Abstract
Oligodendrocyte precursor cells (OPCs) give rise to oligodendrocytes in cerebral white matter. However, the underlying mechanisms that regulate this process remain to be fully defined, especially in adult brains. Recently, it has been suggested that signaling via A-kinase anchor protein 12 (AKAP12), a scaffolding protein that associates with intracellular molecules such as protein kinase A, may be involved in Schwann cell homeostasis and peripheral myelination. Here, we asked whether AKAP12 also regulates the mechanisms of myelination in the CNS. AKAP12 knockout mice were compared against wild-type mice in a series of neurochemical and behavioral assays. Compared to wild-types, 2-month old AKAP12 knockout mice exhibited loss of myelin in white matter of the corpus callosum, along with perturbations in working memory as measured by a standard Y-maze test. Unexpectedly, very few OPCs expressed AKAP12 in the corpus callosum region. Instead, pericytes appeared to be one of the major AKAP12-expressing cells. In a cell culture model system, conditioned culture media from normal pericytes promoted in-vitro OPC maturation. However, conditioned media from AKAP12-deficient pericytes did not support the OPC function. These findings suggest that AKAP12 signaling in pericytes may be required for OPC-to-oligodendrocyte renewal to maintain the white matter homeostasis in adult brain.
Keywords: AKAP12, pericytes, oligodendrocyte, oligodendrocyte precursor cell, white matter
Introduction
Oligodendrocytes and their precursor cells (oligodendrocyte precursor cells: OPCs) play essential roles in maintaining white matter homeostasis. During development, OPCs migrate to their destination from the subventricular zone, and then divide and differentiate to form myelinating oligodendrocytes [1, 2]. Even after adolescence, some OPCs remain in an immature state in the brain [3, 4]. Those residual OPCs may provide a progenitor pool for oligodendrocyte renewal under physiological conditions [5–7] as well as for myelin repair after white matter injury [8–14]. However, the mechanisms that regulate the balance and process of OPC-to-oligodendrocyte renewal in cerebral white matter in mature brain are still relatively unknown.
Recently, we as well as other researchers demonstrated that PKA/CREB signaling plays an essential role in oligodendrocyte regeneration in white matter [6, 15, 16]. PKA associates with A-kinase anchor protein 12 (AKAP12), which is a scaffolding protein for intracellular signal transduction [17, 18]. Importantly, it has been implicated that AKAP12 may regulate Schwann cell homeostasis and myelin maintenance in peripheral nervous system [19]. Therefore, in this study, we ask whether AKAP12 is also involved in OPC-to-oligodendrocyte renewal in cerebral white matter using AKAP12 knockout mice.
Materials and Methods
Animals
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments and procedures were conducted following a Massachusetts General Hospital IACUC-approved protocol. AKAP12 knockout mice were provided from the Gelman Lab at Roswell Park Cancer Institute [20]. Genotyping was done by PCR analysis using genomic DNA from mouse tail biopsies. Genotyping primers for the detection of KO allele were 5′-CGGCTGGGTGTGGCGGACCGCTATCAGGACATAGCG-3′ (forward) and 5′-CTCAGCCTTTGCCAGAATAGGCACTGCCCC-3′ (reverse). Genotyping primers for the detection of wild-type (WT) allele were 5′-CGCTGTACTACTAAGGAGAGTGTTACGC-3′ (forward) and 5′-CCTCCTGGGTCTCAGCCAGTTTCTCAGGGG-3′ (reverse) [20].
Immunohistochemistry
Mouse brains were fixed for 24 hours in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4°C. Then brains were kept in 30% sucrose at 4°C. Coronal sections with a thickness of 16 μm were used for immunohistochemistry in this study. Primary antibody sources were as follows: anti-BrdU antibody (1:50, Oxford Biotechnology), anti-AKAP12 antibody (1:500, obtained from the Gelman Lab at Roswell Park Cancer Institute), anti-GST-pi antibody (1:200, MBL), anti-PDGF-R-α antibody (1:100, SantaCruz or R & D systems), anti-CD13 antibody (1:200, R & D systems), anti-Lectin antibody (1:400, Vector Laboratories) and anti-PDGF-R-β antibody (1:100, R & D systems). For BrdU staining, brain sections were incubated at 37°C for 30 min in 1N HCl to detect BrdU labeling. Sections were incubated overnight with anti-BrdU (1:50, Oxford Biotechnology). Double immunofluorescence staining was performed by simultaneous incubating the sections overnight at 4°C with anti-GST-pi antibody. After washing primary antibody with PBS, the brain sections were then incubated with secondary antibodies (1:200; Jackson Immunoresearch Laboratories) for 1 hour at room temperature. After that, the sections were covered with VECTASHIELD with DAPI (Vector Laboratories). Immunostaining was analyzed with a fluorescence microscope (Nikon) interfaced with a digital charge-coupled device camera and an image analysis system. Z-stack images were obtained with z-interval of 0.4 μm by Confocal Laser scanning microscope (FV3000, Olympus). To obtain cell number data from immunostained brain sections for Figure 3 and Suppl Figure S4, an investigator blinded to the experimental groups counted the number of stained cells in the lateral side of corpus callosum (0.25 mm2 area; bregma +1.18 mm, +0.98 mm, and +0.74 mm).
Figure 3. Oligodendrocyte loss in AKAP12 knockout mouse.
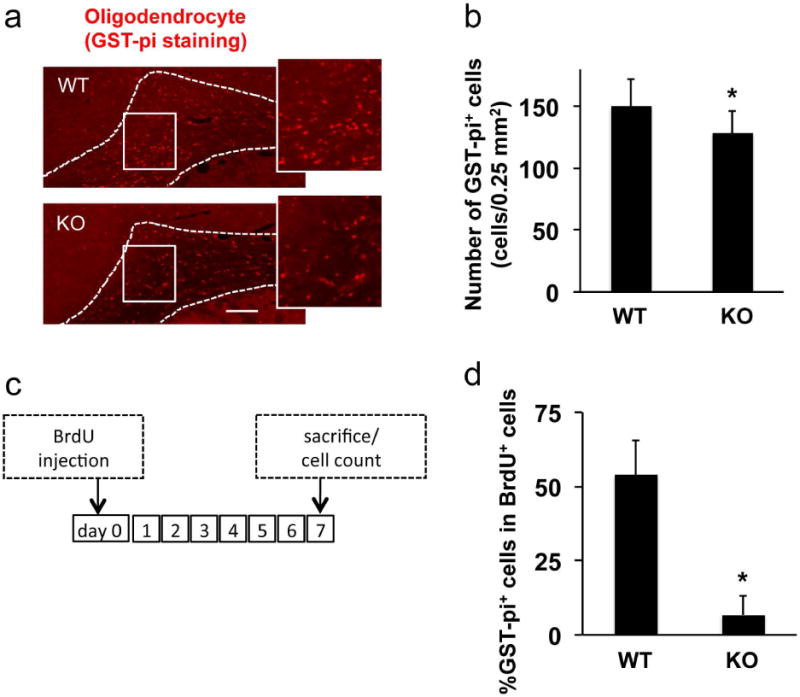
(a-b) Immunostaining using anti-GST-pi antibody showed that the number of oligodendrocytes in white matter (corpus callosum area) in AKAP12 knockout (KO) mice was significantly lower compared to wild-type (WT) mice. Bar=25 μm. Values are mean ± SD. N=5. *P<0.05. (c) Protocols for in vivo OPC-to-oligodendrocyte differentiation assay. Seven days after BrdU injection, brains were taken out and subjected to immunostaining. (d) Double staining of BrdU with anti-GST-pi antibody (oligodendrocyte marker) showed that AKAP12 KO mice exhibited low level of newly generated oligodendrocytes. Values are mean ± SD. N=5. *P<0.05. Please see Supplementary Figure S4 for representative images for GST-pi/BrdU double-staining.
