Abstract
Macrophages are crucial drivers of inflammatory corneal neovascularization and thus are potential targets for immunomodulatory therapies. We hypothesized that therapeutic use of cornea derived mesenchymal stromal cells (cMSCs) may alter the function of macrophages. We found that cMSCs can modulate the phenotype and angiogenic function of macrophages. In vitro, cMSCs induce apoptosis of macrophages while preferentially promoting a distinct CD14hiCD16hiCD163hiCD206hi immunophenotype that has significantly reduced angiogenic effects based on in vitro angiogenesis assays. In vivo, application of cMSCs to murine corneas after injury leads to reduced macrophage infiltration and higher expression of CD206 in macrophages. Macrophages co-cultured (“educated”) by cMSCs express significantly higher levels of anti-angiogenic and anti-inflammatory factors compared to control macrophages. In vivo, injured corneas treated with cMSC-educated macrophages demonstrate significantly less neovascularization compared to corneas treated with control macrophages. Knocking down the expression of PEDF in cMSCs significantly abrogates its modulating effects on macrophages, as shown by the reduced rate of apoptosis, decreased expression of sFLT-1/PEDF, and increased expression of VEGF-A in the co-cultured macrophages. Similarly, cMSCs isolated from PEDF knockout mice are less effective compared to wild type cMSCs at inhibiting macrophage infiltration when applied to wild type corneas after injury. Overall, these results demonstrate that cMSCs therapeutically suppress the angiogenic capacity of macrophages and highlight the role of cMSC secreted PEDF in the modulation of macrophage phenotype and function.
Keywords: Mesenchymal Stromal Cells, Cornea, Macrophages, Inflammation, Angiogenesis, Pigment Epithelial Derived Factor, PEDF
Graphical abstract
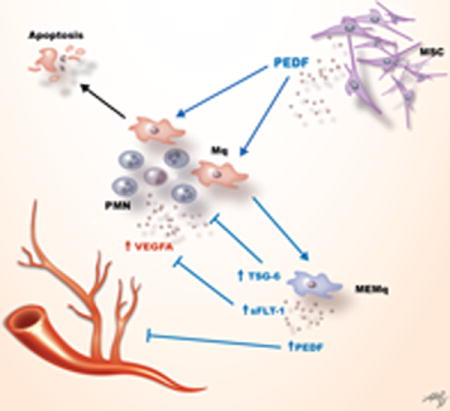
In the setting of severe corneal injury or inflammation, chemokines and cytokines recruit inflammatory cells which in turn induce corneal angiogenesis via VEGF-A. Cell therapy with corneal derived MSCs could modulate this inflammation in part via PEDF by inducing apoptosis of macrophages and promoting a distinct macrophage immunophenotype (cMSC-educated macrophages - cMEMq) that secrete high levels of sFLT-1, PEDF, and TSG-6.
Introduction
The absence of blood vessels and hematopoietic cells including erythrocytes and leukocytes in the cornea is crucial for optical clarity and maintenance of vision [1]To achieve this, the cornea possesses an extensive array of mechanisms by which immune effector cells and neovascular processes are intricately regulated. These mechanisms include local expression of immunomodulatory factors such as Fas ligand, programmed death-ligand 1 (PD-L1) and TGF-β, as well as expression of anti-angiogenic factors such as soluble vascular endothelial growth factor receptor 1 (VEGFR-1; soluble fms-like tyrosine kinase-1), VEGFR3, thrombospondin-1 and pigment epithelium derived factor (PEDF) [2-7].
Corneal neovascularization is often the final consequence of severe corneal infections or inflammation, which alter the balance in favor of angiogenic factors. Under most pathologic conditions that lead to corneal neovascularization, inflammatory cells including neutrophils and macrophages infiltrate the cornea. Macrophages in particular have been shown to play a central role in the development of inflammatory corneal neovascularization by releasing pro-angiogenic and pro-inflammatory cytokines [8-10]. Depleting macrophages largely prevents the development of corneal hemangiogenesis and lymphangiogenesis [11].
Current treatment options for corneal neovascularization include topical anti-inflammatory therapy, mainly corticosteroids, and local administration of anti-VEGF agents [12-14]. While both options can be effective in select situations, they have significant limitations. Corticosteroid-related side effects preclude their long-term use and even their short-term use in many scenarios including infectious and wound healing disorders. Anti-VEGF biological agents, which are used off label for corneal neovascularization, pose a challenge in both administering and maintaining sufficient tissue levels, while also having the potential for adversely affecting the tissue repair processes - given the pleiotropic nature of VEGF signaling [15]. Thus, many patients end up developing corneal neovascularization despite maximal standard therapies, underscoring the need for safer and more effective options in the clinic.
Mesenchymal stromal cells (MSCs) have been investigated therapeutically for a wide range of human diseases based on their immunomodulatory, anti-inflammatory, anti-fibrotic and other tissue repair properties; therefore, providing an attractive candidate for many cell based therapies [16-24]. It has been shown that one of major mechanisms of action of MSCs is through their interactions with the immune system, in particular macrophages [25-27]. In this current study, we hypothesized that corneal derived MSCs (cMSCs) can therapeutically modulate the immunophenotype and angiogenic properties of macrophages by promoting a macrophage phenotype that secretes higher levels of anti-inflammatory and anti-angiogenic factors. We specifically characterize and investigate the mechanistic basis of this therapeutic modulation of macrophages by cMSCs.
Materials and Methods
Mice
All experiments in mice were conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocols were approved by the Animal Care & Use Committee of the University of Illinois at Chicago (UIC; Protocol numbers 15-258 and 14-166). PEDF−/− mice on C57BL/6J background were kindly provided by Dr. Paul J Grippo (UIC). C57BL/6J mice were used as wild type.
