Abstract
Background
Clinical and experimental studies have revealed that liraglutide has multiple anti-diabetes biological effects. However, little is known about its role in autophagy and pancreatic β cell proliferation. This study aimed to assessed the effects of liraglutide on pancreatic β cell proliferation and autophagy in a mouse model of type 2 diabetes.
Material/Methods
The effect of liraglutide on autophagy and proliferation in pancreatic β cells was investigated using a high-fat-fed and streptozotocin-induced mouse model of type 2 diabetes.
Results
Liraglutide significantly improved the symptoms of high-fat-fed (HFD) and streptozotocin (STZ)-induced type 2 diabetic mice, as indicated by body weight gain, reduction of blood glucose and plasma insulin, and enhanced sensitivity to insulin. The results of quantitative real-time polymerase chain reaction and Western blot analysis showed that liraglutide upregulated AGT5 expression and promoted the conversion of LC3-I to LC3-II, thus improving the defective autophagy. In addition, we observed that both mRNA and protein expressions of PCNA and Ki-67 were upregulated by liraglutide treatment. Immunocytochemical staining results showed that the number of PCNA- or Ki-67-positive cells in pancreatic islet tissues in the HFD + STZ + liraglutide group were increased compared with the HFD + STZ group.
Conclusions
These results strongly suggest that liraglutide is able to enhance autophagy and promote pancreatic β cell proliferation. This study improves our insights into the mechanism by which liraglutide treatment relieves diabetes, and provides experimental evidence for clinical utilization of liraglutide in type 2 diabetes treatment.
MeSH Keywords: Autophagy; Cell Proliferation; Diabetes Mellitus, Type 2; Glucagon-Like Peptide 1; Insulin-Secreting Cells; Cell Proliferation
Background
The incidence of type 2 diabetes has been increasing in recent years [1] and has become a major threat to human health around the world. Currently, many drugs are used for type 2 diabetes treatment, such as metformin (an insulin sensitizer), glibenclamide (an insulin secretagogue), pioglitazone (a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist, exenatide (a glucagon-like peptide-1 (GLP-1) receptor agonist, and sitagliptin (a dipeptidyl peptidase-4 (DPP-4) inhibitor) [2]. However, most of these agents cause unpleasant adverse effects. Thus, the study of basic pharmacological mechanisms of oral anti-diabetic agents will be helpful to improve drug efficacy and reduce adverse drug reactions [3]. Streptozotocin (STZ) is used extensively for ameliorating both insulin-dependent and non-insulin-dependent diabetes. Clinically, low-dose STZ is used extensively for inducing a mild impairment of insulin secretion, which is similar to the later stage of type 2 diabetes. The combination of high-fat-fed (HFD) and low-dose STZ model has been considered as an ideal experimental model of type 2 diabetes [4,5].
Glucagon-like peptide-1 (GLP-1) has been shown to regulate glucose ingestion and the synthesis of insulin, and inhibits the apoptosis of islet β cells and slow gastric emptying. GLP-1 is considered a potential therapeutic target for diabetes treatment [6], but GLP-1 cannot be used immediately to treat diabetes since it can be degraded easily by dipeptidyl peptidase IV. Liraglutide, a human GLP-1 analogue, is useful in the clinical treatment of diabetes because that it exhibits the pleiotropic roles of GLP-1 and cannot be degraded easily. It has been reported that liraglutide was used as a once-daily agent for adult patients with type 2 diabetes and was approved by the US Food and Drug Administration [7]. Previous studies suggested that liraglutide maintained glucose homeostasis by moderating gastric emptying, reducing food intake, accelerating glucose-dependent insulin secretion, and promoting pancreatic islet function [8].