Fluoromyelin staining
Fluoromyelin staining is now widely used to assess myelin integrity [21–24]. Coronal sections of 12-μm thicknesses (bregma +0.86mm to +0.50mm) were cut using a cryostat at -20°C and collected on glass slides. Sections were fixed with 4% PFA, and rinsed three times in PBS, then permeabilized in PBT (PBS + 0.2% Triton X-100) for at least 20 minutes. The sections were incubated with FluoroMyelin Green fluorescent myelin stain (1:300, Molecular probes) for 20 minutes at room temperature. Fluoromyelin staining images were viewed on a Nikon upright microscope, and the images from fluoromyelin stains were analyzed using ImageJ. The average pixel intensity from the corpus callosum region (3 brain sections for each animal) was used as a measure of myelin density. Because our initial pilot experiments confirmed there were no significant changes in myelin density between right and left hemispheres, we used a half hemisphere for fluoromyelin staining and the other half hemisphere for western blot experiments.
In vivo OPC differentiation assay
A cell proliferation marker 5-bromodeoxyuridine (BrdU, Sigma-Aldrich) was dissolved in saline. Mice were intraperitoneally injected (50 mg/kg, BrdU) three times a day at 4-hour intervals. The mice were sacrificed 7 days after the BrdU injection. The BrdU staining was conducted according to the method as described above in the “Immunohistochemistry” section.
Western blotting
Tissue samples of corpus callosum and cell culture were dissected in Pro-PREP™ Protein Extraction Solution (Boca scientific). Samples were heated with equal volumes of SDS sample buffer (Novex) including 2-mercaptoethanol at 95°C for 5 min, then each sample (20 μg per lane) was loaded onto 4–20% Tris–glycine gels. After electrophoresis and transferring to polyvinylidene difluoride membranes (Novex), the membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 and 5% skim milk (LabScientific). After incubation with primary antibody against MBP (1:1000, Thermo scientific), PDGF-R-α (1:1000, SantaCruz), AKAP12 (1:5000, obtained from the Gelman Lab at Roswell Park Cancer Institute), or β-actin (1:10000, Sigma-Aldrich), membranes were incubated with peroxidase-conjugated secondary antibodies and visualized by enhanced chemiluminescence (Amersham).
Cognitive test
Spontaneous alternative Y-maze cognitive test is now relatively well-accepted for assessing working memory in mice [6, 25]. In this study, experiments for Y-maze test were conducted between 7:00 AM to 9:00 AM. The maze consists of 3 arms (40 cm long, 9.5 cm high, and 4 cm wide, labeled arm-A, -B, or -C) diverging at a 120° from the central point. Each mouse was placed at the center of the start arm and allowed to move freely through the maze in an 8-minute session. This task was videotaped with a Victor camera (Everio GZ-MG-77-S) and the sequence of arm entries manually recorded in a blinded manner. An actual alternation was defined as entities into all the 3 arms on 3 consecutive runs (eg. ABC, CAB, or BCA but not BAB). The maximum alternation was subsequently calculated by measuring the total number of arm entries minus 2 and the percentage of alternation was calculated as [actual alternation/maximum alternation] ×100% (please see the diagram in Figure 2a). The total number of arms entered during the sessions was shown as locomotor activity. The first experiment (e.g. Figure 2) was conducted by NM, and the second experiment (e.g. Suppl Figure S3) was conducted by YKC.
Figure 2. Learning memory deficits in AKAP12 knockout mouse.
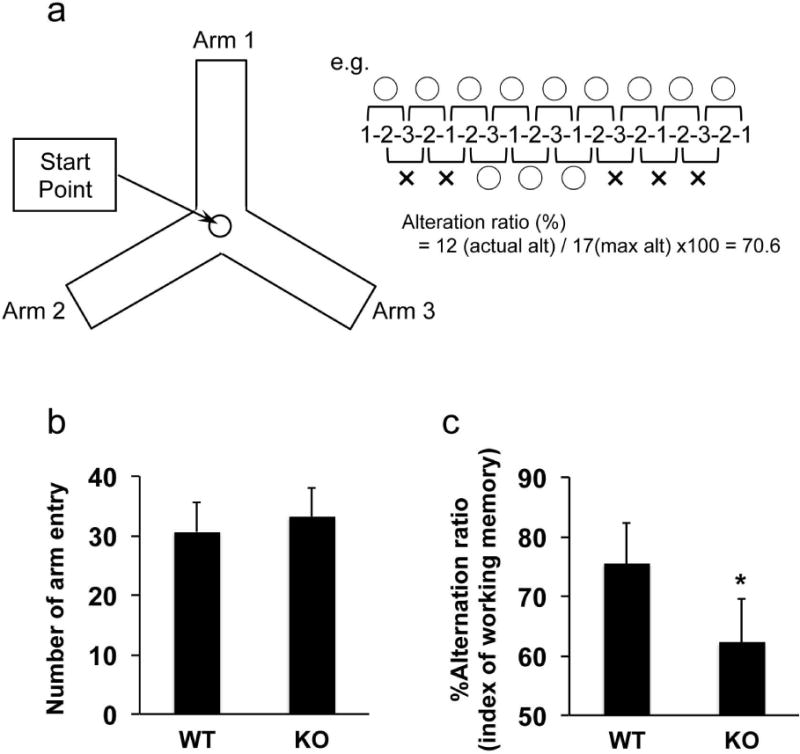
Wild-type (WT) and AKAP12 knockout (KO) mice were subjected to the standard Y-maze test to assess their working memory. (a) Schematics for Y-maze test. Please see the method section as to how to calculate alteration ratio in Y-maze test. (b) There were no differences in the total number of arm entry between WT and KO mice, suggesting that the locomotor activity of AKAP12 KO mice was similar to WT mice. Values are mean ± SD. N=17 for each group. (c) On the other hand, the alternation ratio (index of working memory) in AKAP12 KO mice was significantly lower compared to WT mice. Values are mean ± SD. N=17 for each group. *P<0.05. Please see Supplementary Figure S3 for our repeated experiments with different animals by another operator.