Cell Transplantation
Limbal stem cell deficiency was induced by total corneal epithelial debridement which also creates an inflammatory environment that leads to corneal neovascularization [28, 29]. A 6-0 nylon suture was pre-passed through the lids (in preparation for closing the lids afterwards). Subsequently, 0.2 μl thrombin (Ethicon, Johnson and Johnson Inc.) was placed over the debrided cornea. A total of 5000 cells, resuspended in 3 μl fibrinogen (Ethicon, Johnson and Johnson Inc.), were transferred over the cornea [30, 31]. After gel formation, the suture was tightened to closed the lids and then tied. Erythromycin ophthalmic ointment was applied. The suture was removed after 3 days.
cMSC Culture
Human and mice corneal MSCs were isolated, expanded and characterized as we described before [30] (See Supplementary Figure 1 for PEDF−/− cMSCs). Briefly, corneoscleral button from healthy cadaver eyes or freshly enucleated mouse eyes were washed 5 times with PBS containing 2× Antibiotic-Antimycotic and 2× penicillin- streptomycin (both from Thermo Fisher). After removing the central button with a trephine, the limbus was cut into 3 segments and were placed in 2.4 IU of Dispase II (Thermo Fisher) for 1 hour at 37°C. Intact epithelial sheets were removed from stroma. The limbal segments were cut into small pieces and incubated in collagenase Type I (0.5 mg/ml) (Sigma-Aldrich) in DMEM/F12 media (Thermo Fisher) overnight at 37°C or else used directly for explant culture. The digests were filtered through a 70-μm nylon strainer to obtain a single-cell suspension. They were seeded onto 1% gelatin (Sigma-Aldrich)–coated wells of a 6-well tissue culture plate in alpha MEM media supplemented with 10% fetal bovine serum, 1X L-Glutamine, and 1X NEAA (All from Corning, Manassas, VA). Culture media were changed every other day, and cells were sub-cultured by brief digestion with TrypLE Express (Thermo Fisher) when 80% confluent. At least 3 mouse cMSC lines were used from wild type and PEDF−/− mice. For this study, 5 human cMSC lines were isolated from corneoscleral button of cadaveric donors (C-4977, C-4732, C-6179, C-6436, and C-5422) generously provided by Eversight Eye Bank (Chicago IL). Since the research did not involve human subjects and the tissue was from cadaver donors without any identifying or protected health information, IRB approval was not necessary per guidelines of the Office for the Protection of Research Subjects at UIC. Data from different donors were pooled for statistical analysis.
MSC Conditioned media
Upon reaching 100% confluency in a T175 flask, the MSCs were washed with 30 ml pre-warmed PBS 3 times. The media was then changed to phenol red free alpha MEM media supplemented with 1X L-Glutamine, and 1X NEAA. The conditioned media was collected after 48 hours. The cells were trypsinzed and counted at the same time. The conditioned media was centrifuged with 500 G speed for 15 minutes to remove any cells or debris. The supernatant was transferred to a new tube and was used for experiments or kept at 4° C for up to a week.
MSC Differentiation
After incubation in appropriate differentiation medium (StemPro® Differentiation kits, all from Thermofisher), cMSC differentiation into mesenchymal lineages (osteoblasts and adipocytes) was evaluated using staining with Alizarin Red for calcium and Oil-Red-O for fatty acids, respectively.
Immunostaining
Corneal whole mount immunostaining was done as described before [32]. Briefly, after enucleation of the eye, the cornea was dissected using a spring scissor. It was then fixed with 4% paraformaldehyde at 4° C overnight. After washing with PBS, it was incubated with 20 μg/ml Proteinase-K (Sigma-Aldrich) for 5 minutes at room temperature followed by 100% methanol for another 30 minutes. It was then incubated with 10% serum and 2% BSA at 4° C overnight. Purified anti-mouse CD31 (Biolegend, cat# 102401) was used as primary antibody and Rhodamine-conjugated donkey anti-rat IgG (Jackson ImmunoResearch, Cat# 712-025-153) as secondary antibody. The slides were visualized and photographed using the Zeiss LSM 710 microscope (Carl Zeiss). To make the quantification more objective, we used VesselJ [33] to quantify the ratio of vascularized area to total corneal area.
Tube Formation Assay
Human umbilical vein endothelial cells (HUVEC, Thermo Fisher) were cultivated in Media 200 fortified with low serum growth supplement (LSGS). At passage 4, the cells were detached and resuspended in Media 200 + 10% LSGS which was used as the base media. The 96-well plate were filled with 75 μl Matrigel (Thermo Fisher) and allowed to solidify at 37° C after which HUVECs (2 × 104 cells/75 μl) were gently seeded on top of the gel. Subsequently, 75 μl of conditioned media, complete media or Media 200 was added as the testing condition, positive and negative controls, respectively. After 6 h, network structures were analyzed and photographed at 4× magnification (Leica Microsystems). Total tubule length per image was calculated using Angiogenesis Analyzer plugin of ImageJ (NIH).
Enzyme-Linked Immunosorbent Assay (ELISA)
To get macrophage conditioned media, they were washed thoroughly with PBS after 7 days of education and received fresh complete media. After 24 hours, the media was collected and cells were detached and counted. The conditioned media was cleared of cellular debris by centrifugation at 500 g for 15 min. The supernatant was transferred to a new tube and was used for experiments or kept at 4° C for up to a week.
Human TSG-6 protein levels in the conditioned media were determined by ELISA as described before [34]. Human PEDF (cat# DY1177-05), sFLT-1 (cat# DVR100B) and VEGF-A (cat# DVE00) proteins were detected with commercially available ELISA kits (all from R&D Systems) according to the manufacturer’s protocol. The obtained values were normalized to total cell numbers.
siRNA transfection
After reaching 70 to 80% of confluency, cMSCs were transfected with 50 nM PEDF siRNA (Santa Cruz, Cat# sc-40947) or scrambled siRNA (Dharmacon, Cat# D-001205) in TransIT-TKO siRNA transfection reagent (Mirus, Cat# MIR2150). The transfection efficiency was tested with ELISA on the conditioned media.
Human Macrophage culture
Macrophages were isolated and cultivated as described before [35, 36]. Briefly, peripheral whole blood buffy coats from healthy donors were purchased from Interstate Blood Bank, INC (Memphis, Tennessee). Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coats by density-gradient separation. Monocytes were isolated using anti-human CD14 microbeads (Miltenyi Biotech) and autoMACS Pro Separator (Miltenyi) according to manufacturer’s protocol. Purity of isolated CD14+ cells was over 95% when checked using an Acuri C6 cytometer (BD Biosciences). Purified CD14+ monocytes were plated into the bottom chamber of 6 well 0.4 um pore size transwell cell culture plates (Costar, Cat# 3450) at a concentration of 1 × 106 per well in IMDM media supplemented with 10% human serum blood type AB (Mediatech, Herndon, VA, USA), 1X Non-essential amino acids (NEAA-Lonza), (Thermo Fisher), 1mM sodium pyruvate (Mediatech) and 4 μg/ml recombinant human insulin (Invitrogen). Monocytes were cultured for 7 days at 37° C with 5% CO2 to differentiate to macrophages.On day 7, macrophages were supplemented with fresh media and incubated with cMSCs in the upper chamber of the transwell plate to obtain cMSC educated Macrophages (cMEMqs) at a ratio of 10: 1 of Macrophages:cMSCs, as previously described [36]. After 7 days of co-culture, cMEMqs were collected using Stem Pro Accutase cell detachment (Thermo Fisher) for experiments. For control, macrophages were either cultured in the same media without cMSCs or co-cultured with a non-mesenchymal cell type.
