Abstract
Background
The Reveal LINQ™Insertable Cardiac Monitor (ICM) or Implantable Loop recorder (ILR), is a miniaturized, subcutaneous, single lead, electrocardiographic monitoring device which has been extensively used in the differential diagnosis of unexplained syncope and palpitations in adults.
Patient Description
We describe an asymptomatic 20-month-old boy, noted to have incidental bradycardia on routine examination and in whom Holter monitoring revealed complete heart block (CHB). Over 1 year, the longest recorded pause lengthened from 1.8 seconds to 3.6 seconds.
Results
The Reveal LINQ™ ICM was inserted for long-term monitoring of the CHB. The device will record the electrocardiogram (ECG) continuously for up to 3 years, freezing in its memory any significant arrhythmic events. This will enable the diagnosis of the longest pauses, confirm whether they are lengthening over time and assist with the decision of pacemaker implantation.
Conclusion
The Reveal LINQ™ ICM is much smaller than the conventional loop recorder and has been shown to be ideal for close monitoring of asymptomatic yet potentially dangerous arrhythmias in young children.
Keywords: Implantable loop recorder, insertable loop recorder, bradycardia, complete heart block
Introduction
Congenital CHB (CCHB) in the paediatric population, is a rare and potentially fatal condition with an estimated incidence of 1 in every 15,000 to 20,000 live births.2 It may occur either as a congenital defect with no associated congenital heart lesions (isolated CCHB), combined with congenital heart defects, after surgical treatment of those lesions, or in association with maternal disease and the presence of certain maternal antibodies.3 The classification of isolated CHB as congenital would require the block to be diagnosed prenatally or at birth, however this is rarely the case 3 since affected newborns often appear to have no symptoms and will have accelerated ventricular heart rates approaching those of healthy newborns. Hence isolated CCHB may be easily missed on postnatal clinical examination and may remain undiagnosed until well after birth.3 In older infants, signs of decreased cardiac output due to bradycardia may be noted, such as; dizziness, syncope, pallor, mottling, sedentary attitude, exercise intolerance and palpitations, and the presence of poor growth and development. CCHB may ultimately present with Adams Stokes attacks and may even lead to sudden death.
The only effective treatment for CHB in all age groups is pacemaker (PM) implantation. However, this is not without risks (chiefly infection) and may have detrimental effects on cardiac contractility in the long-term. Paced children look forward to life-long dependence on an implanted device with its leads. The device will need to be changed 8-10 times at least. The leads will need to be revised to follow growth and may also need to be changed, a procedure which carries significant risk. Patients with CCHB usually do very well if left well alone since they have good underlying ventricular escape rhythms with rates that often vary with exercise level.
Hence, the clinical dilemma is focused on identifying the optimal time for commencing PM therapy in order to ensure a favourable risk/benefit ratio for the patients. Timing of the PM implant depends greatly on symptoms and is still controversial at times.2 A study published in the year 2002, identified certain major criteria, which when present, are an indication for PM therapy: syncope, heart failure, left ventricular dilatation, mitral valve regurgitation, complex escape rhythms and ventricular ectopics, prolonged QT interval, and daytime bradycardia of less than 55 beats per minute (bpm).4
There is therefore the need for continuous, long-term monitoring of the cardiac rhythm, throughout the day and night, in order to determine the presence of these markers that would require PM implantation. In this way, a more evidence-based selection of patients for PM therapy can be made.3
Patient Description
We report on the case of a healthy, asymptomatic, 20-month-old boy referred for a cardiology consultation from the community. Routine examination at the age of 5 months had incidentally revealed severe bradycardia with the heart rate ranging between 45 bpm (asleep) and 70 bpm (awake and active). There were no reported episodes of syncope, cyanotic spells, shortness of breath, severe pallor, sweating or absence seizures. The child was well in himself, within the normal range of development and without any evidence of failure to thrive or growth stunting.
He was born via a normal vaginal delivery at 40+ weeks’ gestation without any reported perinatal or postnatal complications. There was no family history of heart disease. The child’s mother was a known case of hypothyroidism on thyroxine replacement. He also had an older brother who was healthy.
On clinical examination, the child was alert and playful at all times. He was pink and well perfused with warm peripheries. The cardiovascular and respiratory examination was unremarkable. His weight was between the 50th and 85th percentile and his height was on the 85th percentile for his age.
A full blood panel including serum electrolytes was normal and a long strip 12-lead electrocardiogram (ECG) appeared to indicate 2nd degree Mobitz II heart block with 2:1 AV conduction, however on closer inspection there was complete AV dissociation (Refer to Fig 1, Fig 2, Fig 3).
Figure 1.

