Abstract
Objective
Most youth with type 1 diabetes do not meet the American Diabetes Association (ADA) and International Society for Pediatric and Adolescent Diabetes (ISPAD) targets for HbA1c, blood pressure (BP), lipids, and BMI. We hypothesized that ISPAD/ADA goal achievement would be associated with better insulin sensitivity (IS) and cardiopulmonary fitness.
Methods
IS was quantified as glucose infusion rate (GIR) from a hyperinsulinemic-euglycemic clamp in youth with type 1 diabetes from the RESISTANT (n=86) and EMERALD (n=41) cohorts (n=127; age 15.7±2.2 years, 52% girls). Cardiopulmonary fitness was measured as peak oxygen consumption (VO2peak/kg) during upright (RESISTANT) or supine (EMERALD) cycle ergometry and were stratified by cycle type. Goal achievement was defined as HbA1c <7.5%, BP <90th percentile, LDL-cholesterol <100 mg/dL, HDL-cholesterol >35 mg/dL, triglycerides <150 mg/dL and BMI <85th percentile. Participants were stratified into 3 groups: achieving 0–3 goals (n=52), 4 goals (n=48) and 5–6 goals (n=27). Differences between groups were examined with generalized linear models.
Results
IS was lower in youth who met 0–3 goals (5.2±3.4 mg/kg/min) vs. those who met 4 goals (7.4±4.1 mg/kg/min, p=0.04) and those who met 5–6 goals (8.5±4.3 mg/kg/min, p=0.003), and remained significant after adjustments for sex and diabetes duration. Upright VO2peak was lower in youth who met 0–3 goals (25.8±4.6 mL/kg/min) vs. those who met 4 goals (33.0±7.8 mL/kg/min, p=0.01) and those who met 5–6 goals (33.2±4.4 mL/kg/min, p=0.004). Similar and significant relationships were observed in EMERALD participants for supine VO2peak.
Conclusions
ADA/ISPAD goal achievement was associated with greater IS and cardiopulmonary fitness.
Introduction
Over 1.25 million American children and adults have type 1 diabetes. This common condition markedly increases excess risk for morbidity and early death from cardiovascular disease (CVD) and diabetic kidney disease (DKD) (1, 2), with atherosclerosis beginning in childhood and adolescence (3). Elevated glucose, blood pressure (BP) and lipid levels are traditionally considered the major contributory factors, but addressing these risk factors in children and adolescents remains difficult (1, 2, 4–8). In fact, most youth with type 1 diabetes in the T1D Exchange Clinic Registry did not meet the American Diabetes Association (ADA) and/or International Society for Pediatric and Adolescent Diabetes (ISPAD) targets for glycosylated hemoglobin (HbA1c), systolic and diastolic blood pressure (SBP/DBP), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), triglycerides (TG) and body mass index (BMI) (9). Furthermore, we previously demonstrated that suboptimal ADA/ISPAD target achievement is associated with worse cardio-renal health in youth with type 1 diabetes (8).
Youth with type 1 diabetes demonstrate lower insulin sensitivity than their normoglycemic peers, and insulin sensitivity is increasingly recognized as an important determinant of micro- and macrovascular disease in type 1 diabetes (10–14). We demonstrated in youth with T1D that higher insulin sensitivity is associated with better peak exercise capacity (VO2peak) (15), and that measures of renal health are associated with VO2peak (16). The insulin resistance of type 1 diabetes is not limited to obese youth and is not typically associated with characteristics of metabolic syndrome (e.g. hypertriglyceridemia, decreased HDL-C and hypertension) (10–14). Furthermore, our previous data demonstrate no correlation between HbA1c and insulin sensitivity in youth with type 1 diabetes (17).