Mouse Macrophage Culture
Mouse peritoneal macrophages were isolated as described previously [37]. In brief, mice were euthanized by CO2 asphyxiation and then cleaned with 70% ethanol and mounted onto a Styrofoam block. Outer skin of the peritoneum was cut with a scissor and forceps and gently pulled back to expose the inner skin lining the peritoneal cavity. Using a 22-gauge needle, 10 ml of ice cold IMDM media was injected into the peritoneal cavity with precaution not to puncture any organ. The media, that contains dislodged cells, was aspirated using a 19-gauge needle. The cell suspension was centrifuged and washed twice. Subsequently, they were purified using F4/80 magnetic beads (Miltenyi) according to the manufacturer’s protocol. They were plated into 6 well cell culture plates at a concentration of 1 × 106 per well in IMDM media supplemented with 10% FBS, 1X NEAA, 1mM sodium pyruvate and 4 μg/ml recombinant human insulin. On day 3, they were supplemented with fresh media and incubated with mouse cMSCs similar to what was described above for the human macrophage co-culture. On day 10, mouse cMEMqs were collected using Accutase cell detachment for experiments.
Flow Cytometry
The cells were detached as described above. They were incubated with Fc block (BD Pharmingen, cat#: 564220 for human and Biolegend TruStain fcX™ [anti-mouse CD16/32], cat# 101319 for mouse) and stained at 4°C for 20 minutes in antibody diluent (PBS with 2% FBS) with cell surface antibodies (Supplementary Table 1). For intracellular staining, fixation buffer (Biolegend, cat# 420801) and intracellular staining perm wash buffer (Biolegend, cat# 421002) were used according to manufacturer’s protocol. Compensation was performed using Ultracomp e-beads (Cat# 01-2222-42) ebiosciences, (San Diego, CA). Flow cytometry data was acquired on the MACSQuant analyzer 10 (Miltenyi) and BD LSR Fortessa (BD). Data were analyzed using FlowJo software (TreeStar).
Fluorescent-Activated Cell Sorting (FACS)
Seven days after the procedure, mice were euthanized. The cornea was digested in 0.25% trypsin (Thermo Fisher). After filtration through a 70 μl nylon strainer, the cells were stained as described in Flow Cytometry section. Cell sorting was done by a Sony cell sorter (Sony Biotechnology SH800).
Apoptosis assay
To measure caspase 3/7 activation, ApoTox-Glo™ Triplex Assay (Promega) was used according to the manufacturer’s protocol.
Real-time quantitative PCR
Total RNA was extracted using the miRCURYtm RNA isolation kit (Exiqon) according to manufacturer’s protocol. After spectrophotometric assessment for quality and concentration (Nanodrop ND-1000; Thermo Scientific), 2 μg of purified RNA was reverse transcribed to single-strand cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc [ABI]) according to the manufacturer’s protocol. TaqMan Universal PCR Master Mix (ABI) or FastStart Universal SYBER GREEN Master (Roche, Germany) was used to make the master-mix. All primers were bought from Integrated DNA technology (IDT; Iowa, USA) (Supplementary Table 2). To detect human sFLT-1, we used 5′-TTTGTTGCAGTGCTCACCTC-3′ (Forward) and 5′-GTTGGGACTGTGGGAAGAAA-3′ (Reverse) primer that only detects splice variant 2 of FLT-1 mRNA. Real-time qPCR was performed using QuantStudio 7 Flex Real-Time PCR System (ABI). The mRNA expression levels were normalized to the expression of a housekeeping gene, Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH). The 2-ΔΔCT method was used to quantify relative mRNA expression.
Data Analysis and Statistical Comparisons
To avoid any observer bias and to increase reproducibility, all the animal surgeries were done by one of the authors who was blinded to the treatment arms. Moreover, all the data collection and analyses were done in a blinded fashion to minimize inter and intra-observer bias. Eyes with infection were excluded from the study. Corneal infection was defined as corneal edema, hypopyon, and exudate within the first 7 days after the procedure. Results are presented as the mean ± SD of three independent experiments. Normality of the data was tested using D’Agostino & Pearson normality test. Based on normality test, Mann-Whitney U-test or 2-sided student’s t-test was performed to determine significance, which was set at p < 0.05. For more than 2 arms comparison, One-way ANOVA with Tukey’s post hoc correction was used. All statistics were performed using GraphPad Prism software 7.0 (GraphPad Software) and Microsoft Excel (Microsoft).
Results
cMSCs modulate macrophage immunophenotype
To evaluate the effects of human cMSCs on the macrophages, we used our previously described co-culture method [35, 36]. Macrophages co-cultured with cMSCs demonstrated reduced expression of CD86 and HLA-DR, markers typically associated with inflammatory macrophages, and at the same time showed increased expression of CD163, CD206, CD14 and CD16, markers associated with an inflammation-resolving macrophage immunophenotype (Fig. 1B) [8, 38-40]. Human cMSC conditioned media was found to induce macrophage apoptosis (Fig. 1C).
Figure 1. cMSCs modulate macrophage immunophenotype.