Cell culture
Primary cultured rat OPCs were prepared according to our previous reports [26, 27]. OPCs were maintained in Neurobasal medium containing 2% B27 supplement, 10 ng/mL PDGF-AA, and 10 ng/mL FGF-2. PDGF-AA and FGF-2 are known to promote OPC proliferation but suppress OPC differentiation. Therefore, during the culture period in the OPC culture media, OPC cultures do not differentiate into mature oligodendrocytes [27–29]. Human brain vascular pericytes [30] were cultured in pericyte basal medium (Sciencell research laboratories) containing 2% fetal bovine serum and pericyte growth supplement (Sciencell research laboratories) onto poly-l-lysine-coated plates. In our previous report, these pericyte cultures were confirmed to express pericyte markers [30].
Media transfer experiment
Pericyte cultures were maintained in Dulbecco’s Modified Eagle Medium (DMEM) for 24 hr. The conditioned media were then collected and centrifuged at 10,000 g for 5 min at 4°C to remove cells and debris. For the media transfer experiments, pericyte-conditioned media (P-CM) were diluted with OPC differentiation media (DMEM with 20 ng/mL CNTF, 30 nM T3, and 4% B27) at a ratio of 50:50 (final concentration of CNTF, T3, and B27 was 10 ng/mL, 15 nM, and 2%, respectively). Control pericyte media were prepared from empty wells and diluted with OPC differentiation media in the same way as P-CM. For AKAP12 knockdown, pericytes were transiently transfected with AKAP12 siRNAs or control siRNAs (50 nM, Thermo Fisher Scientific) using RNAiMax (Thermo Fisher Scientific) according to the manufacturer’s instructions. After recovery for 12 hr, media were changed into DMEM and further cultured for 24 hr and collected medium (P−CMAKAP12-). These conditioned media from pericyte cultures were added to cultured OPCs at 4-5 days after plating. Our previous data confirmed that OPC cultures at this stage expressed the standard OPC markers, such as PDGF-R-α and NG2, but were negative for cell markers of astrocytes or mature oligodendrocytes [27–29]. We also showed that our OPC cultures successfully differentiated into MBP-positive oligodendrocytes 5-7 days after switching culture media from the OPC media to the OPC differentiation media [27–29].
Proteome Profiler™ Human XL Cytokine Array
P-CM and P-CMAKAP12- were concentrated to 100 μl through a centrifugal filter device (3 kDa cut-off, EMD Millipore). P-CM were subjected to Proteome Profiler™ Human XL Cytokine Array kit (R&D systems) according to the manufacturer’s instructions.
Immunocytochemistry
Cells were washed with PBS (pH 7.4), followed by 4% PFA for 15 min. After being further washed in PBS, they were incubated with 3% BSA in PBS for 1 hr. Then cells were incubated with primary antibody against MBP (1:200, Thermo scientific) at 4°C overnight. After washing with PBS, they were incubated with secondary antibodies conjugated with fluorescein isothiocyanate for 1 hour at room temperature. Finally, nuclei were counterstained with DAPI. Images were analyzed with a fluorescence microscope (Nikon) interfaced with a digital charge-coupled device camera and an image analysis system. Cell counting was conducted in a blinded manner by randomly selecting 2 fields for each well. Cell numbers were calculated as the average per 0.25mm2.
Statistical analysis
Two-tailed t-test was used to determine the significant differences between wild-type and knockout groups in vivo. For in vitro experiments, statistical significance was evaluated using one-way ANOVA followed by Tukey’s honestly significant difference test. Data are expressed as mean ± S.D. A p-value of <0.05 was considered statistically significant.
Results
AKAP12 deficiency caused loss of white matter myelin and defects in working memory
First, we checked the levels of AKAP12 expression in the corpus callosum region in both wild-type and AKAP12 knockout mice. Western blot and immunostaining analyses confirmed that AKAP12 knockout mice did not show AKAP12 expression (Figure 1a-b and Supplementary Figure S1). Using this knockout mouse line, we conducted experiments to test our hypothesis that AKAP12 plays an important role in oligodendrocyte maturation in cerebral white matter. Fluoro-myelin staining showed that AKAP12 knockout mice exhibited lower myelin density at the age of 8-weeks (Fig 1c-d), and western blot confirmed that myelin-basic protein (MBP) expression in the white matter (corpus callosum) was also significantly lower in AKAP12 deficient mice (Figure 1e-f). MBP is one of the major proteins that can be observed only in mature oligodendrocytes in brain. Because there are few oligodendrocytes in neonatal mouse brains, there was no significant difference in MBP expression just after birth (e.g. post-natal day 0) between wild-type and AKAP12 knockout mice (Supplementary Figure S2). However, at the age of 4 weeks, AKAP12 knockout mice already exhibited less MBP expression compared to wild-type mice (Supplementary Figure S2).
Figure 1. Loss of white matter myelin in AKAP12 knockout mouse.
