude (Kujala et al., 2015). Impaired GABA neurotransmission has been implicated in a number of brain disorders including schizophrenia (Gonzalez-Burgos et al., 2011) and Huntington’s disease (Reynolds and Sally, 1990) as well as the syndromes associated with FTLD.
Frontotemporal dementia
The subgroup of GABAergic neurons that bind calbindin-D28k are reduced in upper neocortical layers of the frontal and temporal cortex in FTD (Ferrer, 1999), especially in layers II and III (Fig. 6E) (Ferrer, 1999). However, in the same study, the subgroup of GABAergic basket and chandelier neurons that bind parvalbumin were preserved (Ferrer, 1999). The superficial layers II and III are the main source of cortico-cortical feedforward efferent projections and receive feedback projections from deep layers. Gamma oscillations and coherence are reduced between the frontal lobes of patients with bvFTD (Hughes et al., 2013), which may relate to loss of cortical feedforward information processing and cognitive decline (Mann and Paulsen, 2007). GABA concentrations are also decreased in the basal ganglia in bvFTD (Kanazawa et al., 1988). GABAergic approaches to treatment of FTD symptoms warrant further investigation, but evidence of their clinical efficacy is currently lacking.
Progressive supranuclear palsy and corticobasal syndrome
GABAergic interneurons are reduced in PSP. A post-mortem study found a 50–60% decrease in the number of GABAergic neurons (estimated from the number expressing glutamic acid decarboxylase mRNA, by in situ hybridization) in the caudate nucleus, putamen, ventral striatum and pallidum (Fig. 6D) (Levy et al., 1995). Binding to GABAA receptors is reduced in the globus pallidus but preserved in the striatum (Landwehrmeyer and Palacios, 1994; Suzuki et al., 2002). A flumazenil-PET study showed loss of GABAA receptors compared with controls (Fig. 6F) (Foster et al., 2000).
There are case reports of GABA receptor agonists improving speech, eye movements, akinesia and rigidity in PSP (Daniele et al., 1999; Cotter et al., 2010; Dash, 2013; Chang and Weirich, 2014), but in the authors’ experience this phenomenon is very uncommon and there are no randomized placebo controlled studies. There are no reports of post-mortem or in vivo assessments of GABA in CBS.
Towards better symptomatic treatment in frontotemporal lobar degeneration
Despite their overlapping clinical phenotypes and pathological features, the major clinical syndromes associated with FTLD have different neurotransmitter deficits (summarized in Table 1). Restoring these deficits, individually or in combination, has the potential to improve cognitive, behavioural and motor symptoms. However, the evidence base for therapeutic effects is dominated by small, open-label studies in unstratified populations.
To summarize the evidence for selective deficits, FTD causes loss of serotonergic and dopaminergic neurons and receptor densities, whilst noradrenergic and cholinergic pathways are relatively preserved. There is loss of both glutamatergic and GABAergic neurons but the functional consequence of their deficits is unclear, in part because of the complex and dynamic interaction between GABAergic and glutamatergic neurons in cortical circuits. In PSP, the most evident neurotransmitter deficits are dopaminergic, noradrenergic and cholinergic, whilst serotonergic projections appear to be relatively preserved. There is evidence of a glutamatergic and GABAergic deficit, which provide potential avenues for non-dopaminergic therapy. There is limited evidence on the neurotransmitter deficits in CBS, with some evidence of deficits in both cholinergic and dopaminergic pathways.
Although clinical trials and cases series have not shown consistent benefits from the modulation of neurotransmitters in FTLD syndromes, this may be due to weaknesses in research methodology rather than a true lack of effect. For example, many studies use what would now be considered as outdated and inaccurate diagnostic criteria, which reduces the applicability to contemporary patient populations. Many clinical studies are open-labelled and in small series, sometimes fewer than 10 patients, giving little power to detect benefits, let alone guide therapeutic stratification. There is a paucity of replication studies, and where studies contain a ‘conceptual replication’, details in research methodology confound the interpretation of seemingly conflicting results. Much of the research comes from post-mortem brain tissue, which has the advantage of providing concurrent pathological validation of the disorder. However, post-mortem studies have tended to use small series (n < 10), and by the nature of post-mortem material, they cannot provide insights into the early or sequential changes in neurotransmitter systems. Future work will benefit from longitudinal and in vivo studies, exploiting advances in PET ligands (Finnema et al., 2015), ultra-high field MRI and spectroscopy (Agarwal and Renshaw, 2012), and CSF biomarkers. Early PET studies of necessity used non-specific ligands, which may not correspond to the receptor specificities of psychopharmacological agents. This is not to criticise either body of work, but it does impair the direct comparison of imaging and pharmacological studies, even where comparable patient groups are studied. Similarly, future preclinical studies would benefit from within-sample comparisons of different methods, seeking not only cross-validation of biochemical or receptor assays, but also the relationship between different measures, for example neuronal loss, receptor density, and biochemical turnover of a neurotransmitter. Such cross-modal studies would provide a powerful resource to model disease progression and functionally relevant compensatory changes in FTLD.
Further research is required into the effect of FTLD on different neurotransmitter receptors and their subtypes, not only to guide candidate drug selection, but also to determine the progression of changes from early to late stage disease. Without this detailed knowledge, there is a risk that a given drug may be effective at one stage of disease but be counterproductive at another. Such non-linear dose-response effects are common in dopaminergic treatments of Parkinson’s disease (Cools, 2006; Rowe et al., 2008), but the principal of ‘U-shaped’ responses to drug treatment also affect serotonergic (Macoveanu et al., 2013; Hughes et al., 2015) and noradrenergic drugs (Ye et al., 2015). Where drug effects follow a ‘U-shaped’ response, the focal nature of FTLD presents a special challenge. Take the behavioural variant of FTD as an example. If prefrontal and temporal cortex are deficient in a given neurotransmitter (whether neuronal density, receptor density, or afferent projections), but motor, parietal and occipital cortex are not, then any systemic treatment based on restoring that neurotransmitter in frontal and temporal cortex will risk ‘overdosing’ the unaffected areas. This problem is well established in Parkinson’s disease, in the sometimes difficult balance between motor disability and impulse control disorders (Napier et al., 2015). The application of focal treatments to restore biochemical function, such as dopaminergic stem cell transplants or gene therapy to induce dopamine synthesis in striatal cells, can overcome some of the adverse consequences of systemic drug treatment in Parkinson’s disease. However, such localized treatments seem even more challenging in a diffuse lobar cortical disorder. Similarly, the use of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) to restore or enhance focal and selective neurotransmitter systems is having a major impact in drug discovery and systems neuroscience (Roth, 2016), but seems far from direct clinical applications. For the time being, systemic drug delivery is likely to be the mainstay of clinical therapeutics.