Lead II from a 12-lead ECG taken on the 6th June 2016 at standard paper speed of 25 mm/s.
There is AV dissociation with narrow QRS complexes at a rate of about 60 beats per minute.
Note how the PR intervals appear to shorten. The short PR intervals are non-physiologically short. Both these features strongly suggest AV dissociation with the P waves marching through the QRS complexes.
Figure 2.

Lead II from a 12-lead ECG taken on the 6th June 2016 at 21:31 hours with standard paper speed of 25 mm/s.
There is AV dissociation with narrow QRS complexes at a rate of 50 beats per minute.
Figure 3.

Lead II from a 12-lead ECG taken on the 6th June 2016 at 21:32 hours with standard paper speed of 25 mm/s.
The QRS complexes are narrow at a rate of 50 beats per minute. There appears to be 2:1 AV conduction but closer inspection reveals that there is atrio-ventricular dissociation and hence complete heart block. Note how PR intervals appear to shorten and lengthen (not a feature of 2:1 AV conduction).
The PP intervals are not constant. The PP intervals that include a QRS are shorter than the PP intervals that do not include a QRS. This is a well-known but poorly understood phenomenon known as ventriculophasic response and can be considered a non-respiratory sinus arrhythmia. Its mechanism and significance are unclear.
Echocardiography showed normal cardiac contractility, mild tricuspid regurgitation (pressure gradient of <30mmHg) and no mitral regurgitation.
A 24-hour ambulatory ECG monitor reported an average heart rate of 55 bpm (minimum 39 bpm and maximum 88 bpm). The predominant rhythm was CHB with variation in the PP interval. The longest R-R interval was about 1.8 seconds and was recorded at 00:40 hours (Fig 4).
Figure 4.

Three channels from an ambulatory ECG recording taken on the 17th September 2016 at 00:40 hours with standard paper speed of 25 mm/s.
This was the longest pause during this recording. There is AV dissociation with a ventricular pause of about 1.8 seconds. The QRS complexes are narrow at a rate of around 40 beats per minute. There were no premature ventricular beats or ventricular tachycardias recorded.
The child remained well and asymptomatic and was followed up after 1 year with another echocardiogram and a 24-hour ambulatory ECG monitor.
The echocardiogram showed normal anatomy with a heart rate of 50 bpm and dilatation of the left ventricle up to 34 mm. There was no mitral regurgitation and good cardiac function.
The 24-hour ambulatory ECG monitor reported an average heart rate of 49 bpm (minimum 31 bpm and maximum 93 bpm). The predominant rhythm was again CHB with pauses noted after 20:00 hours. The longest R-R interval was documented at 3.6 seconds and occurred at 02.48 hours (Refer to Fig 5).
Figure 5.

Three channels from an ambulatory ECG recording taken on the 6th September 2017 at 02:48 hours with standard paper speed of 25 mm/s.
This was the longest pause during this recording. There is AV dissociation with a ventricular pause of about 3.6 seconds. The QRS complexes are narrow at a rate of around 35 beats per minute. There were no premature ventricular beats or ventricular tachycardias recorded.
Given that one year after the diagnosis of the bradycardia and intermittent CHB, the longest R-R interval detected via the ambulatory ECG monitor appeared to increase from 1.8 seconds to 3.6 seconds, the caring physicians opted to insert a Reveal LINQ™ ICM. This would allow the monitoring of the cardiac rhythm on a continuous basis and hence the detection of longer pauses as well as correlation between the development of any symptoms and the ECG.
Outline of procedure
The procedure was performed under general anaesthesia under cover of intravenous antibiotics administered prior to the procedure. The skin was marked to indicate the intended orientation of the device after a simple procedure to predict whether a large enough electrogram would be detectable (Fig. 6–7).
Figure 6.