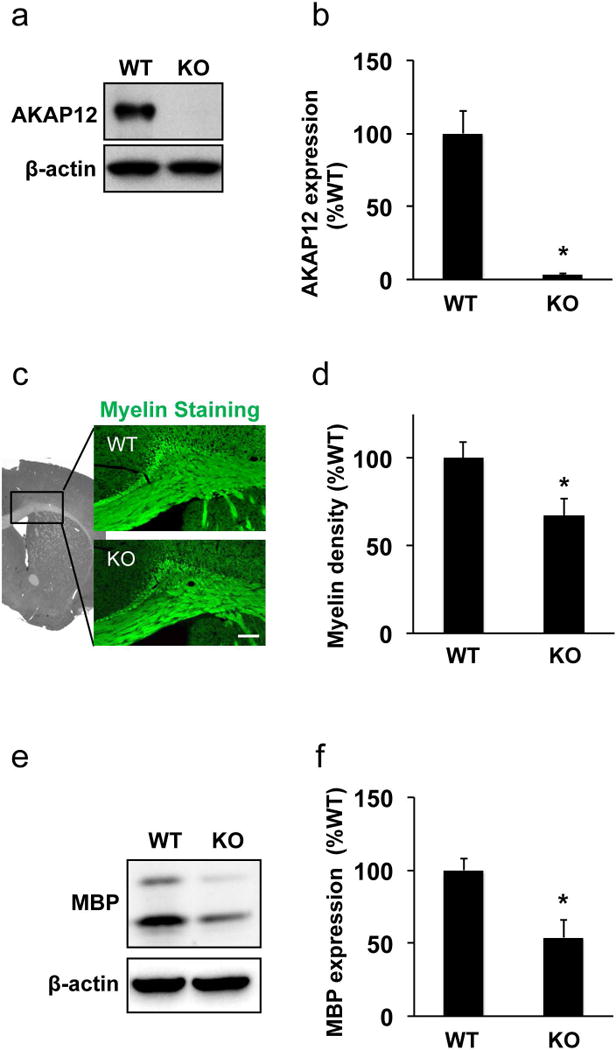
(a-b) Western blot using anti-AKAP12 antibody confirmed AKAP12 expression only in wild-type mouse cerebral white matter (i.e. corpus callosum region). β-actin was used as an internal control. Values are mean ± SD. N=6. *P<0.05. (c-d) Myelin staining using the FluoroMyelin kit showed white matter lesion with myelin damage in AKAP12 knockout (KO) mice. The densitometric analysis confirmed that myelin density in wild-type mice (WT) was significantly higher compared to AKAP12 KO mice. Bar=100 μm. Values are mean ± SD. N=5. *P<0.05. (e-f) White matter samples were subjected to western blot analysis. Expression of myelin-basic protein (MBP), a major protein in white matter myelin, was significantly larger in WT mice. β-actin was used as an internal control. Values are mean ± SD. N=5. *P<0.05.
To assess whether these cellular findings are associated with functional phenotypes, we examined working memory in wild-type and knockout mice with the standard Y-maze test (Figure 2a) because we previously showed that white matter damage caused working memory deficits in the behavioral test [6, 16, 21]. There was no significant difference in the total entry number for Y-maze arms between AKAP12 knockout and wild-type mice (Figure 2b), suggesting that AKAP12 knockout mice exhibit normal locomotor activity. However, the alternation ratio (i.e. index of working memory performance) of AKAP12 knockout mice was significantly lower compared to wild-type mice (Figure 2c). To further confirm the finding, other operator repeated Y-maze experiments with different individuals of wild-type and knockout mice. As shown in Supplementary Figure S3, regardless of operators, AKAP12 knockout mice showed lower working memory performance in the Y-maze test (Supplementary Figure S3).
AKAP12 deficiency dampened OPC-to-oligodendrocyte differentiation
Since CNS myelination is dependent on oligodendrocytes, we asked whether these cells were affected in AKAP12 knockout mice. Quantitative immunostaining demonstrated that the number of mature oligodendrocytes (GST-pi-positive cells) was decreased in the AKAP12 deficient white matter (Figure 3a-b). We also assessed an in-vivo OPC-to-oligodendrocyte differentiation using BrdU labeling. Eight-week-old wild-type or AKAP12 knockout mice were injected with BrdU, and one week later, these mice were sacrificed and the brains were used for immunostaining (Figure 3c). Because BrdU is incorporated into only proliferating cells and because mature oligodendrocytes do not proliferate, this experimental protocol should define newly generated oligodendrocytes by double-staining of BrdU with a mature-oligodendrocyte marker GST-pi. As expected, the percentage of GST-pi/BrdU-double-positive cells within the population of BrdU-positive cells was much lower in AKAP12 knockout mice (Figure 3d), indicating that AKAP12 deficiency interfered with OPC-to-oligodendrocyte progression in the adult corpus callosum. On the contrary, AKAP12 knockout mice showed a higher number of total BrdU-positive cells (Supplementary Figure S4). And in the knockout brain, PDGF-R-α-positive OPCs were the major cell type for BrdU positive cells at 7 days after BrdU injection (Supplementary Figure S4). Taken together, these data suggest that AKAP12 deficiency may cause an imbalance in cell number between OPCs and oligodendrocytes by promoting OPC proliferation but suppressing OPC differentiation.
Pericytes but not OPCs expressed AKAP12
AKAP12 is a scaffolding protein and is associated with PKA [17, 18]. In OPCs, PKA plays essential roles in CREB-related survival signaling [6, 15, 16]. Therefore, we next asked whether the defects of OPC maturation observed in AKAP12 knockout mice could be partly explained by AKAP12 deficiency in OPCs. However, very few OPCs expressed AKAP12 in cerebral white matter (corpus callosum) in wild-type mice (Figure 4a). Cell culture experiments also confirmed that OPCs did not express AKAP12 (Supplementary Figure S5). Instead, pericytes appeared to be one of the major cell types that express AKAP12 in the brain (Figure 4a-b and Supplementary Figure S6). Recently, we demonstrated that pericytes may interact with OPCs in the perivascular region [26]. In fact, AKAP12-expressing pericytes were located closely to OPCs in the corpus callosum (Figure 4a), proposing the possibility that AKAP12 may regulate the trophic support from pericytes to OPCs.
Figure 4. AKAP12 expression in pericytes in vivo.
