Abstract
The first tenet of medicine, “primum non nocere” or “first, do no harm”, is not always compatible with oncological interventions e.g., chemotherapy, targeted therapy and radiation, since they commonly result in significant toxicities. One of the more frequent and serious treatment-induced toxicities is mucositis and particularly oral mucositis (OM) described as inflammation, atrophy and breakdown of the mucosa or lining of the oral cavity. The sequelae of oral mucositis (OM), which include pain, odynodysphagia, dysgeusia, decreased oral intake and systemic infection, frequently require treatment delays, interruptions and discontinuations that not only negatively impact quality of life but also tumor control and survivorship. One potential strategy to reduce or prevent the development of mucositis, for which no effective therapies exist only best supportive empirical care measures, is the administration of agents referred to as radioprotectors and/or chemoprotectors, which are intended to differentially protect normal but not malignant tissue from cytotoxicity. This limited-scope review briefly summarizes the incidence, pathogenesis, symptoms and impact on patients of OM as well as the background and mechanisms of four clinical stage radioprotectors/chemoprotectors, amifostine, palifermin, GC4419 and RRx-001, with the proven or theoretical potential to minimize the development of mucositis particularly in the treatment of head and neck cancers.
Introduction
The term “Medical Machiavellianism” [1] has been coined to describe the predominant ‘first, do harm to do good’ oncologic paradigm of chemotherapy, targeted therapy and radiation administration in which toxicity and benefit are inextricably linked; by contrast, anti-Medical Machiavellianism occurs when toxicity far exceeds benefit.
A quintessential ‘anti-Medical Machiavellian’ iatrogenic toxicity, for which no established effective treatment exists and from which patients tend to suffer greatly, is mucositis and particularly oral mucositis (OM), a term introduced in the late 1980s [2], involving dose-limiting inflammation and ulceration of the oral cavity during chemotherapy, targeted therapy [3], and radiation treatment [4]. While treatment-related mucositis may occur throughout the gastrointestinal tract, this review is focused on OM, which is mucositis that involves the oral cavity, oropharynx, and hypopharynx.
The debilitating sequelae of OM, which include pain, odynodysphagia, dysgeusia, dehydration, malnutrition, and systemic infection [5], [6], [7], are expensive not only in terms of the direct costs to manage them but also in terms of reduced quality of life and survival secondary to dose reduction or therapy discontinuation.
OM occurs in up to 40% of cancer patients that receive chemotherapy and approaches 100% in the treatment of head and neck cancers [8]; the symptoms, which vary in severity from Grade 1 (mild with redness and soreness) to Grade 4 (life-threatening preventing oral intake) (See Table 1), begin after a cumulative exposure to 15 Gy and worsen markedly if the total dose exceeds 60 Gy [9].
Table 1.
World Health Organization (WHO) Oral Mucositis Scale (modified from [10])
| Grade 1 (mild) | Oral soreness, erythema |
|---|---|
| Grade 2 (moderate) | Erythema, ulcers, but oral intake not prevented |
| Grade 3 (severe) | Oral ulcers interfering with oral intake and requiring liquids only |
| Grade 4 (life-threatening) | Oral ulcers to the extent that oral alimentation is impossible |
The pathogenesis of mucositis is related to direct mucosatoxicity from ionizing radiation (IR) and several anticancer agents such as methotrexate, doxorubicin, 5-fluorouracil, busulfan, bleomycin, cisplatin, carboplatin, etoposide, EGFR inhibitors, and selected tyrosine kinase inhibitors [11] in combination with infections, exacerbated by neutropenia, that develop on the damaged mucosa [12]. Methotrexate and etoposide, in particular, are secreted in saliva, which leads to an increased risk of direct mucositis [13].
While evidence of a direct causal link between mucositis and neutropenia is not definitive, the possibility that it is a contributory factor is suggested by a) reduced duration and severity of the OM with topical and systemic GM-CSF treatment (although this data is contradictory [14]) b) predisposition to mucositis with low neutrophil counts (<400 cells/μL) c) high incidence of microbial infections during neutropenia since perturbation of the mucosal barrier results in a portal of entry for pathogens and neutrophil-depleted patients are less able to clear these pathogens d) recovery of the mucosa in tandem with white cell recovery, all of which implies that depletion of neutrophils leads to impaired wound healing and that neutrophil repopulation particularly during the recovery phase accelerates repair [15].
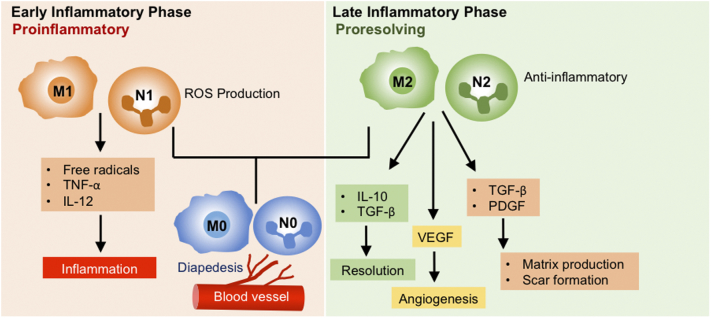
Like macrophages, neutrophils are highly plastic cells, capable of dynamically switching between two main states, N1, inflammatory, and N2, pro-repair, in a context- and time-dependent manner. Immediately following radiation exposure, neutrophils and macrophages adopt N1 and M1 activated phenotypes, respectively, in part due to the recognition and clearance of chemoradiation-induced apoptotic and necrotic cells [16], resulting in prolific expression of pro-inflammatory cytokines such as TNF and IL-6; however, as the wound matures and the dead cells, debris and pathogens are progressively cleared, the ratio of pro-inflammatory N1/M1 to pro-repair N2/M2 changes in favor of the latter, resulting in high expression levels of the anti-inflammatory cytokine, IL-10, and growth factors such as PDGF and TGF-β [17] with subsequent deposition of new extracellular matrix, reepithelialization and neovascularization. (Figure 1)
Figure 1.