The pathogenesis of pancreatic β cell dysfunction in type 2 diabetes is still unclear. β cells in patient with diabetes impairs the ability of the body to respond to a glucose challenge and are unable to mount a well-timed response. During the process of diabetes, and in animal experimental models of type 2 diabetes, insufficient β cell mass is due to an imbalance between the rates of proliferation and apoptosis. The increased rate of apoptosis in diabetic islets may be the leading cause of β cell function impairment. Proliferation cell nuclear antigen (PCNA) and Ki-67 are cell proliferation-related nuclear antigens and are upregulated in the process of cell proliferation [9]. PCNA and Ki-67 reflect the proliferative activity of the tissue cells. PCNA and Ki-67 are widely used as important markers of cell proliferation. Previous studies reported that β cell proliferation capacity is gradually attenuated with age in the condition of normal glucose tolerance. It has been reported that cell proliferation activity is gradually decreased with the development of diabetic pathology, which is one of the reasons for the decline in β cell mass.
In this study, we explored the effect of liraglutide on autophagy and pancreatic β cell proliferation in a high-fat-fed and streptozotocin-induced mouse model of type 2 diabetes.
Material and Methods
Animals
Male C57BL/6J mice, 8 weeks old, were purchased from Huafukang Bioscience Technology Co., Ltd. (Beijing, China). Mice were individually housed at 23±2°C and 50±10% relative humidity with a 12-h light-dark cycle. The mice were acclimatized for 1 week and then randomly divided into 2 initial groups: a control group (normal diet, 62% carbohydrates, 10% fat, and 28% protein) and an HFD group (28% carbohydrates, 59% fat, and 14% protein). After dietary treatments for 8 weeks, the mice in the HFD group were intraperitoneally injected with STZ (50 mg/kg/d) for 5 d, and the control group were injected with normal saline. To validate that the insulin-resistant mouse model was successfully established, fasting blood glucose levels in mice were assessed. Next, the HFD- and STZ-treated mice with a fasting blood glucose level higher than 16.7 mmol/L were randomly subdivided into 2 groups: an HFD + STZ group and an HFD + STZ + liraglutide group. The mice in the HFD + STZ + liraglutide group were injected subcutaneously with liraglutide (0.2 mg/kg/d) for 4 weeks [6], while the mice in the HFD + STZ group were injected subcutaneously with normal saline.
All the experiments were conducted according to the Guidelines for the Care and Use of Medical Laboratory Animals and were approved by the Ethics Committee of the First Affiliated Hospital of Henan University of Science and Technology.
Blood glucose test
Mice was weighed every week, and blood glucose was measured using a glucometer (Roche, Switzerland). Blood samples were collected from the tail vein into a heparin-coated tube.
ELISA
Blood samples were collected into a heparinized container and centrifuged at 600 g for 15 min at 4°C. Plasma insulin levels were determined with a commercial ELISA kit (Life Science Inc., Huston, TX, USA) in accordance with the instructions of the manufacturer.
Intraperitoneal glucose tolerance test (GTT) and insulin tolerance test (ITT)
The GTT and ITT were conducted after overnight fasting. Mice were injected intraperitoneally with glucose (1 g/kg body weight) or insulin (0.25 U/kg body weight). Blood was collected from the tail vein immediately at 0, 30, 60, 90, and 120 min after injection. Glucose tolerance is expressed as the area under the curve of glucose concentrations between 0 and 120 min.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from pancreatic tissues with Trizol reagent (Takara, Japan), and reversely transcribed into cDNA by using the Primescript RT reagent kit (Takara). ATG5, LC3, PCNA, and Ki-67 mRNA levels were measured by PCR with the ABI prism 7900 sequence detection system (Life Technologies, Carlsbad, CA, USA). β-actin was used an internal reference gene. Primers were synthesized by GenScript Corporation (Nanjing, China) and listed as follows: β-actin forward 5′-TGTTGGCATAGAGGTCTTTACGG-3′, reverse 5′-TGGGTATGGAATCCTGTGGCA-3′; ATG5 forward 5′-CCAACTTGCTTCACCCTGTA-3′, reverse 5′-TTTCAGTGGTGTGC CTTCAT-3′; LC3 forward 5′-CATGCCGTCCGAG-AAGACCT-3′, reverse 5′-GATGAGCCGGACATCTTCCACT-3′; PCNA forward 5′-GAGC AACTTGGAATCCCAGAACAGG-3′, reverse 5′-CCAAGCTCC CCACTCGCAGAAAACT-3′; Ki-67 forward 5′-ATTGAACCT GCGGAAGAGCTGA-3′, reverse 5′-GGAGCGCAGGGATATTC CCTTA-3′. The 2−ΔΔCt method was used to quantified the relative mRNA expression of each gene.