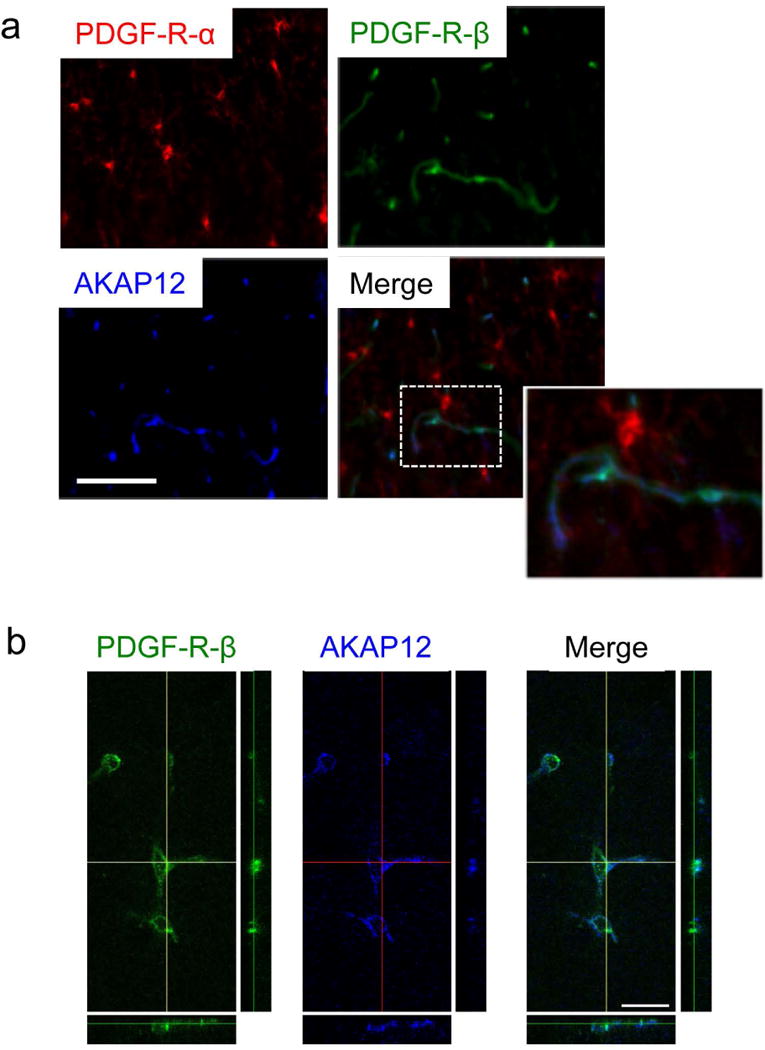
(a) Immunostaining showed that AKAP12 was not expressed in OPCs (PDGF-R-α positive cells) in the corpus callosum region in wild-type mouse brain sections. Instead, AKAP12 was confirmed to be expressed in pericytes (PDGF-R-β positive cells) in the corpus callosum region in wild-type mouse brain sections. Importantly, AKAP12-expressing pericytes were closely located to OPCs in the corpus callosum region. Bar=100 μm. (b) Double-staining of PDGF-R-β and AKAP12 using confocal microscopy confirmed that pericytes express AKAP12. Bar=20 μm. Please see Suppl Figure S6 for additional data from immunostaining with another pericyte marker CD13.
AKAP12-deficient pericytes failed to promote OPC maturation in vitro
To test if pericytic AKAP12 supports OPC differentiation, we conducted media transfer experiments, an approach that is now widely used to study the non-cell autonomous mechanisms of OPC function in vitro [27, 31]. Western blot data confirmed that cultured pericytes expressed AKAP12 (Figure 5a and Supplementary Figure S7). The AKAP12 expressions in pericyte cultures were successfully downregulated by siRNA to AKAP12 - we used two different siRNA sequences for AKAP12 (AKAP12-siRNA #1 and AKAP12-siRNA #2), and both siRNA sequences decreased AKAP12 expression in pericyte cultures (Figure 5a), without changing pericyte viability (Figure 5b).
Figure 5. AKAP12 expression in pericytes in vitro.
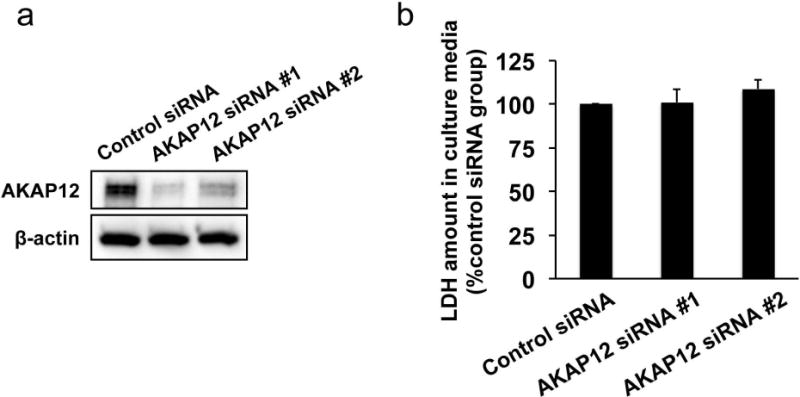
(a) Cultured pericytes expressed AKAP12 and the AKAP12 expression was suppressed by AKAP12 siRNA. β-actin was used as an internal control. (b) AKAP12 knockdown did not affect pericyte survival assessed by LDH assay. LDH amounts in the culture media were not different between control-siRNA-treated pericytes and AKAP12-siRNA-treated pericytes. Values are mean ± SD. N=5.
AKAP12 is known to regulate growth factor production in glial cells [32–34]. Therefore, with the protein array approach using conditioned media of pericyte cultures, we next investigated if AKAP12 is also essential for growth factor secretion in pericytes. Our protein array data indicated that the pattern of growth factor production in AKAP12 deficient pericytes was different from the one in control pericytes (e.g. pericytes that were transfected with scrambled siRNA) (Figure 6 and Supplementary Figure S8). Importantly, some factors that promote OPC maturation, such as BDNF [27, 35], LIF [36, 37], GRO-α [38], and HGF [39], were down-regulated in AKAP12 deficient pericytes (Figure 6 and Supplementary Figure S8). Hence, we next compared the effects of conditioned media from AKAP12 deficient pericytes on the in-vitro OPC differentiation with the one from control pericytes. During the maturation step of OPCs in vitro, cells were maintained in the conditioned media from control pericytes or AKAP12 deficient pericytes. Five days later, cells were subjected to immunostaining or western blotting analyses. Immunostaining with anti-MBP (oligodendrocyte marker) or anti-PDGF-R-α (OPC marker) antibody showed that conditioned media from control pericytes (P-CM) increased the number of MBP-positive cells and decreased the number of PDGF-R-α-positive cells (i.e. promoted the in vitro OPC differentiation) (Figure 7a-b and Supplementary Figure S9). But the in-vitro OPC differentiation was suppressed by AKAP12-deficient P-CM (P−CMAKAP12-) (Figure 7a-b and Supplementary Figure S9). Similarly, western blot showed that the level of MBP expression in P−CMAKAP12- -treated OPCs was lower than the one in P-CM-treated OPCs (Figure 7c-d).
Figure 6. Secreting factors from cultured pericytes.