It is unknown whether ADA/ISPAD goal achievement is associated with insulin sensitivity or VO2peak, two known predictors of cardiovascular disease and mortality, and whether these relationships are independent of measures of renal health. If ADA/ISPAD goal achievement is associated with better insulin sensitivity and exercise capacity, it would support the usefulness of these risk factor targets in clinical practice. Accordingly, the aim of our study was to examine the relationships between achievement of ADA/ISPAD goals (HbA1c, SBP, DBP, LDL-C, TG, HDL-C and BMI) and insulin sensitivity and VO2peak. We hypothesized that ADA/ISPAD goal achievement would be associated with higher insulin sensitivity and VO2peak in youth with type 1 diabetes.
Methods
Participants
Pubertal adolescents between the ages of 12 and 21 years were included from two local studies: RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) and Effects of MEtformin on CardiovasculaR Function in AdoLescents with Type 1 Diabetes (EMERALD). Participants were recruited from type 1 diabetes clinics at the Barbara Davis Center for Childhood Diabetes and from local private practices via advertisements. Eighty-six adolescents with type 1 diabetes from RESISTANT and 41 from EMERALD had data available for analyses of cardiopulmonary fitness, insulin sensitivity, and ISPAD risk factors.
Screening included a history, physical examination with BP measurement, Tanner staging, and fasting laboratory testing (HbA1c and LDL-C). Type 1 diabetes was defined by ADA criteria plus the presence of glutamic acid decarboxylase, islet cell, zinc transporter 8 and/or insulin autoantibodies, as well as insulin requirement. Inclusion criteria included Tanner stage of 2–5. Exclusions included resting BP > 140/90 mm Hg or > 190/100 mm Hg during exercise, hemoglobin < 9 mg/dL, serum creatinine > 1.5 mg/dL, HbA1c > 12% (97 mmol/mol), smoking, asthma requiring medications during exercise or other conditions precluding exercise testing, anti-hypertensive medications, pregnancy, breastfeeding, plans to alter exercise or diet during the study, family history of type 2 diabetes, and use of medications affecting insulin sensitivity (such as oral or inhaled steroids, atypical antipsychotics or non-insulin anti-glycemic medications). Pubertal development was assessed by a pediatric endocrinologist using the criteria established by Tanner and Marshall for pubic hair and breast development. Testicular volume was measured using an orchidometer. BMI percentiles were calculated using the 2000 Centers for Disease Control and Prevention growth chart standards. SBP and DBP percentiles were calculated based on 49,967 normal weight youth in the Pediatric Task Force database (18) and derived from cubic spline and quantile regression methods (19).
All subsequent tests were performed after a 12-h fast and for the 3 preceding days, participants were instructed to refrain from strenuous physical activity and were provided with a CTRC-prepared, fixed-macronutrient, weight-maintenance diet (55% carbohydrates, 30% fat, 15% protein). Participants were instructed to monitor blood glucose at least four times per day and the clamp was rescheduled if fever, significant illness or large urine ketones were present on admission. The study was approved by the University of Colorado Anschutz Medical Campus (UCAMC) Institutional Review Board, and appropriate consent and assent were obtained.
ISPAD/ADA goals
Participants were categorized according to the following ADA and ISPAD targets: HbA1c <7 .5%; BMI < 85th percentile for age and sex; SBP and DBP < 90th percentile for age, sex, and height; LDL-C < 100mg/dL; HDL-C > 35mg/dL; TG < 150mg/dL (20–22). Each participant was categorized by whether the above goals were attained (yes or no), and grouped into those who met 0–3 goals, 4 goals and 5–6 goals. Though the distinction between achieving 0–3, 4 and 5–6 ADA/ISPAD goals is somewhat arbitrary, these cut-offs were decided a priori to ensure sufficient observations for meaningful statistical analyses (Supplemental Figure 1). We also examined ADA/ISPAD goal achievement as a continuous variable (0–6).