We suggest three steps to improve the likelihood of new and effective pharmacological treatments. First, clarification of the links between individual neurotransmitters and specific clinical end-points. We suggest that identifying the neurotransmitter deficits that correlate with clinical severity is essential to guide treatment studies. This evidence may draw on in vivo imaging and CSF studies and post-mortem immunohistochemistry of cases that have been regularly phenotyped during disease progression. This would be a considerable undertaking, but possible if added to existing longitudinal studies (Rohrer et al., 2015b; Woodside et al., 2016).
Second, it is essential to implement stratification of patients in future trials, selecting participants for their relevant symptoms rather than the diagnosis alone. For example, in a trial to demonstrate a clinical effect of serotonergic treatment on impulsivity in bvFTD, based on experimental medicines evidence (Hughes et al., 2015), participants should not merely have bvFTD by consensus criteria, but also have impulsivity; noting that disinhibition is one of six criteria whereas only three are required for the diagnosis. Including patients with bvFTD who are not disinhibited is likely to reduce the power of a symptomatic treatment trial. Moreover, it may be better to include all patients with disinhibition arising from syndromes associated with FTLD in which disinhibition is common but not a diagnostic criterion (including semantic variant PPA, CBS and PSP) (Lansdall et al., 2017). This would increase the power and relevance of the trial to a wider patient group.
Third, future clinical trials need careful selection of relevant outcome tools, especially where drugs are repurposed for new end-points. For example, selective serotonin reuptake inhibitors are licenced for affective disorders but it would be wrong to use a depression rating scale in bvFTD or PSP where the expected effect is on impulsivity. Similarly, cholinesterase inhibitors are licenced for Alzheimer’s disease for their effect on cognition but cognitive function scales would be inappropriate if the intended effect in say PSP were on gait and balance.
For each of the disorders associated with FTLD, it is likely that experimental medicines studies with biomarker based surrogate end-points are needed before randomized placebo controlled clinical trials are started. The evidence presented in this review suggests that there are strong grounds to pursue such experimental medicine studies, drawing on the preclinical psychopharmacology models and patient data, to minimize the risks of clinical trials. There are many candidate end-points, to demonstrate human target engagement and efficacy in the CNS. These may be used singly or in combination, including functional imaging; magnetic resonance spectroscopy (Cai et al., 2012; Muthukumaraswamy et al., 2013); PET imaging of neurotransmitters receptors and occupancy; magneto-/electro-encephalographic physiological indices of oscillatory dynamics (Muthukumaraswamy, 2014), focal function (Hughes et al., 2015) and network interactions (Moran et al., 2011, 2013; Hughes et al., 2013; Gilbert et al., 2016); CSF biomarkers; and neurocognitive batteries (Kehagia et al., 2014).
This review has focused on the symptomatic benefits of restoring neurotransmitters. However, some of these agents, like trazodone, have wider effects on pathogenesis and neuronal survival that may also lead to disease modification or slowing of disease progression (Halliday et al., 2017). Even where the principal effect is symptomatic, this may improve survival, such as the impact of dopaminergic therapy in Parkinson’s disease after its introduction in the late 1960’s (Uitti et al., 1993). Relief of apathy, disinhibition, falls, and dementia in syndromes associated with FTLD might therefore improve survival as well as interim quality of life.
Finally, we note that there has been recent concern regarding international pharma investment in disorders of the CNS (Fineberg et al., 2013). However, we suggest that there is scope and grounds for optimism for progress towards effective symptomatic pharmacological therapies. Such treatments, based on restoring neurotransmitter deficits, would reduce the cost, social and health burden of FTLD.
Funding
This review was funded by the Holt Fellowship (A.M.), the Wellcome Trust (JBR 103838), and the National Institute for Health Research Cambridge Biomedical Research Centre and Cambridge Brain Bank.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-isoxazolepropionic acid
- bvFTD
behavioural variant frontotemporal dementia
- CBD
corticobasal degeneration
- CBS
corticobasal syndrome
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- NMDA
N-methyl d-aspartate
- PPA
primary progressive aphasia
- PSP
progressive supranuclear palsy
- SPECT
single photon emission computed tomography
References
- Agarwal N, Renshaw PF. Proton MR spectroscopy—detectable major neurotransmitters of the brain: biology and possible clinical applications. AJNR Am J Neuroradiol 2012; 33: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani D, Prato F, Fenoglio C, Batelli S, Dusi S, De Mauro S, et al. Association study to evaluate the serotonin transporter and apolipoprotein E genes in frontotemporal lobar degeneration in Italy. J Hum Genet 2008; 53: 1029–33. [DOI] [PubMed] [Google Scholar]
- Alexander SK, Rittman T, Xuereb JH, Bak TH, Hodges JR, Rowe JB. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry 2014; 85: 925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Lenzen M, Friederici AD, Schleicher A, Morosan P, Palomero-Gallagher N, et al. Broca’s region: novel organizational principles and multiple receptor mapping. PLoS Biol 2010; 8: e1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneser JM, Jox RJ, Borasio GD. Inappropriate sexual behaviour in a case of ALS and FTD: successful treatment with sertraline. Amyotroph Lateral Scler 2007; 8: 189–90. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold G, Schwarz J, Tatsch K, Kraft E, Wächter T, Bandmann O, et al. Steele-Richardson-Olszewski-syndrome: the relation of dopamine D2 receptor binding and subcortical lesions in MRI. J Neural Transm 2002; 109: 503–12. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Toledo JB, Appleby DH, Xie SX, Wang LS, Baek Y, et al. Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. J Comp Neurol 2013; 521: 4339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacol Ther 2013; 137: 119–31. [DOI] [PubMed] [Google Scholar]
- Asahina M, Suhara T, Shinotoh H, Inoue O, Suzuki K, Hattori T. Brain muscarinic receptors in progressive supranuclear palsy and Parkinson’s disease: a positron emission tomographic study. J Neurol Neurosurg Psychiatry 1998; 65: 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005; 28: 403–50. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Waterhouse B. Locus coeruleus: from global projection system to adaptive regulation of behavior. Brain Res 2016; 1645: 75–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizabal-Carvallo JF, Jankovic J. Parkinsonism, movement disorders and genetics in frontotemporal dementia. Nat Rev Neurol 2016; 12: 175–85. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442: 916–19. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5_HT receptors and their function. Neuropharmacology 1999; 38: 1083–152. [DOI] [PubMed] [Google Scholar]