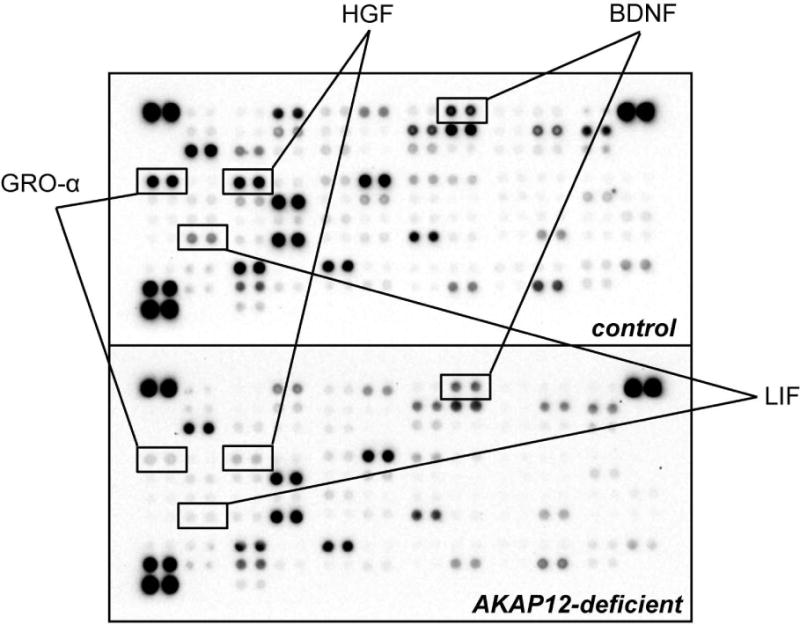
Proteome Profiler™ Human XL Cytokine Array using pericyte-conditioned-media showed that AKAP12 knockdown changed the pattern of secreting factors from pericytes. LIF, BDNF, GRO-α, and HGF are known to promote OPC differentiation. Those factors were downregulated in the AKAP12-deficient pericyte cultures. Please see Suppl Figure S8 for other factors from pericyte cultures.
Figure 7. Pericytic AKAP12 for OPC differentiation in vitro.
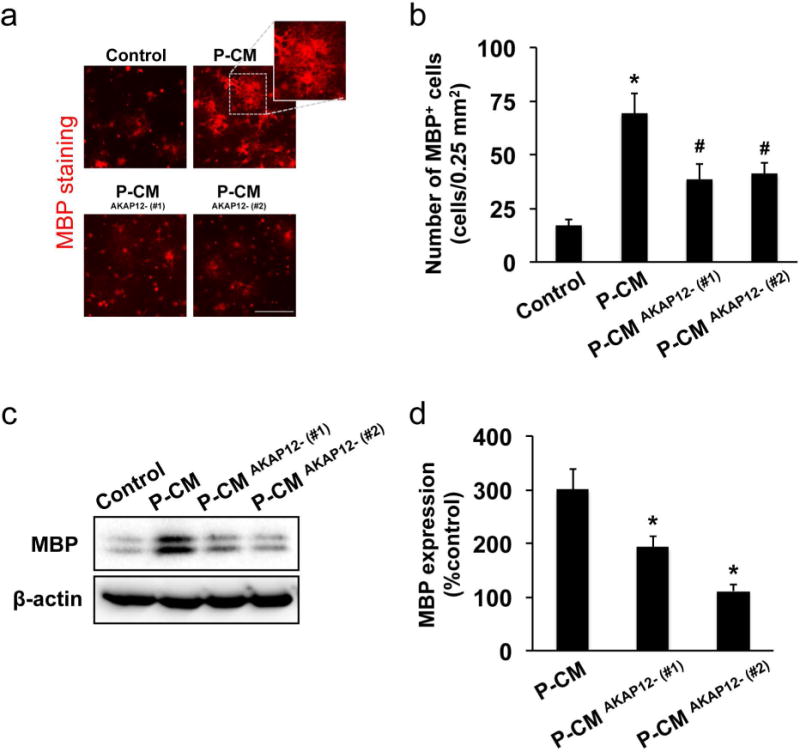
Cultured OPCs were maintained in control media, pericyte-conditioned media (P-CM), or conditioned media from AKAP12-deficient pericytes (P−CMAKAP12-). Five days later, those OPCs were used for immunostaining or western blotting analyses. (a-b) Immunostaining with anti-MBP (oligodendrocyte marker) antibody showed that P-CM increased the number of MBP−positive cells, but P-CMAKAP12- showed less effect on OPC maturation. Bar=100 μm. Values are mean ± SD. N=5. *P<0.05 vs control, and #P<0.05 vs P-CM. (c-d) The levels of MBP expression in P-CM-treated OPCs were also lower compared to ones in P−CMAKAP12- -treated OPCs. Values are mean ± SD. N=4. *P<0.05 vs P-CM.
Discussion
Our data demonstrate that AKAP12 is an important regulator of OPC-to-oligodendrocyte differentiation in adult white matter. AKAP12 knockout mice exhibited significant white matter dysfunction with myelin/oligodendrocyte loss. These white matter defects in AKAP12 knockout mice stem at least partially from a disturbance of normal pericyte-OPC interactions, leading to deficits in the oligodendrocyte maturation process in AKAP12 knockout mice. Working memory depends on the frontal subcortical circuits both in rodents and primates [40–43]. In fact, an impairment of working memory is consistently observed in a mouse model of prolonged cerebral hypoperfusion, which selectively disrupts cerebral white matter [16, 41]. Therefore, the failure of OPC differentiation to mature oligodendrocytes may ultimately cause white matter dysfunction, resulting in working memory deficits.
Our findings suggest that AKAP12 signaling may contribute to the regulation of adult oligodendrocyte regeneration/renewal in white matter in mice. Even in adult brains, regenerative capacities are required to maintain brain function under physiological conditions and/or to repair damaged brain cells under pathological conditions [44–46]. After myelin damage, residual OPCs in the adult brain respond quickly and mediate oligodendrocyte regeneration as a compensatory mechanism [47, 48]. Similar to the processes in the developmental stage, after white matter injury, OPCs would proliferate and migrate to demyelinated areas and then differentiate into mature oligodendrocytes, restoring myelin in damaged white matter [49]. In addition, recent studies have suggested that even under normal conditions, these residual OPCs in adult brain may monitor neighboring micro-environments and proliferate and/or differentiate into mature oligodendrocytes as needed [7]. By providing data that AKAP12 regulates white matter environments to sustain OPC-to-oligodendrocyte renewal in the adult brain, our study may bring novel insights into the mechanisms by which physiological oligodendrogenesis sustains normal white matter function.
Another major novelty in our study is that pericytes support OPC function via AKAP12 signaling. Pericytes are localized at the abluminal side of the perivascular space in microvessels, and play multiple roles in neurovascular function in the brain, such as regulating blood-brain barrier integrity and microcirculation [50, 51]. Recently, we demonstrated that in the perivascular region, pericytes may also support OPC function via secreting soluble factors [26]. Our current study expanded upon our previous study by providing novel data that AKAP12 regulates growth factor production from pericytes, which supports OPC maturation. In addition, our data parallel the findings reported by another group, that AKAP12 in astrocytes regulated the production of several growth factors required for vascular homeostasis [32, 33]. Therefore, AKAP12 may play important roles not only in intra-cellular signaling pathways but also in inter-cellular mechanisms to maintain a normal microenvironment in the brain.