Insulin Sensitivity
Participants were admitted to the inpatient Clinical and Translational Research Center (CTRC) for 12 hours of overnight monitored fasting. Subcutaneous insulin was replaced with a variable-rate overnight intravenous insulin infusion to normalize blood glucose concentration (goal of 100 mg/dL), and prevent the effects of hypoglycemia on insulin sensitivity (17). The following morning, a hyperinsulinemic euglycemic clamp was performed as previously described (23, 24) to quantify insulin sensitivity. Following a 2-hour basal phase, during which the overnight insulin infusion was continued to maintain normoglycemia. Insulin sensitivity (glucose infusion rate [GIR] in mg/kg/min and mg/FFM/min) was calculated from a hyperinsulinemic euglycemic clamp (80 mU/m2/min insulin) (23, 24). Body composition by DXA was performed to determine fat free mass (FFM) (23, 24).
Activity questionnaires and body composition
A 3 day pediatric physical activity recall questionnaire was used to estimate habitual physical activity (23, 24), reported as a 3-d average of daily metabolic equivalents (METS). Body composition was assessed by DEXA scan as previously reported (23, 24).
Exercise testing
Peak oxygen consumption (VO2peak) was measured during a graded bicycle ergometer protocol to exhaustion using a metabolic cart (Medgraphics CPX/D, Medical Graphics Corp., St. Paul, MN, USA) as previously reported (24). An upright bicycle ergometer (Medgraphics, Minneapolis, MN) was used in the RESISTANT study, and a supine bicycle ergometer (Medical Positioning, Inc, Kansas City, MO, USA) in the EMERALD study. Due to the different bicycle ergometer protocols, analyses of VO2peak were stratified by cohort. For all bicycle tests, oxygen consumption (VO2), carbon dioxide production (VCO2) and minute ventilation (VE) were measured, breath-by-breath, at rest and during exercise. Arm BP (by auscultation) and heart rate (by 12-lead ECG) was obtained every minute during exercise. Cardiac status was continuously monitored throughout each test by 12-lead ECG. The respiratory exchange ratio (RER), which is the ratio between the amount of carbon dioxide (CO2) produced in metabolism and oxygen (O2) consumed, was calculated as VCO2/VO2. Participants were excluded if peak RER was ≤ 1.1 with upright cycle ergometer. All participants met the RER criteria with upright cycle ergometer testing (RESISTANT). Conversely, RER > 1.1 was not an absolute exclusion criterion for the EMERALD study due to the use of a supine cycle ergometer, and 3 participants achieved a RER ≤ 1.1 (1.00, 1.00 and 1.03, respectively).
VO2peak was reported in ml/kg/min and ml/lean kg/min as previously reported (23, 24). Blood sugars were closely monitored, and short-acting insulin or carbohydrate given as needed with a goal pre-exercise range of 100–150 mg/dL.
Statistical analysis
Analyses were performed in SAS (version 9.4 or higher; SAS Institute, Cary, NC). Variables were checked for the distributional assumption of normality using normal plots. Results are presented as mean ± standard deviation (SD), median and IQR, or frequencies and percentages, as appropriate. The heterogeneity of the two cohorts (RESISTANT and EMERALD) were evaluated with histograms and QQplots for key variables to ensure it was meaningful to combine the cohorts for analyses. Each participant was categorized based on ADA/ISPAD goals attainment, and stratified by whether 0–3 goals, 4 goals or 5–6 goals were met. We employed linear regression models to examine insulin sensitivity and VO2peak in participants who met 0–3, 4 and 5–6 goals unadjusted and adjusted for sex, pubertal status, diabetes duration and insulin sensitivity. We also examined ADA/ISPAD goal attainment as a continuous variable (0–6). Analyses were considered exploratory and hypothesis generating and adjustments for multiple comparisons were not employed. Linear regression results are presented as β estimate ± standard errors (SE). Significance was based on an α-level of 0.05.