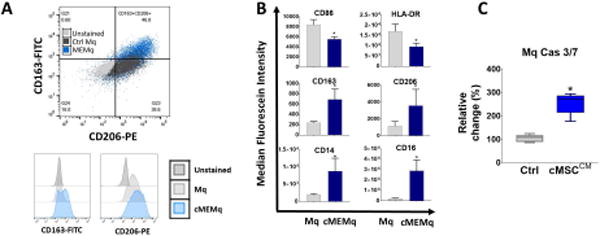
A: Control Mq and cMEMqs were expanded as described in the methods. On day +14, human control Mq or cMEMqs were analyzed by flow cytometry. Cell surface expression of CD86, HLA-DR, CD206 (Mannose receptor), CD163 (scavenger receptor), CD14 and CD16 were determined in control Mq and cMEMqs. Histograms represent MFI (Mean fluorescent intensity) in unstained Mq, control Mq and cMEMqs. B: The mean from independent biological replicated are presented as the bar charts. Educating macrophages with cMSCs reduced the expression of CD86 and HLA-DR, and increased the expression of CD163, CD206, CD14 and CD16 (n=4, 2-sided t-test * P <0.001). The values shown are mean ± SD (error bars). C: Conditioned media from human cMSCs induced more apoptosis in macrophages compared to unconditioned media (n=5, Mann-Whitney U-test: P = 0.0079) Boxes show the interquartile (25%–75%) range, whiskers encompass the range (minimum–maximum), and horizontal lines represent the mean. MFI: median fluorescein intensity, Cas: Caspase, CM: conditioned media, Ctrl: control, Mq: macrophage, cMEMq: cMSC-educated macrophage.
We further investigated the effect of cMSCs on the macrophage immunophenotype in an in vivo corneal injury model. We embedded murine cMSCs in fibrin gels and applied them to the cornea immediately after epithelial injury. Clinical and histologic studies of the corneas after corneal epithelial debridement demonstrated significantly reduced haze with reduced F4/80+ macrophages infiltration in the corneas treated with murine cMSCs compared to those treated with fibrin gels alone (Fig. 2A). To quantify and characterize the infiltrated macrophages, we digested the mice corneas 7 days after treatment and subjected them to flow cytometry. The murine cMSC treated corneas had significantly reduced number of F4/80+ macrophages. However, expression of CD206 was higher in F4/80+ macrophages in the murine cMSC treated corneas compared to fibrin gel treated corneas (P <0.001) (Fig. 2B).
Figure 2. cMSCs modulate macrophage phenotype and recruitment into the corneal stroma after injury.
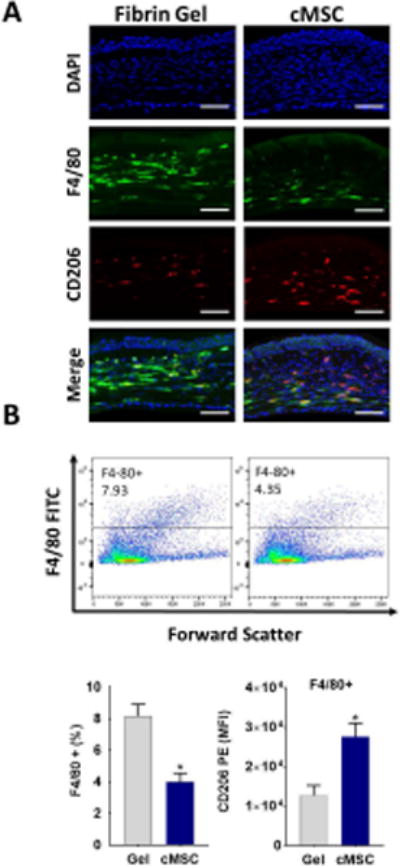
A: Representative immunofluorescence staining after topical application of murine cMSCs to the injured murine cornea, demonstrating significantly reduced infiltration of F4/80+ macrophages, and increased CD206+ cells in the stroma compared to control treated corneas. Scale bar, 50 μm. B: To quantify, corneas were digested and subjected to flow cytometry which demonstrated significantly reduced the percentage of F4/80+ cells to total cells in murine cMSCs treated corneas compared to the control. Among the F4/80+ gated cells, there was the higher expression of CD206 in the cMSCs treated corneas compared to control treated corneas (n=5, 2-sided t-test * P <0.001).
cMSCs modulate the angiogenic properties of macrophages
To investigate the effect of cMSCs on the angiogenic function of macrophages, we examined the protein and gene expression of macrophages after 7 days of co-culture with cMSCs – defined as cMSC educated macrophages (cMEMqs). By gene expression, we found the CD14+CD163+ sorted population of cMEMqs expressed significantly lower levels of VEGF-A, and significantly higher levels of anti-angiogenic genes (sFLT-1, PEDF and TIMP-1) relative to CD14+CD163+ control sorted macrophages (Fig. 3A). Similarly, cMEMqs secreted more sFLT-1, PEDF, and TSG-6, without any significant change in VEGF-A compared to control macrophages (Fig. 3B).
Figure 3. cMSCs modulate the angiogenic properties of macrophages.
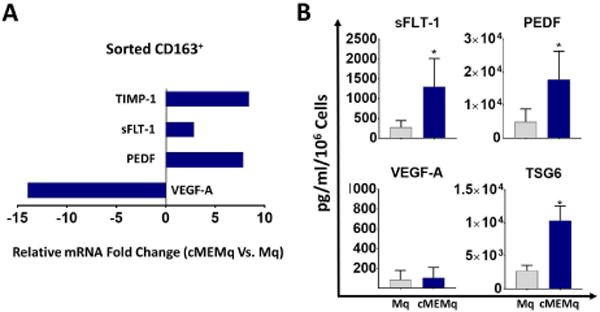
A: CD14+CD163+ population of human cMEMqs were isolated by fluorescence-activated cell sorting and subjected to quantitative RT-PCR demonstrating lower expression of VEGF-A and higher expression of PEDF, sFLT-1, and TIMP-1 compared to CD14+CD163+ sorted control macrophages (n=5, P <0.001 for all comparison). B: The conditioned media from cMEMqs contains more sFLT-1 (1296 ± 715.3 vs. 276.6 ± 168.0 pg/ml), PEDF (17741 ± 8546 vs. 4956 ± 3851 pg/ml) and TSG-6 (10357 ± 2190 vs. 2757 ± 823.7 pg/ml) compared to the conditioned media from control untreated macrophages (n=5). The values shown are mean ± SD (error bars). 2-sided t-test: * P < 0.0001. Mq: macrophage, cMEMq: cMSC-educated macrophage.
To investigate the angiogenic function of macrophages in vivo, we used a limbal stem cell deficiency model. In this model, the entire corneal epithelium is mechanically debrided limbus to limbus which results in severe corneal neovascularization. After 7 days of co-culture with mouse cMSCs, mouse macrophages were embedded in fibrin gels and applied to the cornea after total epithelial injury. Corneas treated with cMSC educated mouse macrophage (murine cMEMq) had significantly less corneal neovascularization compared to control mouse macrophages treated corneas (Fig. 4A). Likewise, the conditioned media from cMEMqs induced significantly less vascular endothelial tubule formation compared to control macrophage conditioned media (Fig. 4B).