- Baron JC, Maziere B, Loc’h C, Cambon H, Sgouropoulos P, Bonnet AM, et al. Loss of striatal [76Br]bromospiperone binding sites demonstrated by positron tomography in progressive supranuclear palsy. J Cereb Blood Flow Metab 1986; 6: 131–6. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8: 45–56. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ. Canonical microcircuits for predictive coding. Neuron 2012; 76: 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63: 182–217. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology 2013; 80: 1148–155. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 2008; 131: 732–46. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Boylan KB, Graff-Radford NR, Dejesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 2012; 135: 765–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999; 53: 795. [DOI] [PubMed] [Google Scholar]
- Borroni B, Grassi M, Agosti C, Premi E, Archetti S, Alberici A, et al. Establishing short-term prognosis in frontotemporal lobar degeneration spectrum: role of genetic background and clinical phenotype. Neurobiol Aging 2010; 31: 270–9. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Procter AW, Mann DMA, Snowden JS, Esiri MM, Neary D, et al. Imbalance of a serotonergic system in frontotemporal dementia: implication for pharmacotherapy. Psychopharmacology. 2008; 196: 603–10. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Boeve BF. Frontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies. Alzheimer Dis Assoc Disord 2007; 21: S79–87. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Knopman DS, Kaufer DI, Grossman M, Onyike C, Graf-Radford N, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2013; 12: 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Playford ED, Quinn N, Mathias CJ, et al. Striatal D2 receptor status in patients with Parkinson’s disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol 1992; 31: 184–92. [DOI] [PubMed] [Google Scholar]
- Brunnström H, Friberg N, Lindberg E, Englund E. Differential degeneration of the locus coeruleus in dementia subtypes. Clin Neuropathol 2011; 30: 104–10. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci 2012; 4: 203–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T 1H-MRS study. Neuropsychopharmacology 2012; 37: 2764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 2007; 10: 1103–9. [DOI] [PubMed] [Google Scholar]
- Carlé NM, Meletis K, Siegle J, Cardin J, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 2011; 17: 537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro ME, Pascual J, Romon T, Pazos A. 5-HT 1B receptor binding in degenerative movement disorders. Brain Res 1998; 790: 323–8. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front Integr Neurosci 2013; 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaal S, Rowe J. Dopamine Transporter (DAT) imaging can be normal with neuropathologically confirmed Cortiobasal Degeneration. J Neurol Neurosurg Psychiatry 2013; 84: e2.59.25346970 [Google Scholar]
- Chandler DJ, Gao WJ, Waterhouse BD. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci USA 2014; 111: 6816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AY, Weirich E. Trial of zolpidem, eszopiclone, and other GABA agonists in a patient with progressive supranuclear palsy. Case Rep Med 2014; 2014: 107064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay Y, Léger L. Brain serotonergic circuitries. Dialogues Clin Neurosci 2010; 12: 471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinaclia G, Landwehrmeyer B. Serotoninergic terminal transporters are differentially affected in Parkinson’s disease and progressive supranuclear palsy: an autoradiographic study with [3H] citalopram. Neuroscience 1993; 54: 691–9. [DOI] [PubMed] [Google Scholar]
- Chow TW. Goals in symptomatic pharmacologic management of frontotemporal lobar degeneration. Am J Alzheimer’s Dis Other Dementias 2002; 17: 267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia R, Rossi C, Frosini D, Volterrani D, Siri C, Pagni C, et al. Dopamine transporter spect imaging in corticobasal syndrome. PLoS One 2011; 6: e18301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciranna L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr Neuropharmacol 2006; 4: 101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev 2006; 30: 1–23. [DOI] [PubMed] [Google Scholar]
- Cotter C, Armytage T, Crimmins D. The use of zolpidem in the treatment of progressive supranuclear palsy. J Clin Neurosci 2010; 17: 385–6. [DOI] [PubMed] [Google Scholar]
- Coull JT, Sahakian BJ, Hodges JR. The α2 antagonist idazoxan remediates certain attentional and executive dysfunction in patients with dementia of frontal type. Psychopharmacology 1996; 123: 239–49. [DOI] [PubMed] [Google Scholar]
- Coyle-Gilchrist ITS, Dick KM, Patterson K, Vázquez Rodríquez P, Wehmann E, Wilcox A, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 2016; 86: 1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer MJ, Simmons A, et al. Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb Cortex 2014; 24: 174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfo E, Albasanz JL, Rodriguez A, Martín M, Ferrer I. Abnormal group I metabotropic glutamate receptor expression and signaling in the frontal cortex in Pick disease. J Neuropathol Exp Neurol 2005; 64: 638–47. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011; 69: 680–94. [DOI] [PubMed] [Google Scholar]
- Daniele A, Moro E, Bentivoglio AR. Zolpidem in progressive supranuclear palsy. N Engl J Med 1999; 341: 543–4. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine—searching for the connections. Br J Pharmacol 2012; 167: 324–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG, Mobius HJ, Stoffler A, Quack G. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease–a unified glutamatergic hypothesis on the mechanism of action. Neurotox Res 2000; 2: 85–97. [DOI] [PubMed] [Google Scholar]
- Dash SK. Zolpidem in progressive supranuclear palsy. Case Rep Neurol Med 2013; 2013: 250865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Li P. The 5-HT6 receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacology 2001; 25: 662–8. [DOI] [PubMed] [Google Scholar]
- Deakin JB, Rahman S, Nestor PJ, Hodges JR, Sahakian BJ. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology 2004; 172: 400–8. [DOI] [PubMed] [Google Scholar]
- Decker JM, Krüger L, Sydow A, Dennissen FJA, Siskova Z, Mandelkow E, et al. The Tau/A152T mutation, a risk factor for frontotemporal spectrum disorders, leads to NR2B receptor mediated excitotoxicity. EMBO Rep 2016; 17: 552–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Gao K, Jankovic J. The role of FUS gene variants in neurodegenerative diseases. Nat Rev Neurol 2014; 10: 337–48. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999; 246 (Suppl 2): II6–15. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 2010; 23: 394–400. [DOI] [PubMed] [Google Scholar]