Neutrophil and Macrophage Polarization During the Early and Late Stages of Mucositis
It is this time- and wound stage-dependence of macrophage and neutrophil function and polarization during the repair process, which probably accounts for the contradictory results with G-CSF (some studies show a positive effect and some no effect), since an increased influx of neutrophils and macrophages may be harmful to resolution in the earlier stages and helpful in later ones. In any case, due to these conflicting results in mucositis, the use of G-CSF is not recommended.
Traditional management of OM, which is mainly anecdotal rather than evidence-based, may consist of ice chips (oral cryotherapy), oral cavity care, multi-agent topical rinses and lozenges (e.g., Magic Mouthwash and PTA lozenges) hydration, nutritional support, systemic pain relief, low-level laser, mouth coating agents like sucralfate, growth colony stimulating factor (G-CSF) and infection surveillance and treatment. Since no single therapy or combination of therapies have been shown to be successful in OM, one strategy is to reduce or prevent its occurrence with chemoradioprotectors, of which there are only three with clinical data, amifostine, palifermin and RRx-001. In this review, the incidence, pathogenesis, symptoms and impact on patients of OM are briefly summarized along with the background on these three chemoradioprotectors and the proposed mechanisms for their beneficial effects.
Incidence and Risk Factors of OM
In general, while mucositis occurs in up to 40% of patients treated with conventional chemotherapy, more than 70% of patients that undergo conditioning therapy for bone marrow transplantation [18] and virtually all head and neck patients that receive simultaneous radiochemotherapy [19], its severity is highly variable and depends on treatment- and patient-specific risk factors (see Table 2). Of the two, treatment-related risk factors, which include dose, location, duration of chemotherapy, and concomitant use of chemotherapy and radiation, are the more impactful; however, since several patient-specific risk factors are potentially modifiable through preventative actions (e.g., poor oral hygiene, the presence of dental appliances, diabetes, oral lesions, malnutrition, neutropenia, folic acid/vitamin B12 deficiencies, concomitant medications, salivary hypofunction/xerostomia [20], tobacco use etc.) they should be identified and addressed before the start of therapy by both an oncologist and a dentist. Other less modifiable patient risk factors include age, gender, body mass index, underlying immune dysfunction, polymorphisms and deficiencies in drug metabolizing enzymes and other epigenetic and genetic factors, which affect the expression or activity of cytokines and transcription factors, such as TGF-β, NF-κB and p53 and genes such as COX-2 and MMPs, all of which are implicated in the development of mucositis [21].
Table 2.
Risk Factors for OM
| Treatment -specific | Patient -specific |
|---|---|
| Type of chemotherapy in particular 5-FU, methotrexate and etoposide | Tobacco abuse |
| Higher doses and continuous infusion especially with 5-FU | Neutropenia |
| Age | |
| Concomitant radiation therapy | Nutritional status |
| Radiation administered directly to the head and neck | Gender |
| Oral health and hygiene | |
| Presence of dentures | |
| Low body mass index | |
| Decreased renal function | |
| Comorbid conditions e.g., diabetes | |
| Genetic polymorphisms or deficiencies in drug metabolizing enzymes | |
| Vitamin B12/folic acid deficiencies | |
| Salivary dysfunction | |
| Concomitant medications e.g., anticholinergics and antidepressants that decrease salivary flow | |
| Higher levels of oral microflora | |
| Previous cancer treatment | |
| Immune dysfunction from an underlying disease state e.g., ulcerative colitis, rheumatoid arthritis, lupus etc. | |
| Epigenetic factors |
Adverse Impact on Patient Outcomes and Quality of Life
While treatment-related nausea, vomiting and cytopenias are the most common adverse events in oncology, mucositis has emerged as the most significant according to an NCCN task force [22]. Its adverse physical, social and psychological impacts on patients are legion and include the need for total parenteral nutrition (TPN), higher risk of systemic infections, increased use of antifungals and opioid analgesics, increased hospitalizations with longer hospital stays, social isolation and depression due to the inability to talk and eat, subsequent dose reductions, treatment interruptions and discontinuations, and as a corollary to dose reductions and treatment interruptions/discontinuations, diminished antitumor responses and shorter survival. The economic consequences are far from trivial as well with the incremental cost exceeding $17,000 (USD) for patients with head and neck cancer [23].
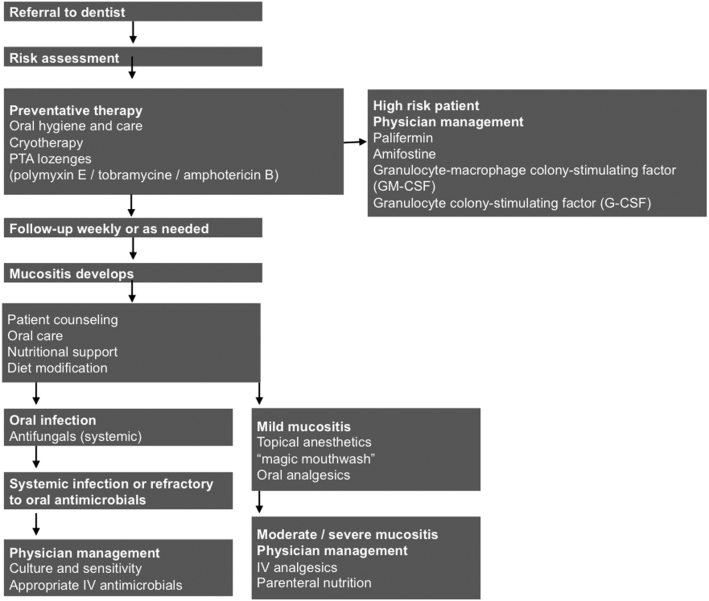
The cornerstone of management for the substantial pain and ulceration, which results and which may persist even after treatment is completed, are parenteral opioid analgesics that themselves carry the inherent risk of aversive side effects such as constipation and decreased alertness [24]. (Figure 2)
Figure 2.