Results
Participant Characteristics and ISPAD and ADA goal achievement
Twenty-four percent of participants had HbA1c < 7.5%, 93% had SBP and DBP < 90th percentile for age, sex and height, 80% had LDL-C < 100mg/dL, 87% had HDL-C > 35mg/dL, 96% had TG < 150mg/dL and 59% had BMI < 85th percentile for age and sex. We stratified the cohort into participants who met 0–3, 4 and 5–6 goals (Table 1). There were no statistically significant differences in age, diabetes duration, sex distribution, Tanner stage, habitual level of physical activity or insulin pump use in participants who met 0–3, 4 or 5–6 goals (Table 1), although participants who met 4 or 5–6 goals had numerically longer diabetes duration compared to participants who only met 0–3 goals (P=NS) (Table 1).
Table 1.
Clinical characteristics of adolescents with type 1 diabetes
| Variables | 0–3 Goals Attained (n=27) | 4 Goals Attained (n=48) | 5–6 Goals Attained (n=52) | P-value (ANOVA/χ2) |
|---|---|---|---|---|
| Age (years) | 15.4±2.1 | 15.8±2.3 | 15.8±2.1 | 0.66 |
| Females (yes, %) | 52% | 52% | 52% | 1.00 |
| Diabetes Duration (years) | 5.5±4.2 | 7.4±3.7 | 7.2±4.3 | 0.12 |
| Tanner Stage (2–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.84 |
| Insulin pump (yes, %) | 46% | 67% | 60% | 0.22 |
| HbA1c (%) | 9.0±1.6a | 8.8±1.4a | 8.2±1.4 | 0.02 |
| SBP (%ile) | 85±19b | 70±26b | 54±29b | <0.0001 |
| DBP (%ile) | 75±19b | 64±21b | 50±20b | <0.0001 |
| LDL-C (mg/dL) | 98±31b | 86±21b | 73±14b | <0.0001 |
| TG (mg/dL) | 112±48 | 79±27c | 71±26c | <0.0001 |
| HDL-C (mg/dL) | 46±10 | 46±10 | 46±8 | 0.94 |
| BMI (%ile) | 85±23b | 71±29b | 58±23b | <0.0001 |
| GIR (mg/kg/min) | 5.3±3.5 | 7.4±4.1c | 8.5±4.3c | 0.01 |
| Steady State Insulin (μU/ml) | 133±71 | 145±59 | 159±117 | 0.51 |
| Mean habitual physical activity (METS) | 59±9 | 65±13 | 63±13 | 0.32 |
Results are presented as mean ± standard deviation (SD), median and IQR
p<0.05 compared to 5–6 goals;
p<0.05 for all pairwise comparisons;
p<0.05 compared to 0–3 goals
Goal achievements and insulin sensitivity
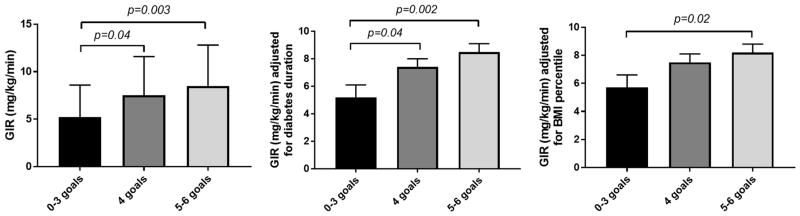
Insulin sensitivity was lower in participants who met 0–3 goals (5.2±3.4 mg/kg/min) vs. those who met 4 goals (7.4±4.1 mg/kg/min, p=0.04) and those who met 5–6 goals (8.5±4.3 mg/kg/min, p=0.003). The between group relationships remained significant after adjusting for diabetes duration and BMI percentile and the adjusted mean insulin sensitivity (mg/kg/min) was significantly lower in participants who attained 0–3 goals compared to those who met 5–6 goals (Figure 1). Similar results were obtained when insulin sensitivity was expressed per kg FFM (mg/kg FFM/min). We also adjusted the models for VO2peak, and the adjusted mean insulin sensitivity (mg/kg/min and mg/kg FFM/min) was lower in participants who attained 0–3 goals compared to those who met 5–6 goals in EMERALD, but not RESISTANT.