- Engelborghs S, Vloeberghs E, Le Bastard N, Van Buggenhout M, Mariën P, Somers N, et al. The dopaminergic neurotransmitter system is associated with aggression and agitation in frontotemporal dementia. Neurochem Int 2008; 52: 1052–60. [DOI] [PubMed] [Google Scholar]
- Engelborghs S, Vloeberghs E, Maertens K, Marescau B, De Deyn PP. Evidence for an association between the CSF HVA:5-HIAA ratio and aggressiveness in frontotemporal dementia but not in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004; 75: 1080. [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology 1997; 203: 829–36. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol 1997; 48: 649–84. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Neurons and their dendrites in frontotemporal dementia. Dement Geriatr Cogn Disord 1999; 10: 55–60. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Haddad PM, Carpenter L, Gannon B, Sharpe R, Young AH, et al. The size, burden and cost of disorders of the brain in the UK. J Psychopharmacol 2013; 27: 761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, Scheinin M, Shahid M, Lehto J, Borroni E, Bang-Andersen B, et al. Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology 2015; 232: 4129–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster NL, Minoshima S, Johanns J, Little R, Heumann ML, Kuhl DE, et al. PET measures of benzodiazepine receptors in progressive supranuclear palsy. Neurology 2000; 54: 1768–73. [DOI] [PubMed] [Google Scholar]
- Foster NL, Wilhelmsen K, Sima AAF, Jones MZ, Damato CJ, Gilman S, et al. Frontotemporal dementia and parkinsonism linked to chromosome 17. Ann Neurol 1997; 41: 706–15. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol 2005; 57: 216–25. [DOI] [PubMed] [Google Scholar]
- Francis PT, Holmes C, Webster MT, Stratmann GC, Procter AW, Bowen DM. Preliminary neurochemical findings in non-Alzheimer dementia due to lobar atrophy. Dementia 1993; 4: 172–7. [DOI] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’ s disease: a review of progress. J Neurol Neurosurg Psychiatry 1999; 66: 137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Narendran R, Mason NS, Vora S, Litschge M, et al. Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology 2009; 34: 624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 2009; 32: 209–24. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Pizzolato G, Bianchetti A, Chierichetti F, Ferlin G, Battistin L, et al. Single photon emission computed tomography with [99Tc]-HM-PAO and [123I]-IBZM in Alzheimer’s disease and dementia of frontal type: preliminary results. Acta Neurol Scand 1994; 89: 199–203. [DOI] [PubMed] [Google Scholar]
- Gaetz M, Edgar J, Wang D, Roberts P. Relating MEG measured motor cortical oscillations to resting γ- aminobutyric acid (GABA) concentration. Neuroimage 2011; 15: 616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Roberts TP, Singh K, Muthukumaraswamy SD. Functional and structural correlates of the aging brain: relating visual cortex (V1) gamma band responses to age-related structural change. Hum Brain Mapp 2012; 33: 2035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain 2008; 131: 1268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Lynch K, Ruan H, Almeida S, Verheyden JM, Seeley WW, et al. Alterations in microRNA-124 and AMPA receptors contribute to social behavioral deficits in frontotemporal dementia. Nat Med 2014; 20: 1444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghika J, Tennis M, Hoffman E, Schoenfeld D, Growdon J. Idazoxan treatment in progressive supranuclear palsy. Neurology 1991; 41: 986–91. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Luthert PJ, Marsden CD. Corticobasal degeneration. Brain 1989; 112: 1171–92. [DOI] [PubMed] [Google Scholar]
- Gilbert JR, Symmonds M, Hanna MG, Dolan RJ, Friston KJ, Moran RJ. Profiling neuronal ion channelopathies with non-invasive brain imaging and dynamic causal models: case studies of single gene mutations. Neuroimage 2016; 124: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Nan B, Wang CN, Wang X, Junck L, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 2010; 74: 1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, et al. NMDA receptors, cognition and schizophrenia—testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology 2012; 62: 1401–12. [DOI] [PubMed] [Google Scholar]
- Gil-Navarro S, Lomeña F, Cot A, Lladó A, Montagut N, Castellví M, et al. Decreased striatal dopamine transporter uptake in the non-fluent/agrammatic variant of primary progressive aphasia. Eur J Neurol 2013; 20: 1459–66. [DOI] [PubMed] [Google Scholar]
- Goforth HW, Konopka L, Primeau M, Ruth A, O’Donnell K, Patel R, et al. Quantitative electroencephalography in frontotemporal dementia with methylphenidate response: a case study. Clin EEG Neurosci 2004; 35: 108–11. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast 2011; 2011: 723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cereb Cortex 2012; 22: 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 2: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday M, Radford H, Zents KAM, Molloy C, Moreno JA, Verity NC, et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 2017: 1768–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LA, Deteresa R, Tobias H, Alford M, Terry RD. Neocortical morphometry and cholinergic neurochemistry in Pick’s disease. Am J Pathol 1988; 131: 507–18. [PMC free article] [PubMed] [Google Scholar]
- Hardman CD, Halliday GM, McRitchie DA, Cartwright HR, Morris JG. Progressive supranuclear palsy affects both the substantia nigra pars compacta and reticulata. Exp Neurol 1997; 144: 183–92. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Role of the serotonin 5-HT2A receptor in learning. Learn Mem 2003; 10: 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw J, Daniel SE, Dickson D, Horoupian DS. Preliminary NINDS neuropathologic criteria for Steele- Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1994; 44: 2015–19. [DOI] [PubMed] [Google Scholar]
- Hazrati LN, Wong JC, Hamani C, Lozano AM, Poon YY, Dostrovsky JO, et al. Clinicopathological study in progressive supranuclear palsy with pedunculopontine stimulation. Mov Disord 2012; 27: 1304–7. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain 2000; 123 (Pt 7): 1410–21. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Black SE, Chow T, Cappell J, Tang-Wai DF, Lanctôt KL. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry 2012; 20: 789–97. [DOI] [PubMed] [Google Scholar]