Immunohistochemistry (IHC)
The pancreatic tissues were embedded in paraffin and sectioned at a thickness of 3 μm and placed on a slide coated with poly-L-lysine. Tissue sections were transferred into the boiling citrate buffer for 15 min, washed by washing buffer, and put in hydrogen peroxide solution. Tissue sections were incubated with anti-PCNA (Abcam, Cambridge, MA, USA) or anti-Ki-67 antibody (Abcam) at 4°C overnight. Sections were washed with PBS buffer for 20 min and cultured in the horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Visualization was carried out using a DAB kit (Boster, Wuhan, China) in accordance with the manufacturer’s protocol and then restained with hematoxylin (Solarbio, Beijing, China). The number of positive cells was counted in 5 random fields from each tissue slide.
Western blot
Total protein was isolated from pancreatic tissues using ice-cold radioimmuno-precipitation assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China). Equal amounts of protein were processed by 14% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore Corporation, Billerica, MA, USA). Then, the membranes were blocked with a 5% skim milk solution and incubated with the appropriate primary antibody, including anti-ATG5 (Abcam), anti-LC3 (Cell Signaling Technology, Boston, USA), anti-PCNA (Abcam), anti-Ki-67 (Abcam), and anti-β-actin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The membranes were washed with TBST buffer for 30 min and incubated with the corresponding secondary antibody (Santa Cruz) at room temperature for 1 h. The bands were visualized using a chemiluminescence detection kit (Pierce, Rockford, IL, USA) after washing for 30 min. The intensity of each band was measured using Image J software (National Institutes of Health, NY, USA) and the relative protein expression was compared with β-actin.
Statistical analysis
All data were analyzed using SPSS 20.0 software and are expressed as the mean ± standard deviation (SD). The t test was used to test the significance of difference between 2 groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Liraglutide relieves diabetes in HFD and STZ-treated mice
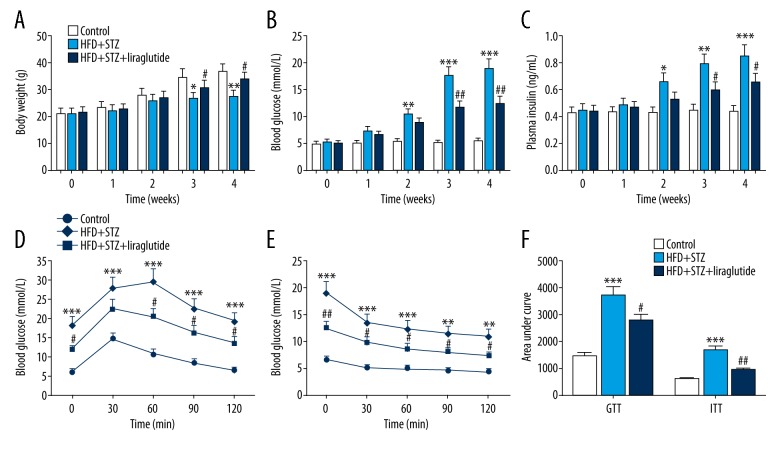
The body weights of mice in the HFD + STZ group were obviously lower than that in the control group. Compared with the HFD + STZ group, the body weights of mice in the HFD + STZ + liraglutide group were increased (Figure 1A). In the control group, blood glucose and plasma insulin levels changed little in the previous 4 weeks, while a significant increase was found in the HFD + STZ group from the second week. At the third and fourth week, the blood glucose and plasma insulin levels in mice treated with HFD + STZ + liraglutide were decreased in comparison with those in mice treated with HFD + STZ (Figure 1B, 1C). The results of GTT (Figure 1D) and ITT (Figure 1E) showed that control mice were tolerant to glucose, whereas HFD + STZ mice had an impaired tolerance to glucose. The level of fasting blood glucose of HFD + STZ mice was higher than those of control mice. Liraglutide-treated mice exhibited a remarkably improved glucose tolerance (Figure 1D). After intraperitoneal injection with insulin, the levels of blood glucose declined in mice treated with HFD + STZ + liraglutide as well as in mice treated with HFD + STZ (Figure 1E). The GTT and ITT areas under the curve after liraglutide treatment were reduced by about 25% and 44%, respectively, indicating that liraglutide improved insulin sensitivity in HFD and STZ-treated mice (Figure 1F).