Figure 1.
Goal achievement is associated with higher IS
Greater goal achievement as a continuous variable was associated with higher insulin sensitivity expressed as mg/kg/min and mg/kg FFM/min (β±SE: 1.21±0.36 mg/kg/min, p=0.001 and 1.42±0.45 mg/kg FFM/min, p=0.002 per 1 goal achievement) after adjusting for sex and pubertal status, and remained significant after further adjustments for BMI percentiles and diabetes duration (β±SE: 1.01±0.37 mg/kg/min, p=0.008 and 1.52±0.48 mg/kg FFM/min, p=0.002 per 1 goal achievement).
Participants’ Characteristics Stratified by RESISTANT and EMERALD Cohort
There were no statistically significant differences in age, sex, diabetes duration or pubertal status observed across goal achievement groups in RESISTANT participants (Supplemental Table 1) or EMERALD participants (Supplemental Table 2). However, like the combined cohort, participants who met 4 goals and 5–6 goals had numerically longer diabetes duration than participants who only met 0–3 goals (P=NS) (Supplemental Table 1–2).
Goal achievements and cardiopulmonary fitness in RESISTANT
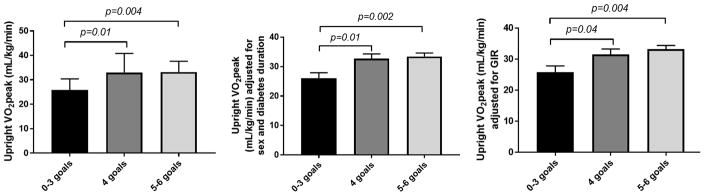
Upright VO2peak was lower in participants in RESISTANT who met 0–3 goals (25.8±4.6 mL/kg/min) vs. those who met 4 goals (33.0±7.8 mL/kg/min, p=0.01) and those who met 5–6 goals (33.2±4.4 mL/kg/min, p=0.004). These relationships remained significant after adjusting for diabetes duration and sex (Figure 2) and insulin sensitivity expressed per kg (mg/kg/min) [Figure 2] or per kg FFM (mg/kgFFM/min) [data not shown]. Similar relationships were observed across the 3 goal achievement groups when upright VO2peak was expressed per kg FFM (mL/kg FFM/min) [data not shown].
Figure 2.
Goal achievement is associated with higher VO2peak in RESISTANT
Greater goal achievement as a continuous variable was associated with higher upright VO2peak (β±SE: 2.38±0.78 ml/kg/min, p=0.005, per 1 goal achievement) after adjusting for sex and pubertal status, and remained significant after further adjustments for insulin sensitivity and diabetes duration (β±SE: 2.50±0.86 ml/kg/min, p=0.007, per 1 goal achievement).
Goal achievements and cardiopulmonary fitness in EMERALD
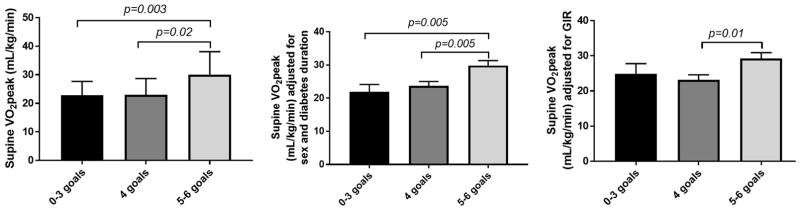
Participants who attained 5–6 goals had higher supine VO2peak (30.1±8.0 mL/kg/min) compared to those who met 4 goals (23.0±5.7 mL/kg/min, p=0.02) and 0–3 goals (22.8±4.9 mL/kg/min, p=0.003). These relationships remained significant after adjusting for diabetes duration and sex (Figure 3). Moreover, adjusted means of supine VO2peak remained significantly lower in participants who met 4 goals vs. 5–6 goals after adjusting for insulin sensitivity expressed per kg (mg/kg/min) [Figure 3] or expressed per kg FFM (mg/lean kg/min) [data not shown]. Similar relationships were observed across the 3 goal achievement groups when for upright VO2peak was expressed per kg FFM (ml/kg FFM/min) [data not shown].