- Hines RM, Hines DJ, Houston CM, Mukherjee J, Haydon PG, Tretter V, et al. Disrupting the clustering of GABAA receptor α2 subunits in the frontal cortex leads to reduced γ-power and cognitive deficits. Proc Natl Acad Sci USA 2013; 110: 16628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Shinotoh H, Shimada H, Aotsuka A, Tanaka N, Ota T, et al. Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain 2010; 133: 2058–68. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA 1987; 84: 5976–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy—the movement disorder society criteria. Mov Disord 2017; 32: 853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holemans S, Javoy F, Agid Y, Laterre EC, Maloteaux JM. [3H]MK-801 binding to NMDA glutamatergic receptors in Parkinson’s disease and progressive supranuclear palsy. Brain Res 1991; 565: 154–7. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat 2003; 26: 331–43. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O, Shannak K. Brain monoamines in progressive supranuclear palsy–comparison with idiopathic Parkinson’s disease. J Neural Transm Suppl 1994; 42: 219–27. [DOI] [PubMed] [Google Scholar]
- Huey E, Putnam K, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 2006; 66: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LE, Ghosh BC, Rowe JB. Reorganisation of brain networks in frontotemporal dementia and progressive supranuclear palsy. Neuroimage Clin 2013; 2: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LE, Rittman T, Regenthal R, Robbins TW, Rowe JB. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain 2015; 138: 1961–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–5. [DOI] [PubMed] [Google Scholar]
- Im JH, Chung SJ, Kim JS, Lee MC. Differential patterns of dopamine transporter loss in the basal ganglia of progressive supranuclear palsy and Parkinson’s disease: analysis with [(123)I]IPT single photon emission computed tomography. J Neurol Sci 2006; 244: 103–9. [DOI] [PubMed] [Google Scholar]
- Invernizzi RW, Garattini S. Role of presynaptic a2-adrenoceptors in antidepressant action: recent findings from microdialysis studies. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 819–27. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol 2016; 79: 272–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javoy-Agid F. Cholinergic and peptidergic systems in PSP. J Neural Transm Suppl 1994; 42: 205–18. [DOI] [PubMed] [Google Scholar]
- Jellinger K. The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1988; 51: 540–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Rademaker A, Weintraub S, Gitelman D, Wienecke C, Mesulam M. Pilot trial of memantine in primary progressive aphasia. Alzheimer Dis Assoc Disord 2010; 24: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncos JL, Hirsch EC, Malessa S, Duyckaerts C, Hersh LB, Agid Y. Mesencephalic cholinergic nuclei in progressive supranuclear palsy. Neurology 1991; 41: 25–30. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Gardberg M, Röyttä M, Seppänen M, Päivärinta M. Normal dopamine transporter SPECT in neuropathologically confirmed corticobasal degeneration. J Neurol 2013; 260: 1410–11. [DOI] [PubMed] [Google Scholar]
- Kalia L, Kalia SK, Salter M. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol 2008; 7: 742–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa I, Kwak S, Sasaki H, Muramoto O, Mizutani T, Hori A, et al. Studies on neurotransmitter markers of the basal ganglia in Pick’s disease, with special reference to dopamine reduction. J Neurol Sci 1988; 83: 63–74. [DOI] [PubMed] [Google Scholar]
- Kasashima S, Oda Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 2003; 105: 117–24. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Housden CR, Regenthal R, Barker RA, Müller U, Rowe J, et al. Targeting impulsivity in Parkinson’s disease using atomoxetine. Brain 2014; 137: 1986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Jesso S. Extrapyramidal syndromes in frontotemporal degeneration. J Mol Neurosci 2011; 45: 336–42. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Morlog D, Light M, Blair M, Davidson W, Jesso S, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord 2008; 25: 178–85. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Ichise M, Ballinger JR, Vines D, Erami SS, Tatschida T, et al. Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov Disord 2002; 17: 303–12. [DOI] [PubMed] [Google Scholar]
- Klaffke S, Kuhn AA, Plotkin M, Amthauer H, Harnack D, Felix R, et al. Dopamine transporters, D2 receptors, and glucose metabolism in corticobasal degeneration. Mov Disord 2006; 21: 1724–7. [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Goetz CG, Litvan I, Jellinger K, Verny M. Pharmacological therapy in progressive supranuclear palsy. Arch Neurol 1998; 55: 1099–102. [DOI] [PubMed] [Google Scholar]
- Kondo T. Drug intervention for freezing of gait resistant to dopaminergic therapy: a pilot study. Park Relat Disord 2006; 12: 63–6. [Google Scholar]
- Kujala J, Jung J, Bouvard S, Lecaignard F, Lothe A, Bouet R, et al. Gamma oscillations in V1 are correlated with GABAA receptor density: a multi-modal MEG and Flumazenil-PET study. Sci Rep 2015; 5: 16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctôt KL, Herrmann N, Ganjavi H, Black SE, Rusjan PM, Houle S, et al. Serotonin-1A receptors in frontotemporal dementia compared with controls. Psychiatry Res Neuroimaging 2007; 156: 247–50. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer B, Palacios JM. Alterations of neurotransmitter receptors and neurotransmitter transporters in progressive supranuclear palsy. J Neural Transm Suppl 1994; 42: 229–46. [DOI] [PubMed] [Google Scholar]
- Lange KW, Kornhuber J, Riederer P. Dopamine/glutamate interactions in Parkinson’s disease. Neurosci Biobehav Rev 1997; 21: 393–400. [DOI] [PubMed] [Google Scholar]
- Lansdall CJ, Coyle-Gilchrist IT, Jones PS, Rodriguez PV, Wilcox A, Wehmann E, et al. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain 2017; 140: 1792–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Salmon E, Garraux G, Peigneux P, Lemaire C, Degueldre C, et al. Fluorodopa uptake and glucose metabolism in early stages of corticobasal degeneration. J Neurol 1999; 246: 1151–8. [DOI] [PubMed] [Google Scholar]
- Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord 2004; 17: 355–9. [DOI] [PubMed] [Google Scholar]
- Leuzy A, Zimmer ER, Dubois J, Pruessner J, Cooperman C, Soucy JP, et al. In vivo characterization of metabotropic glutamate receptor type 5 abnormalities in behavioral variant FTD. Brain Struct Funct 2016; 221: 1387–402. [DOI] [PubMed] [Google Scholar]
- Levy R, Ruberg M, Herrero MT, Villares J, Javoy-Agid F, Agid Y, et al. Alterations of GABAergic neurons in the basal ganglia of patients with progressive supranuclear palsy: an in situ hybridization study of GAD67 messenger RNA. Neurology 1995; 45: 127–34. [DOI] [PubMed] [Google Scholar]
- Liepelt I, Gaenslen A, Godau J, Di Santo A, Schweitzer KJ, Gasser T, et al. Rivastigmine for the treatment of dementia in patients with progressive supranuclear palsy: clinical observations as a basis for power calculations and safety analysis. Alzheimers Dement 2010; 6: 70–4. [DOI] [PubMed] [Google Scholar]
- Litvan I, Blesa R, Clark K, Nichelli P, Atack JR, Mouradian MM, et al. Pharmacological evaluation of the cholinergic system in progressive supranuclear palsy. Ann Neurol 1994; 36: 55–61. [DOI] [PubMed] [Google Scholar]
- Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 1996; 55: 97–105. [DOI] [PubMed] [Google Scholar]