The child is now under general anaesthesia and oriented with his head to the right and his legs to the left of the image. All subsequent images are oriented in a similar manner.
The two red circles indicate the position of two ECG electrodes that had been previously applied to the chest 4 cm apart (see Fig 7 below for further details).
The skin has been marked to indicate the intended orientation of device, in line with the vector between the two ECG electrodes. The entry site will be superior and the device will be directed infero-laterally and leftwards.
An oblique orientation is preferred to a vertical orientation in order to maximise the amplitude of the QRS and hence improve the reliability of the device to sense the QRS complexes. However, the oblique orientation will tend to pull skin outwards and stretch it across the lateral end of the device. This becomes more of an issue with small chests.
Figure 7.

This is a trace obtained by placing ECG electrodes of the left arm and right arm at the two positions indicated by red circles in Fig 6. The ECG machine was set to record Lead I at 2.5 mm/mV on the vertical axis. Paper speed is standard at 25mm/s.
Used in this way, the ECG machine is effectively acting as a simple voltmeter, measuring the potential difference between the two red circles.
The QRS amplitude is 12 mm peak-to-peak, corresponding to 4.8 mV. This is ample magnitude to be detectable by the device.
Had it been small, we would have needed to explore other sites and orientations. This can be accomplished easily by moving the ECG electrodes to different sites and orientations on the patient’s chest until a satisfactory vector with sufficiently large QRS amplitude is found.
Testing in this iterative manner ensures that the device, once implanted between the two red circles, will be able to sense all QRS complexes. Undersensing would result in the false detection of bradycardia and fill up the device memory with false events which would potentially overwrite any true events.
The skin was disinfected and drapes were placed over the chest (Fig. 8). A 1 cm incision was made in the skin (Fig. 9) and the device was inserted beneath the fascia creating a small pocket using the pre-loaded insertion tool provided (as described in Figs. 10–15). The skin was apposed using a subcuticular suture (Fig 16). The procedure was followed by a chest X-ray in order to confirm the location of the device. The child was observed for a few hours in hospital and was found clinically fit for discharge home on the same day.
Figure 8.

Skin is disinfected and drapes are placed over chest to leave site exposed.
Skin markings that indicate the intended insertion site are still visible.
Figure 9.

Skin is incised using the incision tool provided. Skin and subcutaneous tissue is pinched just lateral to incision site to facilitate entry into skin.
Figure 10.
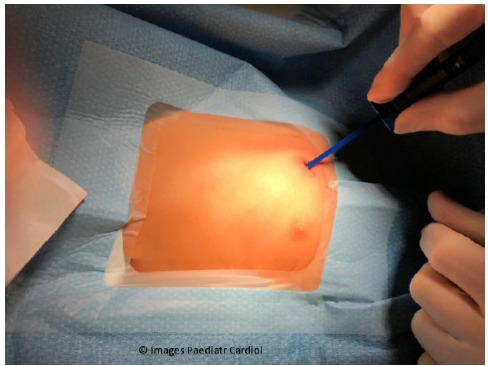
Insertion tool is placed within the incision previously created.
Figure 15.

Insertion tool is pulled out of subcutaneous track leaving the device behind.
Figure 16.

Skin incision is closed using a subcuticular suture (Steristrips may also be used).
Figure 11.

Insertion tool is pushed through the subcutaneous tissue bluntly. It is directed along the route that had been previously determined and marked on the skin.
Note that the insertion tool comes pre-loaded with the device within the handle.
Figure 12.

Insertion tool has now been fully pushed through the subcutaneous tissue.
The device can now be clearly seen within the handle. Note that it is still superficial to the part of insertion tool that is creating the subcutaneous track.
Figure 13.

Insertion tool is now rotated 180 degrees.
This brings the device deep to the part of the insertion tool that is within the subcutaneous track and ready for insertion.
Figure 14.