Flow chart of management Adapted from Napenas [64]
Pathogenesis
The pathogenesis of oral mucositis is thought to involve direct and indirect mechanisms resulting from treatment with chemotherapy and/or radiotherapy that interfere with the division and maturation of epithelial cells.
-
a)
Direct Mechanisms
The epithelial cells of the oral mucosa have a fast turnover rate, usually every 7 to 14 days, which explains their direct susceptibility to apoptosis from cytotoxic therapy and the onset of mucositis approximately two weeks after chemotherapy initiation [25], [26].
-
b)
Indirect Mechanisms
Indirect toxic bystander effects that exacerbate the morbidity of mucositis include the release of pro-inflammatory mediators such as tumor necrosis factor (TNF), interleukin-1 beta (IL-1β) and IL-6 from activated M1 and N1 macrophages and neutrophils, with a concomitant decrease in the anti-inflammatory cytokines IL-10 and TGF-β that would normally act to dampen the inflammatory response as well as secondary infection/colonization from/by Gram-negative bacteria and fungal species. Oral infections also tend to coincide with neutropenia.
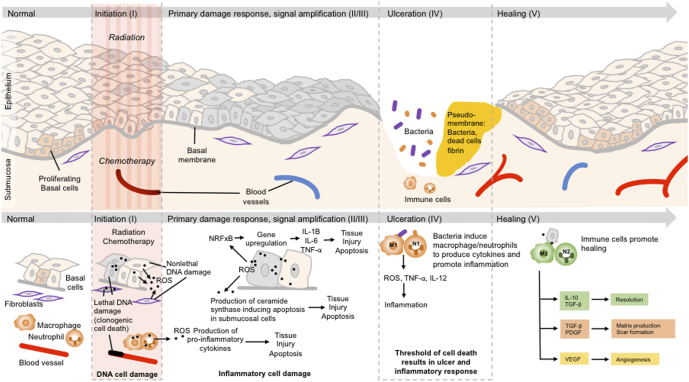
Sonis has proposed a 5-stage model of mucositis, the importance of which is that, while likely incomplete, it identifies potential therapeutic targets (e.g. free radicals, pro-inflammatory cytokines, infection) that could hinder or reverse the development of OM with radio- and chemoprotectors. (See Table 3 and Figure 3)
Table 3.
The 5-phases of Oral Mucositis Adapted from Sonis [27]
| Phase I—Initiation | Chemotherapy or radiation-induced reactive oxygen species (ROS) and lipid peroxidation results in DNA damage, release of pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern molecules (DAMPs) and cellular apoptosis |
| Phase II—Signaling | ROS stimulate the NF-kB pathway, which induces production of proinflammatory cytokines (TNF-α, IL-1β, IL-6) |
| Phase III – Amplification | Proinflammatory cytokines trigger tissue injury, apoptosis, vascular permeability, and activation of cyclooxygenase-2 |
| Phase IV—Ulceration | Ulceration occurs, which serves as a portal of entry for microorganisms. The presence of bacteria activate macrophages and neutrophils to further produce proinflammatory cytokines |
| Phase V—Healing | Signaling from the submucosa promotes epithelial migration, proliferation and differentiation |
Figure 3.
Illustration of the 5 phases of mucositis adapted from Sonis [65]
Amifostine
-
a)
Background
Initially under the auspices of the Manhattan Project during World War II, a systematic screen of 4400 compounds was undertaken at Walter Reed Army Research Institute to identify potential radioprotectors, which culminated in the selection of WR-27213 (the “WR” signifies Walter Reed) or Amifostine on the basis of its superior activity and safety profile [28]. Renamed Ethyol after its acquisition by MedImmune who later sold it to Astra Zeneca and now marketed by Clinigen Group, a UK pharma company, as well as two generic companies, Sun Pharma and Mylan Pharmaceuticals, amifostine has been used and approved clinically to ameliorate the side effects of chemotherapy and radiotherapy. Of the two FDA approvals, the first was in 1996 to reduce the cumulative renal toxicity associated with repeated administration of cisplatin in patients with advanced ovarian cancer or non-small cell lung cancer; the second was in 1999 for radiation-induced xerostomia in Head and Neck cancer [29]. Reportedly, amifostine was provided to the Apollo astronauts on their trip to the Moon in case of exposure to solar flare radiation [30].