Figure 4. cMSC-educated macrophages demonstrate reduced angiogenic properties.
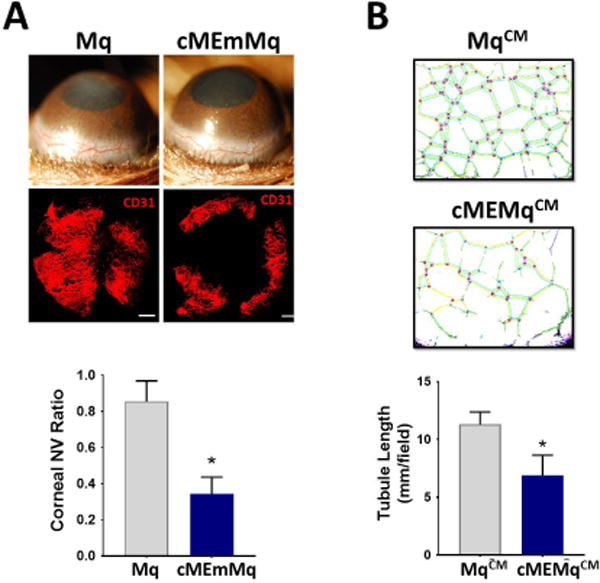
A: Murine macrophages were co-cultured with murine cMSCs as described in methods (cMEmMq). Applying cMEmMqs in fibrin gels to the cornea of wild-type mice after injury resulted in significantly less neovascularization compared to control macrophages on day ten (n=5, 2-sided t-test: * P < 0.0001). Scale bar, 500 μm. B: Conditioned media from cMEMqs inhibited tubule formation by human umbilical vein endothelial cells (HUVEC cells) compared to conditioned media from control untreated macrophages (n=5, 2-sided t-test: * P < 0.0001). Mq: macrophage, cMEmMq: cMSC-educated mouse macrophage, NV: neovascularization, CM: conditioned media.
cMSC derived PEDF is a key mediator of macrophage modulation
To investigate the mechanism of macrophage modulation by cMSCs, we focused on cMSC-secreted PEDF, given its combined anti-angiogenic and anti-inflammatory effects [1, 41, 42]. We isolated cMSCs from PEDF−/− mice and used our in vivo inflammatory injury model as described above to investigate the potential role of PEDF. Injured wild type murine corneas treated with PEDF−/− murine cMSCs demonstrated more macrophage infiltration with reduced expression of CD206 compared to wild type (PEDF+/+) cMSCs treated corneas (Fig. 5A).
Figure 5. cMSC derived PEDF is a key mediator of macrophage modulation.
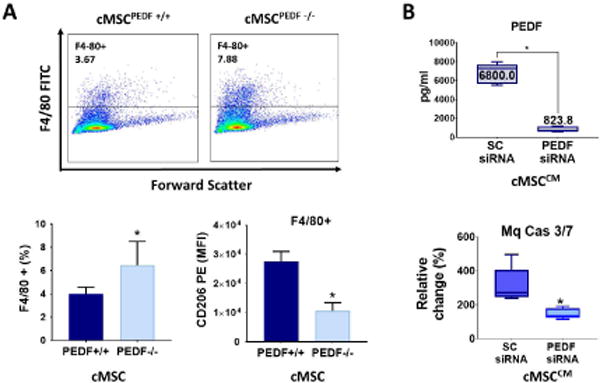
A: Injured wild-type corneas treated either with cMSCs obtained from the PEDF−/− mice (placed in fibrin gels) or from wild-type PEDF +/+ mice were digested and subjected to flow cytometry. Injured wild-type corneas treated with cMSCPEDF−/− had more infiltration of F4/80+ macrophages with lower expression of CD206 compared to the those treated with cMSCPEDF+/+ (n=5; *P = 0.03). B: knocking down PEDF by siRNA decreased PEDF protein by almost 90% compared to scrambled siRNA (n=9, 2-sided t-test: *P<0.0001). Conditioned media from PEDF knockdown cMSCs induced less apoptosis in macrophages compared to conditioned media from control cMSC (n=5, Mann-Whitney U-test: P = 0.007). The values shown in bar graphs are mean ± SD (error bars). Boxes show the interquartile (25%–75%) range, whiskers encompass the range (minimum–maximum), and horizontal lines represent the mean. 2-sided t-test used for all comparison. MFI: median fluorescein intensity, SC: scrambled, Mq: macrophage, Cas: Caspase.
By gene expression, mouse macrophages educated with PEDF −/− cMSCs expressed more iNOS and less arginase-1 with concomitant reduction in the expression of IL-10, PEDF, and sFLT-1 compared to the control macrophages (Fig. 6A). Similar results were found with human cMSC educated macrophages. In particular, human macrophages were co-cultured with PEDF-knockdown cMSCs and found to secrete more VEGF-A, less PEDF and less sFLT-1 compared to conditioned media from macrophages educated with control (scrambled siRNA) cMSCs (Fig. 6B). The rate of apoptosis in macrophages was likewise significantly reduced when they were exposed to the conditioned media from PEDF-knockdown cMSCs compared to control (Fig. 5B). Overall, these results implicate a mechanistic role for PEDF in the cMSC modulation of macrophages and their angiogenic function.
Figure 6. cMSC derived PEDF is a key mediator of macrophage angiogenic function.