Taken together, our current data suggest that AKAP12 signaling is required for oligodendrocyte renewal and white matter homeostasis in the mouse brain. Nevertheless, there are some important caveats in this study. First, we did not provide direct in vivo evidence that pericytic AKAP12 is required for OPC maturation. Genetic manipulation for down- or up-regulating AKAP12 in pericytes in the brain in vivo is necessary for further assessment of pericyte-OPC interactions. In addition, because pericyte dysfunction is now proposed to participate in progressive cognitive decline and dementia [52], the approach in genetic manipulation of pericytic AKAP12 may also give us new insights into the roles of pericytes in cognitive function. Second, we focused on the cell-cell interaction between OPCs and pericytes only. Pericytes constitute one of the major AKAP12-expressing cells in corpus callosum. But other cell types such as astrocytes and cerebral endothelium also express AKAP12, and both astrocytes and cerebral endothelial cells provide several growth factors that regulate OPC function [27, 28, 53, 54]. Therefore, defining the roles of AKAP12 in astrocytes and endothelial cells would be important in understanding the non-cell autonomous regulatory mechanisms of oligodendrocyte maturation. Third, our current study focuses on the roles of AKAP12 in adult brains. However, the myelination in adult white matter may be driven by adult OPCs, whose cell division cycles are slower in post-natal OPCs [55]. Because our supplementary data indicate that AKAP12 may also regulate OPC differentiation during the development as well (Supplementary Figure S2), we plan to conduct more experiments in neonatal mice in the next study to better clarify the roles of AKAP12 on OPC maturation in general. And finally, our current study examined the inter-cellular mechanisms of AKAP12 in OPC maturation. We showed that very few OPCs expressed AKAP12 in the corpus callosum region in adult mice. On the contrary, past studies demonstrated that mature oligodendrocytes may express AKAP12 [56]. Our cell culture experiments also indicated that AKAP12 expression appears during the differentiation step in OPC cultures (Supplementary Figure S5). Because AKAP12 is related to the pro-survival PKA-CREB signaling, it is a plausible hypothesis that AKAP12 supports the survival of mature oligodendrocytes and that AKAP12 deficiency may lead to the suppression of OPC maturation. Our preliminary data showed that AKAP12 knockout mice exhibited more proliferating OPCs compared to wild-type mice (Supplementary Figure S4), which supports the idea that loss of oligodendrocytes due to AKAP12 deficiency increases the number of OPCs as a compensatory response. Therefore, these AKAP12-dependent intracellular mechanisms in survival and proliferation/maturation of oligodendrocyte lineage cells should be carefully examined in future studies for better understanding of the mechanisms of white matter homeostasis.
Conclusion
In conclusion, our current study demonstrates that AKAP12 regulates OPC-to-oligodendrocyte differentiation in cerebral white matter. Even in the adult brains, OPCs provide a progenitor pool for mature oligodendrocytes, and therefore, OPC proliferation and differentiation are critical mechanisms for maintaining white matter function and repairing damaged white matter after injury. A deeper understanding of AKAP12 roles in white matter may lead us to novel therapeutic approaches for augmenting white matter health in CNS disorders including stroke or vascular dementia.
Supplementary Material
Significance Statement.
Cerebral white matter, which primarily consists of axonal bundles ensheathed with myelin by mature oligodendrocytes, plays an important role in passing signals between different areas of gray matter. Although most oligodendrocytes emerge from their precursor cells (oligodendrocyte precursor cells: OPCs) during development, some OPCs remain as an immature state even in adult brains. These residual OPCs are known to serve as a “back-up” for mature oligodendrocytes, but the underlying mechanisms by which OPCs differentiate into oligodendrocytes are still mostly unknown. Here, we report that A-kinase anchor protein 12 (AKAP12), which is a scaffolding protein that associates with intracellular molecules such as protein kinase A, is required for oligodendrocyte renewal in young-adult mice.
Acknowledgments
Supported in part by National Institutes of Health, Research Abroad from the Uehara Memorial Foundation, National Research Foundation of Korea, the World Class University Program, and the Global Research Laboratory Program.
Footnotes
Contributions: Takakuni Maki: Collection of data, Data analysis, Manuscript writing; Yoon Kyung Choi: Collection of data, Data analysis, Manuscript writing; Nobukazu Miyamoto: Collection of data, Data analysis, Manuscript writing; Akihiro Shindo: Collection of data, Data analysis; Anna C. Liang: Collection of data; Bum Ju Ahn: Collection of data, Data analysis; Emiri T. Mandeville: Collection of data, Data analysis; Seiji Kaji: Collection of data; Kanako Itoh: Collection of data; Ji Hae Seo: Collection of data; Irwin H. Gelman: Data analysis and interpretation; Josephine Lok: Data analysis and interpretation; Ryosuke Takahashi: Data analysis and interpretation; Kyu-Won Kim: Data analysis and interpretation; Eng H. Lo: Conception and design, Financial Support, Data analysis and interpretation, Manuscript writing; Ken Arai: Conception and design, Financial Support, Data analysis and interpretation, Manuscript writing.