Plunger is placed within insertion tool and the device is firmly pushed into the subcutaneous track.
Note that the operator’s left hand is stabilizing the insertion tool and keeping it close to the skin incision during the device insertion. Otherwise, the forward push will tend to push out the insertion tool rather than push in the device.
Discussion
ILR/ICM devices have revolutionized cardiac monitoring and have had a tremendous impact on the aetiological diagnosis and management of unexplained syncope, palpitations, suspected atrial fibrillation, cryptogenic stroke and atrial fibrillation management post ablation. In children, they are being increasingly used to diagnose and manage syncope/absences and palpitations. Their use in very small children is still uncommon for most centres. A recent study, evaluates the efficacy of the utilization of miniaturized implantable cardiac devices in the early diagnosis of arrhythmias in children below the age of 6 years.5 It was concluded that this device could be very useful in diagnosing and managing small children with undefined symptoms. The present case report describes the first use of such a device in a very young child in Malta.
Patients with infrequent, short-duration symptoms are unlikely to be diagnosed by conventional 24-hour or even 7-day ambulatory ECG recordings even if these are repeatedly taken. Such patients require long-term monitoring with ILR/ICM devices for ongoing management.6 In our patient, the observed increase in duration of pauses may have been totally incidental. There are no guarantees that the child was not getting longer pauses outside the time that had been captured by the ambulatory recording.
ILR/ICM devices are small, leadless, implantable devices which continuously monitor a modified ECG using two electrodes across their length. They have a longevity of up to 3 years and can communicate wirelessly to the outside world via a dedicated system (either a device programmer or a remote monitoring station). They save the ECG data within their finite memory using a first-in first-out methodology. When the memory buffer fills up, newer data will continuously displace the oldest data. The ECG is stored in permanent memory only if device is activated by patient/bystander (using a dedicated activation device) or if auto-activated (if pre-specified alarm limits are breached). ILR/ICM devices are MR-conditional.
Early devices had a volume of about 10 mL and were about the size of a traditional pen drive but newer devices are considerably smaller with a volume of less than 1.5 mL. The Reveal LINQ™ ICM is ideally suited for easy, minimally-invasive insertion under local anaesthesia, even in a doctor’s office. In children, general anaesthesia is usually used although one of the authors (OA) has inserted an ICM in a 10-year-old child using local anaesthesia only. The small size of the ICM makes it ideally suitable for infants and small children.1
Newer devices have been equipped with remote monitoring capability. The device transmits its data wirelessly to a remote monitoring workstation usually placed at the patient’s bedside. From this workstation, the data is then transmitted via the internet to a central server and then becomes instantly accessible to the patient’s caring team. 79% of physicians state that wireless monitoring devices allow for earlier clinical decision-making and improved patient outcomes.7 Transmissions are either patient-driven (symptomatic events) or automatic (if alarm limits are breached).
The main disadvantages of ILR/ICM devices are: need for minor surgical procedure, difficulty in distinguishing ventricular from supra-ventricular arrhythmias, over-sensing (which fills memory uselessly), under-sensing (which misses arrhythmias) and finally, the high initial cost. Despite these disadvantages, these devices have permanently changed the management of patients with this type of clinical problem and are being increasingly used in children, even if very young.
References
- 1.Hutchinson LJ, Stuart G, Walsh MA. Implantation of the new Medtronic LINQ loop recorder in an infant with ventricular tachycardia. Cardiology in the Young. 2015. August; 25(6):1221-3. [DOI] [PubMed] [Google Scholar]
- 2.Vitali Serdoz L, Cappato R. Congenital complete atrioventricular block in the early pediatric population. Heart Int. 2006. May 28; 2(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman JI. Heart Block in congenital heart disease. Bull N Y Acad Med. 1971. August; 47(8): 885-904. [PMC free article] [PubMed] [Google Scholar]
- 4.Balmer C, Fasnacht M, Rahn M, Molinari L, Bauersfeld U. Long-term follow-up in children with congenital complete atrioventricular block and the impact of pacemaker therapy. Europace. 2002. October; 4(4):345–9. [DOI] [PubMed] [Google Scholar]
- 5.Placici S, Drago F, Milioni M, et al. Miniaturized implanatable loop recorder in small patients: an effective approach to the evaluation of subjects at risk of sudden death. Pacing Clin Electrophysiol. 2016. July; 39(7): 669-74. [DOI] [PubMed] [Google Scholar]
- 6.Krahn AD, Klein GJ, Yee R, Takle-Newhouse T, Norris C, Use of an extended monitoring strategy in patients with problematic syncope. Circulation. 1999. January 26;99(3):406-10. [DOI] [PubMed] [Google Scholar]
- 7.Crossley GH, Boyle A, Vitense H, et al. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011. March 8;57(10):1181-1189. [DOI] [PubMed] [Google Scholar]