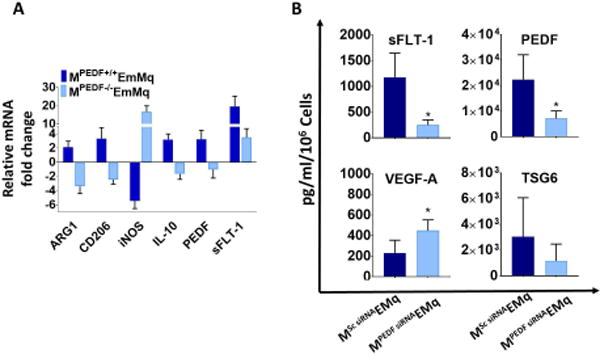
A: RT-qPCR shows mouse macrophages educated with wild type PEDF+/+ cMSCs, expressed more arginase-1, CD206, IL-10, PEDF, and sFLT-1; and less iNOS mRNA compared to control untreated macrophages. Conversely, macrophages educated with PEDF −/− cMSCs, expressed less arginase-1, CD206, IL-10, PEDF, and sFLT-1 with more iNOS (n=5; P <0.001 for all comparison). B: Macrophages educated with PEDF knockdown cMSCs secreted more VEGF-A (448.3 ± 104.1 vs. 229.4 ± 125.1 pg/ml; P = 0.03) and less PEDF (7162.1 ± 2918.0 vs. 22373 ± 9683.0 pg/ml; P = 0.02) and sFLT-1 (249.1 ± 98.54 vs. 1172 ± 473.4 pg/ml; P = 0.008) compared to those trained with control cMSCs. The mean TSG-6 expression although decreased was not statistically significant (1160 ± 1309.0 vs. 3035 ± 3032.0 pg/ml) (P = 0.32). The values shown in bar graphs are mean ± SD (error bars). 2-sided t-test used for all comparison. MPEDF EmMq: cMSCPEDF-educated mouse macrophage, MSc siRNA EMq: cMSCScrambled siRNA educated macrophage, MPEDF siRNA EMq: cMSCPEDF siRNA educated macrophage.
Discussion
In this study, we focused on the modulatory effects of cMSC on macrophages and show that cMSC-educated macrophages have significantly reduced angiogenic effects both in vitro and in vivo. cMSCs specifically induce markers that are associated with the anti-inflammatory and pro-resolving function of the macrophages. Mechanistically, we found cMSC secreted PEDF as an important mediator of its macrophage modulation.
It is well accepted that the therapeutic effects of MSCs, most commonly derived from the bone marrow, are mediated largely through their immunomodulatory and anti-inflammatory properties [43-47]. MSCs have been shown to modulate both innate and adaptive immune mechanisms [48-51]. They have also been implicated in angiogenesis and wound repair [52-54]. Macrophages, in particular, have been studied as one of the major mediators of MSCs’ immunomodulatory and regenerative effects [25-27, 55-60]. The current study provides the first direct evidence that MSCs derived from the cornea modulate the immunophenotype and angiogenic function of macrophages in a distinct manner. An important finding of our studies is that cMSCs induce apoptosis of macrophages in vitro as a potential mechanism of suppressing the monocytic inflammatory response. Likewise, cMSC treated corneas in vivo demonstrated significantly reduced macrophage recruitment.
Macrophages exhibit extraordinary phenotypic plasticity during different stages of inflammation and repair, where they dynamically orchestrate an immune and later a reparative response specific for that tissue [61]. Traditionally, macrophages have been classified into subtypes, such as classic versus alternatively activated, or M1 and M2, mostly defined by in vitro cytokine stimulation and subsequent marker/cytokine expression profile [8]. This study provides new insights into the distinct phenotype of macrophages “educated” by cMSCs. Our results demonstrated that cMSCs promote markers of M2 (CD163, CD206) while suppressing those associated with M1 phenotype (CD86, HLA-DR). However, it may be simplistic to consider macrophage plasticity just as a simple binary phenomenon [62]. A satisfactory paradigm should take into account the specific function of the macrophages, along with spatial and temporal attributes of their environment, including the stage of inflammation and their tissue of residence [61]. Consistent with their dynamic nature, macrophages could be both anti-angiogenic and/or pro-angiogenic, depending on the stage of inflammation, playing a critical and complex role in regulating angiogenesis at sites of tissue injury [63-65]. We have shown that cMSCs promote macrophages with a distinct phenotype favorable for the terminal stages of corneal tissue repair and remodeling. Specifically, cMSC-educated macrophages demonstrated high expression of CD163, CD206, CD14 and CD16 consistent with a profile of macrophages that help resolve inflammation in part by phagocytizing apoptotic cells, including neutrophils [39, 66, 67].
In the cornea, macrophages have been shown to play a central role in the development of inflammatory corneal neovascularization and depleting macrophages largely prevents the development of hemangiogenesis and lymphangiogenesis [9, 11]. In our study, cMSCs, when used therapeutically, not only reduced macrophage infiltration after injury in vivo but also in co-culture experiments in vitro, enhanced the macrophages’ secretion of anti-angiogenic factors (sFLT-1 and PEDF), consistent with the role of these macrophages during the latter phases of corneal tissue repair and remodeling [64, 65, 68]. It is well-known that macrophages have remarkable plasticity based on the spatial and temporal attributes of their environment, including the stage of inflammation and their tissue of residence [62]. Therefore, the observed phenotype of cMEMqs are not likely to be end stage and can potentially change with time.
Our findings also implicate cMSC derived PEDF as an important mediator of their modulating effects on macrophages. During skin wound healing, PEDF is typically found in the remodeling phase where regression of neo-vessels takes place, a phenomenon that is important not only in the cornea but also in vascularized tissues such as the skin [69]. PEDF selectively inhibits the formation and promotes the regression of neo-vessels but has no detrimental effect on mature vascular structures [41]. The inhibitory effect of PEDF on VEGF-induced angiogenesis is mediated in part via γ-secretase-dependent cleavage of the C terminus of VEGF receptor (VEGFR)-1, which in turn inhibits VEGFR-2-induced angiogenesis [70]. PEDF has likewise been recognized to exert anti-inflammatory effects in part by inhibiting macrophage recruitment and activation [42, 71]. Our results demonstrate that cMSC derived PEDF is not only involved in the induction of macrophage apoptosis, but also contributes to the development of anti-inflammatory macrophages which, in the terminal stage of repair, function by removing immune cells, granulation tissue and unnecessary neovasculature.
As noted earlier, we only studied cMSCs in the context of their therapeutic effects as a cell therapy and did not specifically examine the role of cMSCs that already exist in the cornea during inflammation. We also focused specifically on cornea-derived MSCs and did not study MSCs from other sources. However, it has been shown that bone marrow derived MSCs are also able to modulate macrophage immunophenotype and function [26, 27, 35, 36, 47, 49, 55, 58, 72, 73]. Further studies are needed to determine if MSCs from other sources can similarly modulate the angiogenic function of macrophages.
Overall, this study advances our understanding of the mechanisms by which cMSCs modulate macrophages and specifically adds PEDF to the list of important mediators (such as TSG-6, stanniocalcin 1, and prostaglandin E2) which MSCs secrete to modulate macrophages [26, 34, 74-77]. It will be interesting to further examine the role of PEDF in mediating the therapeutic effects of MSCs from other source including the bone marrow.