Disclosure of Potential Conflicts of Interest: None
References
- 1.Thomas JL, Spassky N, Perez Villegas EM, et al. Spatiotemporal development of oligodendrocytes in the embryonic brain. J Neurosci Res. 2000;59:471–476. doi: 10.1002/(SICI)1097-4547(20000215)59:4<471::AID-JNR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Qi Y, Stapp D, Qiu M. Origin and molecular specification of oligodendrocytes in the telencephalon. Trends Neurosci. 2002;25:223–225. doi: 10.1016/s0166-2236(02)02145-8. [DOI] [PubMed] [Google Scholar]
- 3.Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama A, Komitova M, Suzuki R, et al. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto N, Tanaka R, Shimura H, et al. Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab. 2010;30:299–310. doi: 10.1038/jcbfm.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto N, Pham LD, Hayakawa K, et al. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke. 2013;44:2573–2578. doi: 10.1161/STROKEAHA.113.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes EG, Kang SH, Fukaya M, et al. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matute C, Domercq M, Perez-Samartin A, et al. Protecting white matter from stroke injury. Stroke. 2013;44:1204–1211. doi: 10.1161/STROKEAHA.112.658328. [DOI] [PubMed] [Google Scholar]
- 9.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 10.Chari DM, Blakemore WF. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307–313. [PubMed] [Google Scholar]
- 11.Li L, Harms KM, Ventura PB, et al. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang RL, Chopp M, Roberts C, et al. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J Cereb Blood Flow Metab. 2011;31:614–625. doi: 10.1038/jcbfm.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang RL, Chopp M, Roberts C, et al. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One. 2012;7:e48141. doi: 10.1371/journal.pone.0048141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph MJ, Caliaperumal J, Schlichter LC. After Intracerebral Hemorrhage, Oligodendrocyte Precursors Proliferate and Differentiate Inside White-Matter Tracts in the Rat Striatum. Transl Stroke Res. 2016;7:192–208. doi: 10.1007/s12975-015-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi C, Muramatsu R, Fujimura H, et al. Prostacyclin promotes oligodendrocyte precursor recruitment and remyelination after spinal cord demyelination. Cell Death Dis. 2013;4:e795. doi: 10.1038/cddis.2013.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto N, Maki T, Pham LD, et al. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelman IH. The role of SSeCKS/gravin/AKAP12 scaffolding proteins in the spaciotemporal control of signaling pathways in oncogenesis and development. Front Biosci. 2002;7:d1782–1797. doi: 10.2741/A879. [DOI] [PubMed] [Google Scholar]
- 18.Gelman IH, Tombler E, Vargas J., Jr A role for SSeCKS, a major protein kinase C substrate with tumour suppressor activity, in cytoskeletal architecture, formation of migratory processes, and cell migration during embryogenesis. Histochem J. 2000;32:13–26. doi: 10.1023/a:1003950027529. [DOI] [PubMed] [Google Scholar]
- 19.Ji Y, Tao T, Cheng C, et al. SSeCKS is a suppressor in Schwann cell differentiation and myelination. Neurochem Res. 2010;35:219–226. doi: 10.1007/s11064-009-0045-2. [DOI] [PubMed] [Google Scholar]
- 20.Akakura S, Huang C, Nelson PJ, et al. Loss of the SSeCKS/Gravin/AKAP12 gene results in prostatic hyperplasia. Cancer research. 2008;68:5096–5103. doi: 10.1158/0008-5472.CAN-07-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo JH, Miyamoto N, Hayakawa K, et al. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanaan A, Farahani R, Douglas RM, et al. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. American journal of physiology Regulatory, integrative and comparative physiology. 2006;290:R1105–1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- 23.Berlanga ML, Phan S, Bushong EA, et al. Three-dimensional reconstruction of serial mouse brain sections: solution for flattening high-resolution large-scale mosaics. Frontiers in neuroanatomy. 2011;5:17. doi: 10.3389/fnana.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korn MJ, Cramer KS. Distribution of glial-associated proteins in the developing chick auditory brainstem. Developmental neurobiology. 2008;68:1093–1106. doi: 10.1002/dneu.20645. [DOI] [PubMed] [Google Scholar]
- 25.Choi YK, Maki T, Mandeville ET, et al. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med. 2016;22:1335–1341. doi: 10.1038/nm.4188. [DOI] [PubMed] [Google Scholar]
- 26.Maki T, Maeda M, Uemura M, et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett. 2015;597:164–169. doi: 10.1016/j.neulet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyamoto N, Maki T, Shindo A, et al. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J Neurosci. 2015;35:14002–14008. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai K, Lo E. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:4351–4356. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham LD, Hayakawa K, Seo JH, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi Y, Maki T, Liang AC, et al. p38 MAP kinase mediates transforming-growth factor-beta1-induced upregulation of matrix metalloproteinase-9 but not -2 in human brain pericytes. Brain Res. 2014;1593:1–8. doi: 10.1016/j.brainres.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Shi Y, Jiang X, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SW, Kim WJ, Choi YK, et al. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 33.Choi YK, Kim JH, Kim WJ, et al. AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha. J Neurosci. 2007;27:4472–4481. doi: 10.1523/JNEUROSCI.5368-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Yan M, Hu L, et al. Involvement of Src-suppressed C kinase substrate in experimental autoimmune encephalomyelitis: a link between release of astrocyte proinflammatory factor and oligodendrocyte apoptosis. J Neurosci Res. 2010;88:1858–1871. doi: 10.1002/jnr.22355. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Cejudo J, Gutierrez-Fernandez M, Otero-Ortega L, et al. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke. 2015;46:221–228. doi: 10.1161/STROKEAHA.114.006692. [DOI] [PubMed] [Google Scholar]
- 36.Rowe DD, Collier LA, Seifert HA, et al. Leukemia inhibitor factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia. The European journal of neuroscience. 2014;40:3111–3119. doi: 10.1111/ejn.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer R, Wajant H, Kontermann R, et al. Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia. 2014;62:272–283. doi: 10.1002/glia.22605. [DOI] [PubMed] [Google Scholar]
- 38.Kadi L, Selvaraju R, de Lys P, et al. Differential effects of chemokines on oligodendrocyte precursor proliferation and myelin formation in vitro. Journal of neuroimmunology. 2006;174:133–146. doi: 10.1016/j.jneuroim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Hu Z, Li T, Zhang X, et al. Hepatocyte growth factor enhances the generation of high-purity oligodendrocytes from human embryonic stem cells. Differentiation; research in biological diversity. 2009;78:177–184. doi: 10.1016/j.diff.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Sarti C, Pantoni L, Bartolini L, et al. Persistent impairment of gait performances and working memory after bilateral common carotid artery occlusion in the adult Wistar rat. Behavioural brain research. 2002;136:13–20. doi: 10.1016/s0166-4328(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 41.Shibata M, Yamasaki N, Miyakawa T, et al. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38:2826–2832. doi: 10.1161/STROKEAHA.107.490151. [DOI] [PubMed] [Google Scholar]
- 42.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. Journal of neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 43.Nordahl CW, Ranganath C, Yonelinas AP, et al. White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal of cognitive neuroscience. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmichael ST. Emergent properties of neural repair: elemental biology to therapeutic concepts. Ann Neurol. 2016;79:895–906. doi: 10.1002/ana.24653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang ZG, Chopp M. Promoting brain remodeling to aid in stroke recovery. Trends in molecular medicine. 2015;21:543–548. doi: 10.1016/j.molmed.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maki T, Liang AC, Miyamoto N, et al. Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci. 2013;7:275. doi: 10.3389/fncel.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh K, Maki T, Lok J, et al. Mechanisms of cell-cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. 2015;1623:135–149. doi: 10.1016/j.brainres.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 2014;24:371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyamoto N, Pham LD, Seo JH, et al. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71:1055–1066. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai K, Lo E. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. Journal of neuroscience research. 2010;88:758–821. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young KM, Psachoulia K, Tripathi RB, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao F, Fei M, Cheng C, et al. Spatiotemporal patterns of SSeCKS expression after rat spinal cord injury. Neurochem Res. 2008;33:1735–1748. doi: 10.1007/s11064-008-9617-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