Figure 1.
Liraglutide relieves diabetes in HFD and STZ-treated mice. Effects of liraglutide on body weight (A), blood glucose (B), plasma insulin (C), glucose tolerance (D), and insulin tolerance (E). (F) The area under the glucose or insulin tolerance curve generated in D and E. Data are presented as mean ±SD (n=6). * P<0.05 vs. control, ** P<0.01 vs. control, *** P<0.001 vs. control, # P<0.05 vs. HFD + STZ group, ## P<0.01 vs. HFD + STZ group.
Liraglutide treatment improves defective autophagy
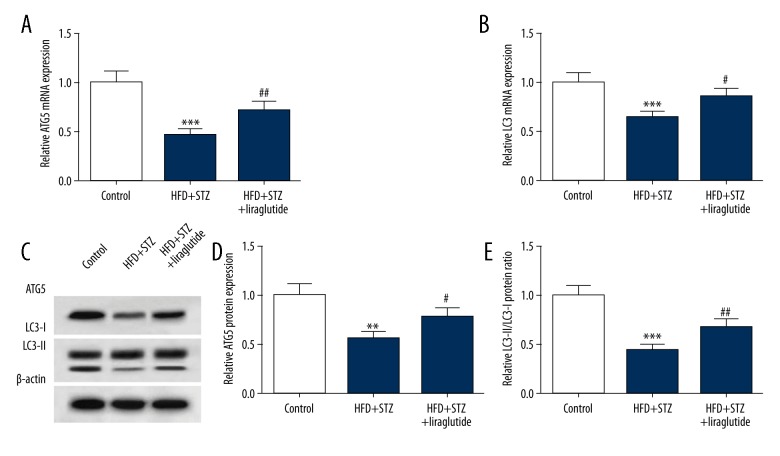
To study the effect of liraglutide on autophagy in mice, we assessed the expression of ATG5 and LC3. The result of qRT-PCR revealed that the expression of ATG5 mRNA in the HFD + STZ + liraglutide group (0.72±0.08) was markedly higher than that in the HFD + STZ group (0.46±0.06) (Figure 2A). Compared with the HFD + STZ group (0.65±0.07), the LC3 mRNA level was increased in the HFD + STZ + liraglutide group (0.86±0.09) (Figure 2B). Representative images of Western blots are shown in Figure 2C. The protein expression of ATG5 was downregulated in mice treated with HFD and STZ (0.58±0.07). The expression level of ATG5 protein in the HFD + STZ + liraglutide group (0.78±0.09) was increased compared with the HFD + STZ group (0.57±0.07) (Figure 2D). Compared with the HFD + STZ group (0.44±0.05), the relative LC3-II/LC3-I protein ratio was increased in the HFD + STZ + liraglutide group (0.69±0.08) (Figure 2E). These data show that liraglutide treatment enhanced ATG5 expression and the conversion of LC3-I to LC3-II in mice treated with HFD and STZ.