Conclusion
Our study reveals that corneal derived MSCs can therapeutically modulate the immunophenotype and the angiogenic function of the macrophages in part through PEDF. These results highlight the potential of tissue specific MSCs for targeted immunomodulatory and anti-angiogenic cell-based therapies.
Supplementary Material
Significance Statement.
Macrophages play a central role in pathologic corneal neovascularization. We found that mesenchymal stromal cells from the cornea (cMSCs) therapeutically modulate the inflammatory and angiogenic functions of macrophages. In particular, cMSCs induce macrophage apoptosis and preferentially promote macrophages that secrete high levels of anti-angiogenic factors. We further demonstrate that cMSC modulation of macrophages significantly depends on cMSC secreted pigment epithelium-derived factor.
Acknowledgments
We thank Lauren Kalinoski for assistance in creating the schematics, Ruth Zelkha for assistance with imaging, Tara Nguyen for assistance with animal work, and Dr. Balaji Ghanesh for assistance with flow cytometry. The authors also thank Dr. Luisa DePietro at University of Illinois at Chicago and Myriam Bouchlaka at University of Wisconsin-Madison for helpful scientific discussions and comments. We thank Dr. Paul J Grippo for sharing the mice.
Grants
This work was supported by Clinical Scientist Development Program Award K12EY021475 (ME), R01 EY024349-01A1 (ARD) and Core grant EY01792 from NEI/NIH; MR130543 (ARD) from US Department of Defense, U.S. ARMY, Vision for Tomorrow (ARD), University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520 (PH), unrestricted grant to the department from RPB; Eversight (providing both seed funding and human corneal research tissue) and UW-Madison Don Anderson fund for GVHD research, and Crystal Carney Fund for Leukemia Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ contributions:
Medi Eslani: Conception and design, Financial support, Administrative support, Provision of study material or patients, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Ilham Putra: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Xiang Shen: Administrative support, Provision of study material or patients, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Judy Hamouie: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Asha Tadepalli: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Khandaker N. Anwar: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
John A. Kink: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Samaneh Ghassemi: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Gaurav Agnihotri: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Sofiya Reshetylo: Collection and/or assembly of data, Data analysis and interpretation, Final approval of manuscript.
Alireza Mashaghi: Provision of study material or patients, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Reza Dana: Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Peiman Hematti: Conception and design, Financial support, Administrative support, Provision of study material or patients, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Ali R. Djalilian: Conception and design, Financial support, Administrative support, Provision of study material or patients, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Disclosure of Potential Conflicts of Interest
Ali Djalilian discloses consultant/advisory role with Novartis, Vomaris, Abbvie.
All other authors indicate no potential conflicts of interest.
References
- 1.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Transactions of the American Ophthalmological Society. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 2.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67:1503–1508. doi: 10.1097/00007890-199906270-00001. [DOI] [PubMed] [Google Scholar]
- 5.Streilein JW, Yamada J, Dana MR, et al. Anterior chamber-associated immune deviation, ocular immune privilege, and orthotopic corneal allografts. Transplantation proceedings. 1999;31:1472–1475. doi: 10.1016/s0041-1345(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 6.Cursiefen C, Masli S, Ng TF, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Investigative ophthalmology & visual science. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, Yamagami S, Tanaka K, et al. Immunologic mechanisms of corneal allografts reconstituted from cultured allogeneic endothelial cells in an immune-privileged site. Investigative ophthalmology & visual science. 2009;50:3151–3158. doi: 10.1167/iovs.08-2530. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Maruyama K, Nakazawa T, Cursiefen C, et al. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Investigative ophthalmology & visual science. 2012;53:3145–3153. doi: 10.1167/iovs.11-8010. [DOI] [PubMed] [Google Scholar]
- 10.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. The Journal of clinical investigation. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng SF, Dastjerdi MH, Ferrari G, et al. Short-term topical bevacizumab in the treatment of stable corneal neovascularization. American journal of ophthalmology. 2012;154:940–948.e941. doi: 10.1016/j.ajo.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevenson W, Cheng SF, Dastjerdi MH, et al. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin) The ocular surface. 2012;10:67–83. doi: 10.1016/j.jtos.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Archives of ophthalmology. 2009;127:381–389. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig Y, Bock F, Horn F, et al. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2009;247:1375–1382. doi: 10.1007/s00417-009-1099-1. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy. 2017;19:1–8. doi: 10.1016/j.jcyt.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh JY, Kim MK, Shin MS, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem cells. 2006;24:315–321. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 19.Robey P. “Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Research. 2017:6. doi: 10.12688/f1000research.10955.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 21.Tolar J, Le Blanc K, Keating A, et al. Concise review: hitting the right spot with mesenchymal stromal cells. Stem cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanathan S, Keating A. Overcoming the challenges of conducting translational research in cell therapy. Frontiers of medicine. 2011;5:333–335. doi: 10.1007/s11684-011-0166-2. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh KR, Frazier T, Rowan BG, et al. Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert review of clinical immunology. 2013;9:175–184. doi: 10.1586/eci.12.96. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz EM. Advancing regenerative medicine the translational way. Science translational medicine. 2013;5:177fs179. doi: 10.1126/scitranslmed.3005873. [DOI] [PubMed] [Google Scholar]
- 25.Fontaine MJ, Shih H, Schafer R, et al. Unraveling the Mesenchymal Stromal Cells’ Paracrine Immunomodulatory Effects. Transfusion medicine reviews. 2016;30:37–43. doi: 10.1016/j.tmrv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem cells. 2013;31:2042–2046. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 27.Pawelczyk E, Jordan EK, Balakumaran A, et al. In vivo transfer of intracellular labels from locally implanted bone marrow stromal cells to resident tissue macrophages. PloS one. 2009;4:e6712. doi: 10.1371/journal.pone.0006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afsharkhamseh N, Movahedan A, Gidfar S, et al. Stability of limbal stem cell deficiency after mechanical and thermal injuries in mice. Experimental eye research. 2016;145:88–92. doi: 10.1016/j.exer.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang JH, Putra I, Huang YH, et al. Limited versus total epithelial debridement ocular surface injury: Live fluorescence imaging of hemangiogenesis and lymphangiogenesis in Prox1-GFP/Flk1::Myr-mCherry mice. Biochimica et biophysica acta. 2016;1860:2148–2156. doi: 10.1016/j.bbagen.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eslani M, Putra I, Shen X, et al. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investigative ophthalmology & visual science. 2017;58:5507–5517. doi: 10.1167/iovs.17-22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu S, Hertsenberg AJ, Funderburgh ML, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Science translational medicine. 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao R, Lim S, Ji H, et al. Mouse corneal lymphangiogenesis model. Nature protocols. 2011;6:817–826. doi: 10.1038/nprot.2011.359. [DOI] [PubMed] [Google Scholar]