-
b)
Mechanism and Toxicities
A phosphorylated aminothiol prodrug [31], amifostine is dephosphorylated by the enzyme, alkaline phosphatase that is at high levels in normal tissues but at low levels in tumors and thus converted to its active free radical-scavenging sulfhydryl metabolite, WR-1065, which provides a selective mechanism for protection of non-CNS tissues (since amifostine does not cross the blood brain barrier). Other mechanisms for preferential protection of normal tissues include increased uptake in the salivary glands and kidneys. As a free radical scavenger, amifostine quenches DNA damaging species from ionizing radiation and alkylating agents. (Figure 4) However, in animal studies it was observed that amifostine diffuses into malignant tissues [32], albeit more slowly than normal tissues, which raises a still-lingering question mark, even nearly 30 years after its approval, about the potential of amifostine to also protect tumors from radiation and chemotherapy cytotoxicity. In addition to this potential for tumor cytoprotection, its use in the clinic is limited by severe toxicities such as emesis and hypotension.
Figure 4.
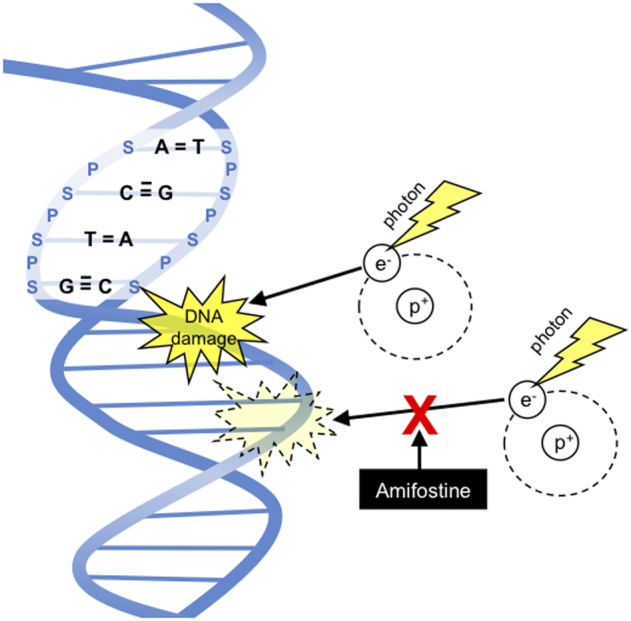
Amifostine as a thiol-based free radical scavenger, which prevents DNA damage
Palifermin
-
a)
Background
Palifermin or Kepivance is a recombinant human form of Keratinocyte growth factor (KGF) originally derived from embryonic lung fibroblasts and developed by Amgen that acts specifically on the differentiation, proliferation and survival of epithelial cells, which express the KGF receptor, throughout the GI tract [33]. In 2004, the FDA approved the use of palifermin [34] to reduce the incidence and duration of severe oral mucositis in patients with hematological malignancies [35]. However, palifermin has not been approved for use in head and neck cancers likely due a lack of improvement in narcotic use, patient-reported pain, or chemoradiotherapy compliance despite a significant reduction in the severity and duration of oral mucositis [36], [37].
-
b)
Mechanism and Toxicities
Palifermin binds to the KGF receptor [38], which induces the differentiation of epithelial cells and mediates a host of biological effects including upregulation of Th2 cytokines, inhibition of epithelial cell apoptosis, prevention of DNA strand breaks and tissue remodeling [39]. While the FDA package insert [40]states under its Warnings and Precautions section that palifermin is associated with the proliferation of KGF-receptor bearing malignant cells as well as secondary cancers in laboratory animals, a long term follow up of 672 patients treated with palifermin or placebo (428 palifermin and 244 placebo) with a median follow-up time of 7.9 years demonstrated no differences in the incidence of secondary malignancies [41]. Palifermin is generally well tolerated with mild-to-moderate adverse events of the skin and the mouth [42].
GC4419
-
a)
Background
Excessive formation of reactive oxygen species such as superoxide (SO) radical O2 plays a central role in the pathogenesis of mucositis. The antioxidant enzyme superoxide dismutase (SOD) detoxifies superoxides via transformation to the relatively stable and poorly reactive oxidant, hydrogen peroxide (H2O2). However, during radiation therapy, the overproduction of superoxide radicals so overwhelms native SOD enzymes that SO-mediated damage results, which suggest that exogenous administration of superoxide dismutase, will reduce the toxicity of radiation and chemotherapy. Indeed, during the 1970s, Orgotein, a Cu-Zn superoxide dismutase, was used successfully to ameliorate radiation-induced toxicity in bladder tumors [43]. However, use of SODs in the clinic has been limited by problems of immunogenicity when derived from non-human sources, large size (MW ~30,000) [44] and a short half-life, since they are prone to hydrolysis in vivo, and therefore require continuous infusion [45].
As an alternative to these large unstable immunogenic proteins, synthetic small molecule SOD mimetics (SODm) have been developed. One example of a non-peptide SODm is GC4419, a manganese-based low molecular weight small molecule that selectively dismutates superoxide anions but not other biologically relevant oxidizing species from Galera Therapeutics, Inc. In a recently completed a multi-site phase 2b clinical trial with 223 head and neck cancer patients, GC4419 resulted in a 92% reduction in the duration of oral mucositis (P = 0.024) and has received FDA fast track designation [46].
-
b)
Mechanism and Toxicities
The selectivity of GC4419 for superoxide is due to the reduced manganese (II) ion in the center of the molecule, which is alternately reduced by superoxide anion to Mn (III) and re-oxidized to Mn (II) by protonated superoxide via a one-electron pathway. Superoxide is the only kinetically competent oxidant/reductant in the redox cycle of Mn: Other one-electron radicals such as nitric oxide and oxygen are too sluggish to oxidize Mn (II) while the more potent peroxynitrite, hydrogen peroxide and hypochlorite, which transfer two electrons, are not able to oxidize it [47]. (Figure 5)
Figure 5.