- Litvan I, Phipps M, Pharr VL, Hallett M, Grafman J, Salazar A. Randomized placebo-controlled trial of donepezil in patients with progressive supranuclear palsy. 2001; 57: 467–73. [DOI] [PubMed] [Google Scholar]
- MacKenzie IRA, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010; 119: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoveanu J, Hornboll B, Elliott R, Erritzoe D, Paulson OB, Siebner H, et al. Serotonin 2A receptors, citalopram and tryptophan-depletion: a multimodal imaging study of their interactions during response inhibition. Neuropsychopharmacology 2013; 38: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996; 14: 301–7. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci 2007; 30: 343–9. [DOI] [PubMed] [Google Scholar]
- Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, et al. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. Am J Neuroradiol 2001; 22: 1813–24. [PMC free article] [PubMed] [Google Scholar]
- Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson’s disease: a pilot open-label study. Mov Disord 2009; 24: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazere J, Meissner WG, Mayo W, Sibon I, Lamare F, Guilloteau D, et al. Progressive supranuclear palsy: in vivo SPECT imaging of presynaptic vesicular acetylcholine transporter with [123I]-iodobenzovesamicol. Radiology 2012; 265: 537–43. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 2008; 153: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ruge W, Iwangoff P, Reichlmeier K. Neurochemical enzyme changes in Alzheimer’s and Pick’s disease. Arch Gerontol Geriatr 1984; 3: 161–5. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 2000; 130: 1007S–15S. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry 2007; 15: 84–7. [DOI] [PubMed] [Google Scholar]
- Mirsky JB, Heuer HW, Jafari A, Kramer JH, Schenk AK, Viskontas IV, et al. Anti-saccade performance predicts executive function and brain structure in normal elders. Cogn Behav Neurol 2011; 24: 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Inagaki T, Horiguchi J. Fluvoxamine for the treatment of depression and parkinsonism in progressive supranuclear palsy. Int J Psychiatry Clin Pract 2002; 6: 45–7. [DOI] [PubMed] [Google Scholar]
- Moran RJ, Campo P, Symmonds M, Stephan KE, Dolan RJ, Friston KJ, et al. Free energy, precision and learning: the role of cholinergic neuromodulation Europe PMC Funders Group. J Neurosci 2013; 33: 8227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RJ, Symmonds M, Stephan KE, Friston KJ, Dolan RJ. An in vivo assay of synaptic function mediating human cognition. Curr Biol 2011; 21: 1320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Rivastigmine in frontotemporal dementia. Drugs Aging 2004; 21: 931–7. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms: a randomized, controlled, open 14-month study. Eur Neurol 2003a; 49: 13–19. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Bava A. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Demen 2003b; 18: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Oda M, Komori T, Arai N, Takanashi M, Mizutani T, et al. Lewy bodies in progressive supranuclear palsy. Acta Neuropathol 2002; 104: 273–8. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 1986; 319: 774–6. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Karaconji T, Hardman CD, Halliday GM. Excessive dopamine neuron loss in progressive supranuclear palsy. Mov Disord 2008; 23: 607–10. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. The use of magnetoencephalography in the study of psychopharmacology (pharmaco-MEG). J Psychopharmacol 2014; 28: 815–29. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA 2009; 106: 8356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JFM, Wilson SJ, Nutt DJ, Hamandi K, Lingford-Hughes A, et al. Elevating endogenous GABA levels with GAT-1 blockade modulates evoked but not induced responses in human visual cortex. Neuropsychopharmacology 2013; 38: 1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S, Arai H, Iwamoto N, Ohwada J, Ichimiya Y, Nakamura M, et al. A juvenile case of frontotemporal dementia: neurochemical and neuropathological investigations. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19: 1251–61. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Tanji H, Nomura H, Saito H, Itoyama Y, Kimura I, et al. PET study of cerebral glucose metabolism and fluorodopa uptake in patients with corticobasal degeneration. J Neurol Sci 1996; 139: 210–17. [PubMed] [Google Scholar]
- Napier TC, Corvol J, Grace AA, Roitman JD. Linking neuroscience with modern concepts of impulse control disorders in Parkinson’s disease. Mov Disord 2015; 30: 141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Olney JW. NMDA receptor function, memory, and brain aging. Dialogues Clin Neurosci 2000; 2: 219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology 2008; 200: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd S, Curtin D, Waite AJ, Roberts K, Pender N, Reid V, et al. C9ORF72 expansion in amyotrophic lateral sclerosis/frontotemporal dementia also causes Parkinsonism. Mov Disord 2012; 27: 1072–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odawara T, Shiozaki K, Iseki E, Hino H, Kosaka K. Alterations of muscarinic acetylcholine receptors in atypical Pick’s disease without Pick bodies. J Neurol Neurosurg Psychiatry 2003; 74: 965–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med 2012; 53: 399–406. [DOI] [PubMed] [Google Scholar]
- Olanow CW. The scientific basis for the current treatment of Parkinson’s disease. Annu Rev Med 2004; 55: 41–60. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 2002; 3: 715–27. [DOI] [PubMed] [Google Scholar]
- Oyanagi C. Comparison of striatal dopamine D2 receptors in Parkinson’s disease and progressive supranuclear palsy patients using [I] iodobenzofuran single-photon emission computed tomography. J Neuroimaging 2002; 12: 316–24. [DOI] [PubMed] [Google Scholar]
- Oyanagi K, Tsuchiya K, Yamazaki M, Ikeda K. Substantia nigra in progressive supranuclear palsy, corticobasal degeneration, and parkinsonism-dementia complex of Guam: specific pathological features. J Neuropathol Exp Neurol 2001; 60: 393–402. [DOI] [PubMed] [Google Scholar]
- Padovani A, Agosti C, Premi E, Bellelli G, Borroni B. Extrapyramidal symptoms in frontotemporal dementia: prevalence and clinical correlations. Neurosci Lett 2007; 422: 39–42. [DOI] [PubMed] [Google Scholar]
- Pal PK, Wszolek ZK, Kishore A, De La Fuente-Fernandez R, Sossi V, Uitti RJ, et al. Positron emission tomography in pallido-ponto-nigral degeneration (PPND) family (frontotemporal dementia with parkinsonism linked to chromosome 17 and point mutation in tau gene). Park Relat Disord 2001; 7: 81–8. [DOI] [PubMed] [Google Scholar]