Figure 2.
Liraglutide treatment enhances autophagy in HFD and STZ-treated mice. (A, B) ATG5 and LC3 mRNA expression levels were detected by qRT-PCR. (C) Western blot analysis was conducted to detect ATG5 and LC3 protein expression. (D) Liraglutide upregulated the protein expression of ATG5 and accelerated the conversion of LC3-I to LC3-II. Data are presented as mean ±SD (n=6). ** P<0.01 vs. control, *** P<0.001 vs. control, # P<0.05 vs. HFD + STZ group, ## P<0.01 vs. HFD + STZ group.
Liraglutide treatment promotes pancreatic β cell proliferation
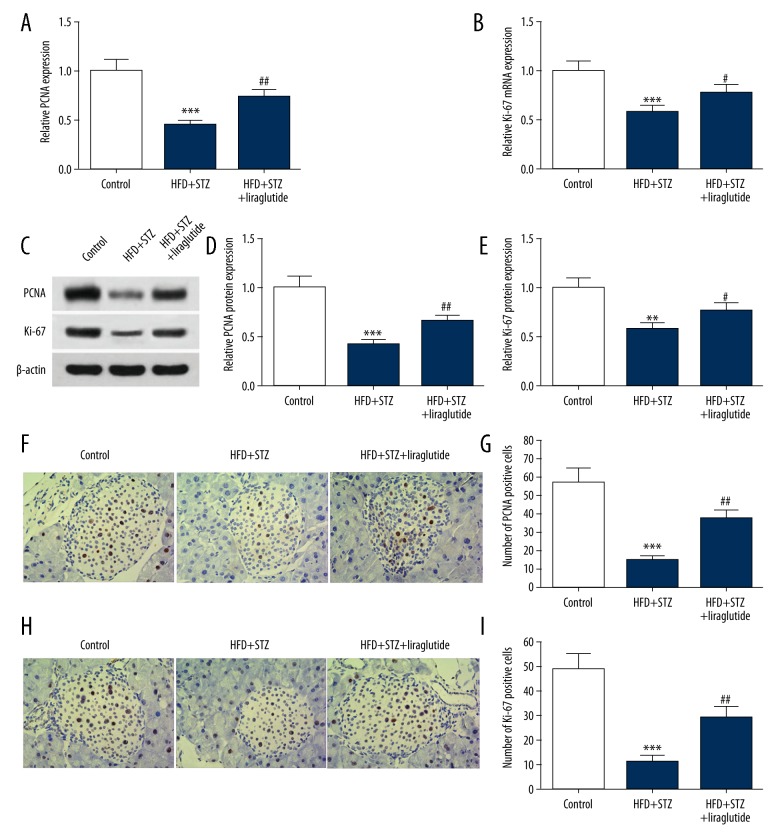
To evaluate the pancreatic β cell proliferation in mice, the mRNA and protein level of PCNA and Ki-67 were tested. As shown in Figure 3A and 3B, compared with the control group, the expressions of PCNA and Ki-67 mRNA were decreased in the HFD + STZ group (0.45±0.05; 0.58±0.07). Liraglutide treatment upregulated PCNA and Ki-67 mRNA level in mice treated with HFD + STZ. Representative images of Western blots are shown in Figure 3C. The results of quantification analysis show that the protein levels of PCNA and Ki-67 were significantly upregulated in the HFD + STZ + liraglutide group (0.65±0.07; 0.77 ± 0.08) compared with the HFD + STZ group (0.41±0.05; 0.58±0.07) (Figure 3D, 3E). The results of IHC are shown in Figure 3F, 3H. The number of PCNA- or Ki-67-positive cells in the HFD + STZ group (14.7±2.5; 11.5±2.3) was obviously lower than that in the control group (56.8 ± 7.3; 48.7±6.6). The number of PCNA- or Ki-67-positive cells in the HFD + STZ + liraglutide group (37.2±4.9; 29.4±4.0) were increased in comparison with the HFD + STZ group (Figure 3G, 3I).