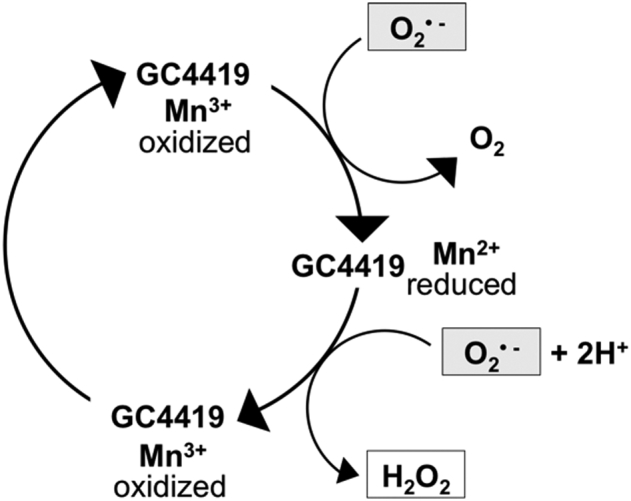
Redox cycling of the catalytic metal, Mn, in GC4419
In a Phase 1b/2a trial of GC4419 a maximum tolerated dose was not reached and only two dose-limiting toxicities, grade 3 gastroenteritis and vomiting with hyponatremia, were observed [48].
One potential “toxicity” of GC4419 and all SOD mimetics is tumor protection since antioxidants like SOD inhibit the formation of free radicals, which are cytotoxic. However, because GC4419 selectively dismutates superoxide into H2O2, which is an oxidant in its own right, tumor inhibition is more likely than tumor protection.
RRx-001
-
a)
Background
Similar to amifostine, RRx-001 also originated in a military setting, having been derived from 1,3,3-Trinitroazetidine or TNAZ, a melt cast explosive [49]. Developed by EpicentRx, a San Diego, California-based biotech, RRx-001 is under investigation in a Phase II clinical trial called QUADRUPLE THREAT (NCT02489903) as a chemosensitizer of platinum resistant/refractory small cell carcinoma and a high-grade neuroendocrine carcinoma and as a radioprotector in case of nuclear emergency at the Armed Forces Radiobiologic Research Institute (AFRRI). Preliminary data from the QUADRUPLE THREAT trial indicate that in 24 enrolled patients RRx-001 was associated with 4.2% of Grade 3/4 neutropenia (1/24) and a 0% rate of febrile neutropenia compared to a historical rate of neutropenia of 79.8% [50] and an 11% rate of febrile neutropenia for platinum plus etoposide, which may be indicative of bone marrow chemoprotection. Preclinical data also provide evidence of radioprotection [51] and platinum chemoprotection [52] A Phase II proof of concept randomized chemoradioprotection trial for oral mucositis in head and neck cancer is in preparation and scheduled to start at end of 2017 with three weekly doses of RRx-001 preceding the start of cisplatin and radiotherapy (RT) and continued RRx-001 dosing during platinum + RT rest periods.
-
b)
Mechanism and Toxicities
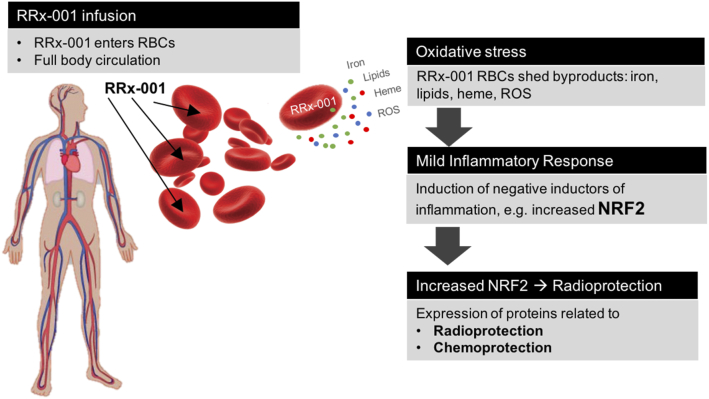
The mechanism of chemoradioprotection is red blood cell-based, since RRx-001 binds to and oxidatively modifies hemoglobin (Hb). The result of Hb oxidation is deposition of iron and heme in the red blood cell membrane with subsequent shedding of iron and lipid-laden microvesicles in normal tissues as an RBC self-protection mechanism against reactive oxygen species (ROS) accumulation. The deposition of these Fenton-active microvesicles in healthy tissues such as the bone marrow end induces a slight oxidative stress, which results in upregulation of Nrf2-dependent antioxidant enzymes [53]. Similar to ischemic preconditioning, first described in the dog myocardium [54], whereby periods of ischemia and reperfusion prior to a prolonged period of ischemia dramatically increases ischemic tolerance in solid organs such as the heart, liver, kidney, and bones, this form of RRx-001-mediated oxidative priming to induce the endogenous Nrf2 antioxidant machinery in normal cells preconditions them to better withstand subsequent oxidative insults. As both a single agent and in combination with radiation and chemotherapy, RRx-001 is well tolerated and to date has not been associated with any dose-limiting toxicities in over 200 treated patients [55]. (Figure 6)
Figure 6.
Schema of RRx-001-mediated oxidative preconditioning, resulting in potential chemoprotection and radioprotection
Conclusion and Possible Future Directions
According to Sonis’ characterization, on whose model the current 5 step pathogenesis of OM is based, mucositis is a toxicity that “largely defies effective intervention” [56]. In light of the lack of progress to treat it despite an extended clinical legacy, which reaches as far back as Marie Curie’s discovery of radium in 1897 [8], and on the premise that an ounce of prevention is worth a pound of cure, a chemo-and radioprotection strategy would seem to make eminent sense.