- Pascual J, Berciano J, Grijalba B, del OE, Gonzalez AM, Figols J, et al. Dopamine D1 and D2 receptors in progressive supranuclear palsy: an autoradiographic study. Ann Neurol 1992; 32: 703–7. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Ghosh BCP, Hughes L, Carpenter RHS, Barker RA, Rowe JB. Saccadic latency in Parkinson’s disease correlates with executive function and brain atrophy, but not motor severity. Neurobiol Dis 2011; 43: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 2012; 76: 116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering-Brown SM, Richardson AMT, Snowden JS, McDonagh AM, Burns A, Braude W, et al. Inherited frontotemporal dementia in nine British families associated with intronic mutations in the tau gene. Brain 2002; 125: 732–51. [DOI] [PubMed] [Google Scholar]
- Pierot L, Desnos C, Blin J, Pierrot L, Scherman D, Javoy-agid F, et al. D1 and D2-type dopamine receptors in patients with Parkinson’s disease and progressive supranuclear palsy. J Neurol Sci 1988; 86: 291–306. [DOI] [PubMed] [Google Scholar]
- Pijnenburg YAL, Sampson EL, Harvey RJ, Fox NC, Rossor MN. Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. Int J Geriatr Psychiatry 2003; 18: 67–72. [DOI] [PubMed] [Google Scholar]
- Pirker S, Perju-Dumbrava L, Kovacs GG, Traub-Weidinger T, Asenbaum S, Pirker W. Dopamine D2 receptor SPECT in corticobasal syndrome and autopsy-confirmed corticobasal degeneration. Park Relat Disord 2013; 19: 222–6. [DOI] [PubMed] [Google Scholar]
- Pirker S, Perju-Dumbrava L, Kovacs GG, Traub-Weidinger T, Pirker W. Progressive dopamine transporter binding loss in autopsy-confirmed corticobasal degeneration. J Parkinsons Dis 2015; 5: 907–12. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol 2012; 22: 239–58. [DOI] [PubMed] [Google Scholar]
- Poujois A, Vidailhet M, Trocello JM, Bourdain F, Gaymard B, Rivaud-Péchoux S. Effect of gabapentin on oculomotor control and parkinsonism in patients with progressive supranuclear palsy. Eur J Neurol 2007; 14: 1060–2. [DOI] [PubMed] [Google Scholar]
- Premi E, Archetti S, Pilotto A, Seripa D, Paghera B, Padovani A, et al. Functional genetic variation in the serotonin 5-HTTLPR modulates brain damage in frontotemporal dementia. Neurobiol Aging 2015; 36: 446–51. [DOI] [PubMed] [Google Scholar]
- Procter AW, Qurne M, Francis PT. Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord 1999; 10: 80–4. [DOI] [PubMed] [Google Scholar]
- Rae CL, Nombela C, Rodríguez PV, Ye Z, Hughes LE, Jones PS, et al. Atomoxetine restores the response inhibition network in Parkinson’s disease. Brain 2016; 139: 2235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Robbins TW, Hodges JR, Mehta MA, Nestor PJ, Clark L, et al. Methylphenidate (’Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology 2006; 31: 651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, et al. Distinct subtypes of behavioral-variant frontotemporal dementia based on patterns of network degeneration HHS public access. JAMA Neurol 2016; 73: 1078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sieradzan K, Peyro-Saint-Paul H, Thalamas C, Brefel-Courbon C, Senard JM, et al. Efaroxan, an alpha-2 antagonist, in the treatment of progressive supranuclear palsy. Mov Disord 1998; 13: 673–6. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Möbius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348: 1333–41. [DOI] [PubMed] [Google Scholar]
- Revesz T, Sangha H, Daniel SE. The nucleus raphe interpositus in the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Brain 1996; 119 (Pt 4): 1137–43. [DOI] [PubMed] [Google Scholar]
- Reynolds G, Sally P. Brain GABA levels in asymptomatic Huntington’s disease. N Engl J Med 1990; 323: 682–2. [DOI] [PubMed] [Google Scholar]
- Riedl L, Mackenzie IR, Forstl H, Kurz A, Diehl-Schmid J. Frontotemporal lobar degeneration: current perspectives. Neuropsychiatr Dis Treat 2014; 10: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin A, Quitkin F, Kane J, Struve F, Klein DF. Are prophylactic antiparkinson drugs necessary? A Controlled Study of procyclidine withdrawal. Arch Gen Psychiatry 1978; 35: 483–9. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Laine M, Kaasinen V, Norvasuo-Heila MK, Nagren K, Helenius H. Striatal dopamine transporter and extrapyramidal symptoms in frontotemporal dementia. Neurology 2002; 58: 1489–93. [DOI] [PubMed] [Google Scholar]
- Rittman T, Coyle-Gilchrist IT, Rowe JB. Managing cognition in progressive supranuclear palsy. Neurodegener Dis Manag 2016; 6: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ESJ, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 2008; 33: 1028–37. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT, Albala B, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with alzheimers-disease. Neurology 1998; 50: 136–45. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Isaacs AM, Mizlienska S, Mead S, Lashley T, Wray S, et al. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 2015a; 14: 291–301. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the genetic frontotemporal dementia initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 2015b; 14: 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Paviour D, Bronstein AM, O’Sullivan SS, Lees A, Warren JD, et al. Progressive supranuclear palsy syndrome presenting as progressive nonfluent aphasia: a neuropsychological and neuroimaging analysis. Mov Disord 2010; 25: 179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. DREADDs for neuroscientists. Neuron 2016; 89: 683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Rittman T. The basal ganglia in cognitive disorders. In: Husain M, Schott J, editors. Oxford textbook of cognitive neurology and dementia. Oxford: Oxford University Press; 2016. p. 69–80. [Google Scholar]
- Rowe JB, Hughes L, Ghosh BCP, Eckstein D, Williams-Gray CH, Fallon S, et al. Parkinson’s disease and dopaminergic therapy—differential effects on movement, reward and cognition. Brain 2008; 131: 2094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Saunders JR, Durantou F, Robbins TW. Systemic idazoxan impairs performance in a non-reversal shift test: implications for the role of the central noradrenergic systems in selective attention. J Psychopharmacol 1996; 10: 188–94. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacol 2005; 30: 633–9. [DOI] [PubMed] [Google Scholar]
- Ruberg M, Javoy-Agid F, Hirsch E, Scatton B, LHeureux R, Hauw JJ, et al. Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurol 1985; 18: 523–9. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Coull JJ, Hodges JR. Selective enhancement of executive function by idazoxan in a patient with dementia of the frontal lobe type. J Neurol Neurosurg Psychiatry 1994; 57: 120–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 2009; 10: 211–23. [DOI] [PubMed] [Google Scholar]
- Sarac H, Zagar M, Davorka V, Henigsberg Ne, Bilic E, Pavlisa G. Magnetic resonance imaging and magnetic resonance spectroscopy in a patient with amyotrophic lateral sclerosis and frontotemporal dementia. Coll Antropol 2008; 32: 205–10. [PubMed] [Google Scholar]
- Sawle GV, Brooks DJ, Marsden CD, Frackowiak RSJ. Corticobasal degeneration—a unique pattern of regional cortical oxygen hypometabolism and striatal fluorodopa uptake demonstrated by positron emmission tomography. Brain 1991; 114: 541–56. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol 2015; 25: R1051–6. [DOI] [PubMed] [Google Scholar]