Figure 3.
Liraglutide promotes pancreatic β cell proliferation. (A, B) PCNA and Ki-67 mRNA expression levels in pancreatic tissues were detected by qRT-PC. (C) Western blot results of PCNA and Ki-67 protein are shown. (D, E) Quantification of the protein levels of PCNA and Ki-67 shown in C. (F, H) Representative images of IHC are shown (magnification 200×). (G, I) Quantification of PCNA and Ki-67-positive cells in F and H. Data are presented as mean ±SD (n=6). ** P<0.01 vs. control, *** P<0.001 vs. control, # P<0.05 vs. HFD + STZ group, ## P<0.01 vs. HFD + STZ.
Discussion
Many studies have demonstrated that HFD and STZ-treated mice have elevated glucose and plasma insulin levels and impaired ability to maintain glucose homeostasis, as well as weakened autophagy and pancreatic β cell apoptosis [4,10,11]. In our study, mice were treated with HFD for 8 weeks to induce insulin resistance and then injected with STZ to induce mild impairment of pancreatic β cells.
Autophagy is a cellular event that can remove excess or damaged organelles and maintain internal hemostasis, consequently modulating the proliferation, development, and senescence of cells, and hypermetabolism [12]. Autophagy plays a critical role in sustaining intracellular homeostasis and cell integrity via improving the state of insulin resistance, modulating cellular lipid metabolism and regulating the activation of the innate immune response [13]. Previous studies suggest that autophagy can be accurately regulated by autophagy-related genes [14]. Among these ATG genes, ATG5 protein in a conjugated form with ATG12 and ATG8 (LC3) are associated with the early stages of autophagosome formation [15,16]. A growing number of studies have demonstrated that autophagy is related to the pathogenesis of many diseases, including cancer, infections, diabetes, and neurodegenerative disorders [17]. Several studies hypothesized that autophagy not only protect pancreatic β cells from apoptosis induced by external stimuli, but also maintain the structure, number, and functionality of β cells, as well as internal homeostasis [18].
Cell survival and death are complex cellular processes involving inflammation, oxidative stress, apoptosis, and autophagy [19]. The interactions between apoptosis, autophagy, and endoplasmic reticulum stress aggravate the pathological process of type 2 diabetes [20]. Serving as an adaptive pathway reaction to the stress, the intracellular protein and organelles were degraded by the activated autophagy. In a previous study, autophagy was shown to be an effective therapeutic target for the treatment of diabetic nephropathy [13]. The autophagy protein ATG5 plays an essential role in the formation of the autophagosome via promoting the lipidation of microtubule-associated protein LC3, which is involved in the expansion and completion of the autophagosome [21]. In the present study, we demonstrated that the expression of ATG5 and conversion of LC3-II to LC3-I were significantly enhanced by liraglutide, indicating that liraglutide enhanced autophagy in HFD and STZ-induced type 2 diabetic mice.
Liraglutide reduced hyperglycemia in type 2 diabetes mouse models by improving pancreatic β cell function [22]. A study involving patients with type 2 diabetes showed that liraglutide can improve β cell function and recovered insulin secretion, whereas another study considered it would be effective in patients with insulin secretion incapacity [13,23]. It has been reported that liraglutide can facilitate the proliferation and reduce the apoptosis of β cells after alloxan treatment [24,25]. Wang et al. found that liraglutide can repair β cell function and relieve diabetes via activating AMPK signaling [26]. Yin et al. reported that liraglutide can protect INS-1 cells from free fatty acid-induced apoptosis by promoting autophagy [27]. However, large dosages and/or long-term usage of liraglutide can cause gastrointestinal diseases, thyroid cancer, and pancreatitis in patients with type 2 diabetes [28–30]. In this study, we discovered that the expressions of PCNA and Ki-67 were upregulated by liraglutide, suggesting that liraglutide can enhance proliferation of pancreatic β cells.