Unfortunately, as a field, radio- and chemoprotection also carries a lot of historical baggage, since its inception approximately 7 decades ago, when the amino acid cysteine was identified as a putative radioprotector in 1949 [57]. In all of this time, despite multiple potential candidates, including antioxidants, plant extracts, antihypertensives, steroid hormones, statins and cytokines [58], many of which have failed in clinical trials due to acute toxicities [59], [60], the only one to be approved as both a chemo- and radioprotector is amifostine [61]; however, its reputation and, by extension the whole chemo- and radioprotection field of which amifostine serves as the prototype and standard bearer, has suffered by virtue of its multiple toxicities including nausea, vomiting and hypotension, underwhelming clinical outcomes and the still-lingering possibility, to date never absolutely disproven, that amifostine also protects tumors and reduces therapeutic gain [62].
Hence, these black marks on amifostine and the resultant shortfall of candidates in clinical evaluation contribute to the impression that chemoradioprotection is a therapeutic and commercial dead-end, which, in turn, disincentivizes R&D and serves to establish a vicious cycle of disinterest and disinvestment. However, the magnitude of the unmet need in mucositis, absence of competition and potential market of at least 500,000 plus patients per year [63], which is the annual worldwide incidence of head and neck (H&N) cancer, since almost all H&N patients receive loco-regional radiotherapy also presents a high-reward opportunity for private sector investors e.g., pharmaceutical companies and venture capital firms. In addition to H&N cancer, a market also exists for patients treated with commonly prescribed chemotherapeutic agents that are associated with mucositis such as methotrexate, doxorubicin, 5-fluorouracil, etoposide, cisplatin, and carboplatin.
In terms of future directions, one potential strategy is to treat with a cocktail of RRx-001, amifostine, palifermin, CG4419, which has shown promise in the clinic, and potentially other agents as well such as corticosteroids and hematopoietic factors on the premise that they may act synergistically to protect normal tissues given their non-overlapping mechanisms of action. Moreover, RRx-001, as a chemoprotector as well as a radioprotector, may attenuate the dose-limiting toxicities of amifostine. If successful, and anticancer activity is not adversely affected, the immediate result will likely be a paradigm shift from quantity of life to quality of life-centered treatment where the same regimens are given with the same or better efficacy but with considerably less toxicity.
References
- 1.Oronsky B, Carter C, Scicinska A, Oronsky A, Oronsky N, Lybeck M, Scicinski J. Medical Machiavellianism: the tradeoff between benefit and harm with targeted chemotherapy. Oncotarget. 2016;7(8):9041–9045. doi: 10.18632/oncotarget.6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaveli López B, Gavaldá Esteve C, Sarrión Pérez MG. Dental treatment considerations in the chemotherapy patient. J Clin Exp Dent. 2011;3:e31–42. [Google Scholar]
- 3.Sankhala K, Mita A, Kelly K, Mahalingam D, Giles F, Mita M. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 2009;4(2):135–142. doi: 10.1007/s11523-009-0107-z. [DOI] [PubMed] [Google Scholar]
- 4.Alterio D, Jereczek-Fossa BA, Fiore MR, Piperno G, Ansarin M, Orecchia R. Cancer treatment-induced oral mucositis. Anticancer Res. 2007;27(2):1105–1125. [PubMed] [Google Scholar]
- 5.McGuire DB, Altomonte V, Peterson DE, Wingard JR, Jones RJ, Grochow LB. Patterns of mucositis and pain in patients receiving preparative chemotherapy and bone marrow transplantation. Oncol Nurs Forum. 1993;20:1493–1502. [PubMed] [Google Scholar]
- 6.Elad S, Zadik Y, Yarom N. Oral Complications of Nonsurgical Cancer Therapies. Atlas Oral Maxillofac Surg Clin North Am. 2017;25(2):133–147. doi: 10.1016/j.cxom.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Crowder SL, Douglas KG, Pepino Y, Sarma KP, Arthur AE. Nutrition impact symptoms and associated outcomes in post-chemoradiotherapy head and neck cancer survivors: a systematic review. J Cancer Surviv. 2018 doi: 10.1007/s11764-018-0687-7. [DOI] [PubMed] [Google Scholar]
- 8.Sonis ST. Oral mucositis in head and neck cancer: risk, biology, and management. Am Soc Clin Oncol Educ Book. 2013 doi: 10.14694/EdBook_AM.2013.33.e236. [DOI] [PubMed] [Google Scholar]
- 9.Plevová P. Prevention and treatment of chemotherapy- and radiotherapy-induced oral mucositis: a review. Oral Oncol. 1999;35:453–470. doi: 10.1016/s1368-8375(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 10.WHO . World Health Organization; Geneva: 1979. WHO Handbook for Reporting Results for Cancer Treatment. [Google Scholar]
- 11.Parkhill AL. Oral Mucositis and Stomatitis Associated with Conventional and Targeted Anticancer Therapy. J Pharmacovigilance. 2013;1:4. [Google Scholar]
- 12.Peterson DE. Research advances in oral mucositis. Curr Opin Oncol. 1999;11:261–266. doi: 10.1097/00001622-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Olif A, Blayer WA, Poplak DG. Methotrexate induced oral mucositis and salivary methotrexate concentration. Cancer Chemother Pharmacol. 1979;2:225–226. doi: 10.1007/BF00258300. [DOI] [PubMed] [Google Scholar]