- Sedaghat F, Gotzamani-Psarrakou A, Dedousi E, Arnaoutoglou M, Psarrakos K, Baloyannis I, et al. Evaluation of dopaminergic function in frontotemporal dementia using I-FP-CIT single photon emission computed tomography. Neurodegener Dis 2007; 4: 382–5. [DOI] [PubMed] [Google Scholar]
- Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry 2011; 82: 476–86. [DOI] [PubMed] [Google Scholar]
- Selden N. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998; 121: 2249–57. [DOI] [PubMed] [Google Scholar]
- Seppi K, Scherfler C, Donnemiller E, Al E, Contribution O. Topography of dopamine transporter availability in progressive supranuclear palsy: a voxelwise [123i]β-cit spect analysis. Arch Neurol 2006; 63: 1154–60. [DOI] [PubMed] [Google Scholar]
- Servello D, Zekaj E, Saleh C, Menghetti C, Porta M. Long-term follow-up of deep brain stimulation of peduncolopontine nucleus in progressive supranuclear palsy: report of three cases. Surg Neurol Int 2014; 5: S416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotoh H, Namba H, Yamaguchi M, Fukushi K, Nagatsuka SI, Iyo M, et al. Positron emission tomographic measurement of acetylcholinesterase activity reveals differential loss of ascending cholinergic systems in Parkinson’s disease and progressive supranuclear palsy. Ann Neurol 1999; 46: 62–9. [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol 2006; 6: 108–13. [DOI] [PubMed] [Google Scholar]
- Siuda J, Fujioka S, Wszolek ZK. Parkinsonian syndrome in familial frontotemporal dementia. Park Relat Disord 2014; 20: 957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren M, Minthon L, Passant U, Blennow K, Wallin A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer’s disease. Neurobiol Aging 1998; 19: 379–84. [DOI] [PubMed] [Google Scholar]
- Skillback T, Farahmand B, Bartlett JW, Rosen C, Mattsson N, Nagga K, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014; 83: 1945–53. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Markesbery WR. Altered serotonergic and cholinergic synaptic markers in Pick’s disease. Arch Neurol 1991; 48: 796–9. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Woeltx VM, Marksebery WR. Alterations in brain monoamine oxidase activity in aging, Alzheimer’s Disease, and Pick’s Disease. Arch Neurol 1991; 48: 718–21. [DOI] [PubMed] [Google Scholar]
- Stamelou M, Höglinger G. A review of treatment options for progressive supranuclear palsy. CNS Drugs 2016; 30: 629–36. [DOI] [PubMed] [Google Scholar]
- Stamelou M, Matusch A, Elmenhorst D, Hurlemann R, Eggert KM, Zilles K, et al. Nigrostriatal upregulation of 5-HT2A receptors correlates with motor dysfunction in progressive supranuclear palsy. Mov Disord 2009; 24: 1170–5. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Desmond TJ, Albin RL, Frey KA. Cholinergic vesicular transporters in progressive supranuclear palsy. Neurology 2002; 58: 1013–18. [DOI] [PubMed] [Google Scholar]
- Teipel S, Raiser T, Riedl L, Riederer I, Schroeter ML, Bisenius S, et al. Atrophy and structural covariance of the cholinergic basal forebrain in primary progressive aphasia. Cortex 2016; 83: 124–35. [DOI] [PubMed] [Google Scholar]
- Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol 2014; 16: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitti RJ, Ahlskog JE, Maraganore DM, Muenter MD, Atkinson EJ, Cha RH, et al. Levodopa therapy and survival in idiopathic Parkinson’s disease: Olmsted County project. Neurology 1993; 43: 1918–26. [DOI] [PubMed] [Google Scholar]
- Vermeiren Y, Le Bastard N, Van Hemelrijck A, Drinkenburg WH, Engelborghs S, De Deyn PP. Behavioral correlates of cerebrospinal fluid amino acid and biogenic amine neurotransmitter alterations in dementia. Alzheimers Dement 2013; 9: 488–98. [DOI] [PubMed] [Google Scholar]
- Vermeiren Y, Janssens J, Aerts T, Martin JJ, Sieben A, Van Dam D, et al. Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer’s disease. J Alzheimers Dis 2016; 53: 1079–96. [DOI] [PubMed] [Google Scholar]
- Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann Neurol 2011; 69: 375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 2013; 77: 736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmus BA, Sekar DR, McCutchen E, Schellenberg GD, Roberts RC, McMahon LL, et al. Tau-Mediated NMDA receptor impairment underlies dysfunction of a selectively vulnerable network in a mouse model of frontotemporal dementia. J Neurosci 2014; 34: 16482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren NM, Piggott MA, Greally E, Lake M, Lees AJ, Burn DJ. Basal ganglia cholinergic and dopaminergic function in progressive supranuclear palsy. Mov Disord 2007a; 22: 1594–600. [DOI] [PubMed] [Google Scholar]
- Warren NM, Piggott MA, Lees AJ, Burn DJ. The basal ganglia cholinergic neurochemistry of progressive supranuclear palsy and other neurodegenerative diseases. J Neurol Neurosurg Psychiatry 2007b; 78: 571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren NM, Piggott MA, Perry EK, Burn DJ. Cholinergic systems in progressive supranuclear palsy. Brain 2005; 128: 239–49. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Gibson R, Coppola R, Jones DW, Molchan S, Sunderland T, et al. The distribution of cerebral muscarinic acetylcholine receptors in vivo in patients with dementia. A controlled study with 123IQNB and single photon emission computed tomography. Arch Neurol 1991; 48: 169–76. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol 2005; 62: 1402–8. [DOI] [PubMed] [Google Scholar]
- Williams S, Boksa P. Gamma oscillations and schizophrenia. J Psychiatry Neurosci 2010; 35: 75–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004; 5: 483–94. [DOI] [PubMed] [Google Scholar]
- Wood PL, Etienne P, Lal S, Nair NP, Finlayson MH, Gauthier S, et al. A post-mortem comparison of the cortical cholinergic system in Alzheimer’s disease and Pick’s disease. J Neurol Sci 1983; 62: 211–17. [DOI] [PubMed] [Google Scholar]
- Woodside J, Lamb R, Chelban V, Burn D, Church A, Gerhard A, et al. PROSPECT: a UK-based longitudinal observational study of PSP, CBD, MSA and atypical Parkinsonism syndromes [abstract]. Mov Disord 2016; 31: S87–8. [Google Scholar]
- Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol 2001; 101: 256–70. [DOI] [PubMed] [Google Scholar]
- Yates CM, Simpson J, Maloney AFJ, Gordon A. Neurochemical observations in a case or Pick’s disease. J Neurol Sci 1980; 48: 257–63. [DOI] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, et al. Improving response inhibition in Parkinson’s disease with atomoxetine. Biol Psychiatry 2015; 77: 740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama JS, Bonham LW, Sturm VE, Adhimoolam B, Karydas A, Coppola G, et al. The 5-HTTLPR variant in the serotonin transporter gene modifies degeneration of brain regions important for emotion in behavioral variant frontotemporal dementia Serotonin transporter in bvFTD. Neuroimage Clin 2015; 9: 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Rittman T, Nombela C, Fois A, Coyle-Gilchrist I, Barker RA, et al. Different decision deficits impair response inhibition in progressive supranuclear palsy and Parkinson’s disease. Brain 2016; 139: 161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.