- 33.Rabiolo A, Bignami F, Rama P, et al. VesselJ: A New Tool for Semiautomatic Measurement of Corneal Neovascularization. Investigative ophthalmology & visual science. 2015;56:8199–8206. doi: 10.1167/iovs.15-17098. [DOI] [PubMed] [Google Scholar]
- 34.Bartosh TJ, Ylostalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouchlaka MN, Moffitt AB, Kim J, et al. Human Mesenchymal Stem Cell-Educated Macrophages are a Distinct High IL-6 Producing Subset That Confer Protection in Graft-Versus-Host-Disease and Radiation Injury Models. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017 doi: 10.1016/j.bbmt.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental hematology. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Wang H, Levine YA, et al. Identification of CD163 as an antiinflammatory receptor for HMGB1-haptoglobin complexes. JCI insight. 2016:1. doi: 10.1172/jci.insight.85375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zizzo G, Cohen PL. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. Journal of immunology. 2013;190:5237–5246. doi: 10.4049/jimmunol.1203017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends in molecular medicine. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 42.Zamiri P, Masli S, Streilein JW, et al. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Investigative ophthalmology & visual science. 2006;47:3912–3918. doi: 10.1167/iovs.05-1267. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Coulson-Thomas VJ, Ferreira TG, et al. Mesenchymal stem cells for treating ocular surface diseases. BMC ophthalmology. 2015;15(Suppl 1):155. doi: 10.1186/s12886-015-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kao WW, Coulson-Thomas VJ. Cell Therapy of Corneal Diseases. Cornea. 2016;35(Suppl 1):S9–S19. doi: 10.1097/ICO.0000000000001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, et al. Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. The ocular surface. 2016;14:121–134. doi: 10.1016/j.jtos.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forrester JV, Steptoe RJ, Klaska IP, et al. Cell-based therapies for ocular inflammation. Progress in retinal and eye research. 2013;35:82–101. doi: 10.1016/j.preteyeres.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Choi H, Lee RH, Bazhanov N, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Current molecular medicine. 2013;13:856–867. doi: 10.2174/1566524011313050016. [DOI] [PubMed] [Google Scholar]
- 49.Ko JH, Lee HJ, Jeong HJ, et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:158–163. doi: 10.1073/pnas.1522905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roddy GW, Oh JY, Lee RH, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 52.Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem cell research & therapy. 2016;7:37. doi: 10.1186/s13287-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golpanian S, Wolf A, Hatzistergos KE, et al. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiological reviews. 2016;96:1127–1168. doi: 10.1152/physrev.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He S, Gleason J, Fik-Rymarkiewicz E, et al. Human Placenta-Derived Mesenchymal Stromal-Like Cells Enhance Angiogenesis via T Cell-Dependent Reprogramming of Macrophage Differentiation. Stem cells. 2017;35:1603–1613. doi: 10.1002/stem.2598. [DOI] [PubMed] [Google Scholar]
- 55.Prockop DJ, Youn Oh J. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mass E, Ballesteros I, Farlik M, et al. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353 doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature communications. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salgado AJ, Reis RL, Sousa NJ, et al. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Current stem cell research & therapy. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 60.Mattar P, Bieback K. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Frontiers in immunology. 2015;6:560. doi: 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piccinini AM, Zuliani-Alvarez L, Lim JM, et al. Distinct microenvironmental cues stimulate divergent TLR4-mediated signaling pathways in macrophages. Science signaling. 2016;9:ra86. doi: 10.1126/scisignal.aaf3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly J, Ali Khan A, Yin J, et al. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. The Journal of clinical investigation. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiller KL, Freytes DO, Vunjak-Novakovic G. Macrophages modulate engineered human tissues for enhanced vascularization and healing. Annals of biomedical engineering. 2015;43:616–627. doi: 10.1007/s10439-014-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jenner W, Motwani M, Veighey K, et al. Characterisation of leukocytes in a human skin blister model of acute inflammation and resolution. PloS one. 2014;9:e89375. doi: 10.1371/journal.pone.0089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zizzo G, Hilliard BA, Monestier M, et al. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. Journal of immunology. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu WK, Georgiadis A, Copland DA, et al. IL-4 regulates specific Arg-1(+) macrophage sFlt-1-mediated inhibition of angiogenesis. The American journal of pathology. 2015;185:2324–2335. doi: 10.1016/j.ajpath.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Wietecha MS, Krol MJ, Michalczyk ER, et al. Pigment epithelium-derived factor as a multifunctional regulator of wound healing. American journal of physiology Heart and circulatory physiology. 2015;309:H812–826. doi: 10.1152/ajpheart.00153.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai J, Jiang WG, Grant MB, et al. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. The Journal of biological chemistry. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 71.Faia L, Becerra P, Amaral J, et al. The Effects of Pigment Epithelial Derived Factor on Murine Macrophages. Investigative ophthalmology & visual science. 2009;50:4283. [Google Scholar]
- 72.Kim J, Breunig MJ, Escalante LE, et al. Biologic and immunomodulatory properties of mesenchymal stromal cells derived from human pancreatic islets. Cytotherapy. 2012;14:925–935. doi: 10.3109/14653249.2012.684376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espagnolle N, Balguerie A, Arnaud E, et al. CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem cell reports. 2017;8:961–976. doi: 10.1016/j.stemcr.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee RH, Yu JM, Foskett AM, et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh JY, Roddy GW, Choi H, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanellis J, Bick R, Garcia G, et al. Stanniocalcin-1, an inhibitor of macrophage chemotaxis and chemokinesis. American journal of physiology Renal physiology. 2004;286:F356–362. doi: 10.1152/ajprenal.00138.2003. [DOI] [PubMed] [Google Scholar]
- 77.Mohammadipoor A, Lee RH, Prockop DJ, et al. Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Translational research : the journal of laboratory and clinical medicine. 2016;177:127–142. doi: 10.1016/j.trsl.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