- 14.Cartee L, Petros WP, Rosner GL, Gilbert C, Moore S, Affronti ML, Hoke JA, Hussein AM, Ross M, Rubin P. Evaluation of GM-CSF mouthwash for prevention of chemotherapy-induced mucositis: a randomized, double-blind, dose-ranging study. Cytokine. 1995;7(5):471–477. doi: 10.1006/cyto.1995.0064. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy GM, Awde JD, Gtardi H. Risk factors associated with mucositis in cancer patients receiving 5-FU. Oral Oncol. 1998;34:484–490. doi: 10.1016/s1368-8375(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 16.Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20(48):7085–7095. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- 17.Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int J Mol Sci. 2017;17:18(7). doi: 10.3390/ijms18071545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonis ST, Sonis AL, Lieberman A. Oral complications in patients receiving treatment for malignancies other than of the head and neck. J Am Dent Assoc. 1978;97:468–472. doi: 10.14219/jada.archive.1978.0304. [DOI] [PubMed] [Google Scholar]
- 19.Jansma J, Vissink A, Spijkervet FK, Roodenburg JL, Panders AK. Vermey a, Szabo BG, Gravenmade EJ, Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer. 1992;70:2171–2180. doi: 10.1002/1097-0142(19921015)70:8<2171::aid-cncr2820700827>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Peterson DE, Bensadoun RJ, Roila F. ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl. 6):vi78–84. doi: 10.1093/annonc/mdr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung YL, Wang AJ, Yao LF. Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: implications for increasing therapeutic gain in cancer radiotherapy. Mol. Cancer Ther. 2004;3:317–325. [PubMed] [Google Scholar]
- 22.Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG, Elting L, Mittal BB, Schattner MA, Spielberger R. NCCN Task Force Report: Prevention and Management of Mucositis in Cancer Care. J Natl Compr Canc Netw. 2008;6(Supplement 1) [PubMed] [Google Scholar]
- 23.Nonzee NJ, Dandade NA, Patel U, Markossian T, Agulnik M, Argiris A, Patel JD, Kern RC, Munshi HG, Calhoun EA. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008;113:1146–1152. doi: 10.1002/cncr.23714. [DOI] [PubMed] [Google Scholar]
- 24.Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46(6):452–456. doi: 10.1016/j.oraloncology.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Lockhart PB, Sonis ST. Alterations in the oral mucosa caused by chemotherapeutic agents. A histologic study. J Dermatol Surg Oncol. 1981;7:1019–1025. doi: 10.1111/j.1524-4725.1981.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 26.Verdi CJ. Cancer therapy and oral mucositis. Drug Saf. 1993;9:185–195. doi: 10.2165/00002018-199309030-00004. [DOI] [PubMed] [Google Scholar]
- 27.Sonis S. New thoughts on the initiation of mucositis. Oral Dis. 2010;16:597–600. doi: 10.1111/j.1601-0825.2010.01681.x. [DOI] [PubMed] [Google Scholar]
- 28.Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007;12(19-20):794–805. doi: 10.1016/j.drudis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Eisbruch A. Amifostine in the Treatment of Head and Neck Cancer: Intravenous Administration, Subcutaneous Administration, or None of the Above. J Clin Oncol. 2011;29(2):119–121. doi: 10.1200/JCO.2010.31.5051. [DOI] [PubMed] [Google Scholar]
- 30.Hall EJ. 4th ed. J. B Lippincott Co.; Philadelphia, PA: 1994. Radiobiology for the Radiologist; p. 186. [Google Scholar]
- 31.Santini V, Giles FJ. The potential of amifostine: from cytoprotectant to therapeutic agent. Haematologica. 1999;84(11):1035–1042. [PubMed] [Google Scholar]
- 32.Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anti-Cancer Drugs. 2002;13:181–209. doi: 10.1097/00001813-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Seyboth B, Mathew S, Gilheeney SW, Chou AJ, Drill E, Kobos R. Retrospective Evaluation of Palifermin Use in Nonhematopoietic Stem Cell Transplant Pediatric Patients. J Pediatr Hematol Oncol. 2017;39(4):e177–e182. doi: 10.1097/MPH.0000000000000791. [DOI] [PubMed] [Google Scholar]
- 34.Lauritano D, Petruzzi M, Di Stasio D, Lucchese A. Clinical effectiveness of palifermin in prevention and treatment of oral mucositis in children with acute lymphoblastic leukaemia: a case-control study. Int J Oral Sci. 2014;6:27–30. doi: 10.1038/ijos.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan A, Sonis S. Emerging therapies for the prevention and treatment of oral mucositis. Expert Opin Emerg Drugs. 2014;19(3):343–351. doi: 10.1517/14728214.2014.946403. [DOI] [PubMed] [Google Scholar]
- 36.Le QT, Kim HE, Schneider CJ, Muraközy G, Skladowski K, Reinisch S, Chen Y, Hickey M., Mo M., Chen MG. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: A randomized, placebo-controlled study. J Clin Oncol. 2011;29(20):2808–2814. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 37.Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: A randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815–2820. doi: 10.1200/JCO.2010.32.4103. [DOI] [PubMed] [Google Scholar]
- 38.Blijlevens N, Sonis S. Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann Oncol. 2007;18(5):817–826. doi: 10.1093/annonc/mdl332. [DOI] [PubMed] [Google Scholar]