Conclusions
In summary, our findings demonstrate that liraglutide can relieve diabetes in HFD and STZ-induced type 2 diabetic mice by increasing proliferation and enhancing autophagy of pancreatic β cells. Our study further elucidates the mechanism by which liraglutide exerts anti-diabetic effects in vivo and may provide a promising therapeutic strategy for diabetes treatment.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the United Foundation of the National Natural Science Foundation of China (No. U1404805)
References
- 1.Gotham K, Unruh K, Lord C. Depression and its measurement in verbal adolescents and adults with autism spectrum disorder. Autism. 2014;19(4):491–504. doi: 10.1177/1362361314536625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng C, Zhou W, Wang T, et al. A Novel TGR5 activator WB403 promotes GLP-1 secretion and preserves pancreatic β-cells in type 2 diabetic mice. Plos One. 2015;10(7):e0134051. doi: 10.1371/journal.pone.0134051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aranda-González I, Moguel-Ordóñez Y, Chel-Guerrero L, et al. Evaluation of the antihyperglycemic effect of minor steviol glycosides in normoglycemic and induced-diabetic wistar rats. J Med Food. 2016;19(9):844–52. doi: 10.1089/jmf.2016.0014. [DOI] [PubMed] [Google Scholar]
- 4.Shen KP, Su CH, Lu TM, et al. Effects of Grifola frondosa non-polar bioactive components on high-fat diet fed and streptozotocin-induced hyperglycemic mice. Pharm Biol. 2015;53(5):705–9. doi: 10.3109/13880209.2014.939290. [DOI] [PubMed] [Google Scholar]
- 5.Ahn EH, Kim DW, Shin MJ, et al. Tat-ATOX1 inhibits streptozotocin-induced cell death in pancreatic RINm5F cells and attenuates diabetes in a mouse model. Int J Mol Med. 2016;38(1):217–24. doi: 10.3892/ijmm.2016.2599. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Ao N, Du J, et al. Protective effect of liraglutide against ER stress in the liver of high-fat diet-induced insulin-resistant rats. Endocrine. 2015;49(1):106–18. doi: 10.1007/s12020-014-0480-y. [DOI] [PubMed] [Google Scholar]
- 7.Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide – the FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362(9):774–77. doi: 10.1056/NEJMp1001578. [DOI] [PubMed] [Google Scholar]
- 8.Tong W, Ju L, Qiu M, et al. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol Res. 2016;46(9):933–43. doi: 10.1111/hepr.12634. [DOI] [PubMed] [Google Scholar]
- 9.Rashidi B, Rad JS, Rad LR. Immunohistochemical (Ki-67) study of endometrial maturation in mice after use of phosphodiesterase type 5 inhibitor. Adv Biomed Res. 2015;4(1):154. doi: 10.4103/2277-9175.161581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Gao S, Niu J, et al. Cannabinoid 2 receptor agonist improves systemic sensitivity to insulin in high-fat diet/streptozotocin-induced diabetic mice. Cell Physiol Biochem. 2016;40(5):1175–85. doi: 10.1159/000453171. [DOI] [PubMed] [Google Scholar]
- 11.Sendrayaperumal V, Subramanian S. Zinc-morin complex improves pancreatic β-cell function in the HFD-STZ induced experimental type 2 diabetes. Der Pharma Chem. 2017;7(5):1–10. [Google Scholar]
- 12.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–48. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Liu G, Shen H, et al. Liraglutide inhibits autophagy and apoptosis induced by high glucose through GLP-1R in renal tubular epithelial cells. Int J Mol Med. 2015;35(3):684–92. doi: 10.3892/ijmm.2014.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong K, Kwon HY, Jeong MS, et al. CNOT2 promotes degradation of p62/SQSTM1 as a negative regulator in ATG5 dependent autophagy. Oncotarget. 2017;8(28):46034–46. doi: 10.18632/oncotarget.17682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang K, Liu M, Lin G, et al. Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death. Oncotarget. 2016;7(18):25652–67. doi: 10.18632/oncotarget.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim NY, Han BI, Lee M. Cytoprotective role of autophagy against BH3 mimetic gossypol in ATG5 knockout cells generated by CRISPR-Cas9 endonuclease. Cancer Lett. 2016;370(1):19–26. doi: 10.1016/j.canlet.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Zhou SW, Zhang M, Zhu M. Liraglutide reduces lipid accumulation in steatotic L-02 cells by enhancing autophagy. Mol Med Rep. 2014;10(5):2351–57. doi: 10.3892/mmr.2014.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Jie WU, Hong WU, et al. Liraglutide protects pancreatic β-cells against free fatty acidsin vitroand affects glucolipid metabolism in apolipoprotein E−/− mice by activating autophagy. Mol Med Rep. 2015;12(3):4210–18. doi: 10.3892/mmr.2015.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: The Four Horsemen of the Apocalypse. Int Urol Nephrol. 2017;49(5):837–44. doi: 10.1007/s11255-016-1488-4. [DOI] [PubMed] [Google Scholar]
- 20.Demirtas L, Guclu A, Erdur FM, et al. Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J Med Res. 2016;144(4):515–24. doi: 10.4103/0971-5916.200887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graf MR, Jia W, Johnson RS, et al. Autophagy and the functional roles of Atg5 and beclin-1 in the anti-tumor effects of 3beta androstene 17alpha diol neuro-steroid on malignant glioma cells. J Steroid Biochem Mol Biol. 2009;115(3):137–45. doi: 10.1016/j.jsbmb.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Abdulreda M, Rodriguez-Diaz R, Caicedo A, Berggren PO. Liraglutide compromises pancreatic β cell function in a humanized mouse model. Cell Metab. 2016;23(3):541–46. doi: 10.1016/j.cmet.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gough SC, Jain R, Woo VC. Insulin degludec/liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Rev Endocr Metab. 2016;11(1):7–19. doi: 10.1586/17446651.2016.1113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Minami K, Kudo M, et al. Liraglutide improves pancreatic Beta cell mass and function in alloxan-induced diabetic mice. PLoS One. 2015;10(5):e0126003. doi: 10.1371/journal.pone.0126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai W, Fu Z, Aylor K, et al. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high fat diet fed rats. Am J Physiol Endocrinol Metab. 2016;311(3):E640–48. doi: 10.1152/ajpendo.00205.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Sun Y, Lin P, et al. Liraglutide activates AMPK signaling and partially restores normal circadian rhythm and insulin secretion in pancreatic islets in diabetic mice. Biol Pharm Bull. 2015;38(8):1142–49. doi: 10.1248/bpb.b15-00024. [DOI] [PubMed] [Google Scholar]
- 27.Jia JY, Yan BL, Ming MC, Yang W. Liraglutide improves the survival of INS-1 cells by promoting macroautophagy. Int J Endocrinol Metab. 2013;11(3):184–90. doi: 10.5812/ijem.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thong KY, Gupta PS, Blann AD, Ryder RE. The influence of age and metformin treatment status on reported gastrointestinal side effects with liraglutide treatment in type 2 diabetes. Diabetes Res Clin Pract. 2015;109(1):124–29. doi: 10.1016/j.diabres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Gallo M. Thyroid safety in patients treated with liraglutide. J Endocrinol Invest. 2013;36(2):140–45. doi: 10.1007/BF03346749. [DOI] [PubMed] [Google Scholar]
- 30.Jensen TM, Saha K, Steinberg WM. Is there a link between liraglutide and pancreatitis? A post hoc review of pooled and patient-level data from completed liraglutide type 2 diabetes clinical trials. Diabetes Care. 2015;38(6):1058–66. doi: 10.2337/dc13-1210. [DOI] [PubMed] [Google Scholar]