- 39.Beaven AW, Shea TC. The effect of Palifermin on chemotherapy and radiation therapy induced mucositis: a review of the current literature. Support Cancer Care. 2007;4:188–197. doi: 10.3816/SCT.2007.n.014. [DOI] [PubMed] [Google Scholar]
- 40.FDA Approved Drug Products Kepivance, Palifermin. https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/125103lbl.pdf
- 41.Stiff PJ, Leinonen M, Kullenberg T, Rudebeck M, de Chateau M, Spielberger R. Long-Term Safety Outcomes in Patients with Hematological Malignancies Undergoing Autologous Hematopoietic Stem Cell Transplantation Treated with Palifermin to Prevent Oral Mucositis. Biol Blood Marrow Transplant. 2016;22(1):164–169. doi: 10.1016/j.bbmt.2015.08.018. [Epub 2015 Aug 22] [DOI] [PubMed] [Google Scholar]
- 42.Vadhan-Raj S, Goldberg JD, Perales M-A, Berger DP, Brink MR. Clinical applications of palifermin: amelioration of oral mucositis and other potential indications. J Cell Mol Med. 2013;17(11):1371–1384. doi: 10.1111/jcmm.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menander-Huber KB, Edsmyr F, Huber W. Orgotein (superoxide dismutase): a drug for the amelioration of radiation-induced side effects. A double-blind, placebo-controlled study in patients with bladder tumours. Urol Res. 1978;6(4):255–257. doi: 10.1007/BF00262630. [DOI] [PubMed] [Google Scholar]
- 44.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140(3):445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley DP, Lennon PJ, Neumann WL, Weiss RH. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalytic activity of macrocyclic manganese(ii) complexes. J Am Chem Soc. 1997;119:6522–6528. [Google Scholar]
- 46.Galera therapeutics, press release. http://www.galeratx.com/data-galera-therapeutics-223-patient-phase-2b-clinical-trial-gc4419-presented-2018-multidisciplinary-head-neck-cancers-symposium
- 47.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 48.Anderson CM, Sonis ST, Lee CM, Adkins D, Allen BG, Sun W, Agarwala SS, Venigalla ML, Chen Y, Zhen W. Phase 1b/2a Trial of the Superoxide Dismutase Mimetic GC4419 to Reduce Chemoradiotherapy-Induced Oral Mucositis in Patients With Oral Cavity or Oropharyngeal Carcinoma. Int Radiat Oncol Biol Phys. 2018;100(2):427–435. doi: 10.1016/j.ijrobp.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oronsky B, Scicinski J, Ning S, Peehl D, Oronsky A, Cabrales P, Bednarski M, Knox S. Rockets, radiosensitizers, and RRx-001: an origin story part I. Discov Med. 2016;21(115):173–180. [PubMed] [Google Scholar]
- 50.Sandler A, Langer CJ, Bunn PA, Einhorn LH. Phase III study comparing irinotecan and cisplatin to cisplatin and etoposide for previously untreated extensive small cell lung Cancer: interim safety analysis. Lung Cancer. 2003;41(Suppl. 2):282. [Abstract O-280] [Google Scholar]
- 51.Ning S, Bednarski M, Oronsky B, Scicinski J, Saul G, Knox SJ. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials. Cancer Res. 2012;72(10):2600–2608. doi: 10.1158/0008-5472.CAN-11-2303. [DOI] [PubMed] [Google Scholar]
- 52.Oronsky B, Reid TR, Larson C, Carter CA, Brzezniak CE, Oronsky A, Cabrales P. RRx-001 protects against cisplatin-induced toxicities. J Cancer Res Clin Oncol. 2017;17 doi: 10.1007/s00432-017-2416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ning S, Sekar TV, Scicinski J, Oronsky B, Peehl DM, Knox SJ, Paulmurugan R. Nrf2 activity as a potential biomarker for the pan-epigenetic anticancer agent, RRx-001. Oncotarget. 2015;6(25):21547–21556. doi: 10.18632/oncotarget.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 55.Reid T, Oronsky B, Scicinski J, Scribner CL, Knox SJ, Ning S, Peehl DM, Korn R, Stirn M, Carter CA. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol. 2015;16(9):1133–1142. doi: 10.1016/S1470-2045(15)00089-3. [DOI] [PubMed] [Google Scholar]
- 56.Sonis S, Treister N, Chawla S, Demetri G, Haluska F. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116(1):210–215. doi: 10.1002/cncr.24696. [DOI] [PubMed] [Google Scholar]
- 57.Patt HM, Tyree EB, Straube RL, Smith DE. Cysteine protection against X-irradiation. Science. 1949;110:213–214. doi: 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- 58.Kamran MZ, Ranjan A, Kaur N. Sur, Tandon V. Radioprotective Agents: Strategies and Translational Advances. Med Res Rev. 2016;36(3):461–493. doi: 10.1002/med.21386. [DOI] [PubMed] [Google Scholar]
- 59.Bump EA, Malaker K. CRC Press; Boca Raton, FL: 1998. Radioprotectors: chemical, biological and clinical perspective. [Google Scholar]
- 60.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 61.Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004;3:261–264. doi: 10.1016/j.radonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Koukourakis MI. Amifostine: is there evidence of tumor protection? Semin Oncol. 2003;30(6 Suppl. 18):18–30. doi: 10.1053/j.seminoncol.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Xie X, O'Neill W, Pan Q. Immunotherapy for head and neck cancer: the future of treatment? Expert Opin Biol Ther. 2017;17(6):701–708. doi: 10.1080/14712598.2017.1315100. [DOI] [PubMed] [Google Scholar]
- 64.Napenas JJ, Shetty KV, Streckfus CF. Oral mucositis: review of pathogenesis, diagnosis, prevention, and management. Gen Dent. 2007;55(4):335–344. [quiz 345-6, 376] [PubMed] [Google Scholar]
- 65.Sonis ST. A biological approach to mucositis. J Support Oncol. 2004;2(1):21–32. [discussion 35-36] [PubMed] [Google Scholar]