Figure 3.
Goal achievement is associated with higher VO2peak in EMERALD
Greater goal achievement as a continuous variable was associated with higher supine VO2peak (β±SE: 2.12±0.81 ml/kg/min, p=0.01, per 1 goal achievement) after adjusting for sex and pubertal status, and remained significant after further adjustments for insulin sensitivity and diabetes duration (β±SE: 2.01±0.95 ml/kg/min, p=0.04, per 1 goal achievement).
Individual goal achievements, insulin sensitivity and cardiopulmonary fitness
We also examined the relationships between individual ISPAD/ADA risk factors, insulin sensitivity and VO2peak (Supplemental Table 3). Achieving the BMI percentile target was the only risk factor target that individually conferred significantly greater insulin sensitivity and VO2peak. Insulin sensitivity correlated with HbA1c, BMI percentiles, triglycerides, LDL-C and DBP. Supine VO2peak (RESISTANT) correlated with BMI percentiles, SBP and DBP percentiles, whereas upright VO2peak (EMERALD) only correlated with DBP percentiles (Supplemental Table 3).
Discussion
ISPAD/ADA target achievement was suboptimal in two contemporary cohorts of adolescents with type 1 diabetes. Only 9% of participants met all six goals, and 21% met only three or less of the six ISPAD/ADA targets. Achieving 5–6 goals was associated with higher insulin sensitivity and exercise capacity, possibly emphasizing the importance of aggressive risk factor control. The reverse could also be true, that improving fitness and/or insulin sensitivity may improve aspects of the ISPAD/ADA targets. Research suggests that regular exercise in youth with type 1 diabetes can help achieve several risk factor goals, including reducing HbA1c (25), triglycerides and total cholesterol (25). Furthermore, some studies in type 2 diabetes demonstrate improved insulin sensitivity with high intensity interval training (26), but it is unclear whether the same effects of exercise on insulin sensitivity are observed in youth with type 1 diabetes. Regardless, achieving ISPAD/ADA goals may improve long-term cardiovascular health.
We previously demonstrated reduced peak exercise capacity, cardiac and vascular function in otherwise healthy, non-obese adolescents with type 1 diabetes, compared with well-matched nondiabetic controls of similar BMI, pubertal stage, and habitual level of physical activity (24). Low fitness levels in adults with and without diabetes are associated with CVD mortality and decreased longevity (27, 28). The results from studies investigating whether improved fitness translates to a reduction in cardiovascular mortality in adults with type 2 diabetes are inconsistent (29, 30). No such data are, however, available in youth with type 1 diabetes. Furthermore, we found that insulin sensitivity correlated strongly with VO2peak in youth with type 1 diabetes (24). In this report, we present for the first time a relationship between ADA/ISPAD goal achievements, insulin sensitivity and cardiopulmonary fitness. The differences we observed in VO2peak across the ADA/ISPAD groups remained significant even after adjusting for insulin sensitivity. This observation suggests that cardiopulmonary fitness is independently associated with achieving more ADA/ISPAD goals, while insulin sensitivity was not independently associated with ADA/ISPAD goals after adjusting for VO2peak.
Contemporary cohorts of adolescents and adults with type 1 diabetes demonstrate suboptimal ADA and ISPAD goal achievements respectively (31, 32). The ISPAD and ADA target achievement in our cohort is similar to that reported in the T1D Exchange study for the 13–20 year old age group, with 21% of their participants meeting the HbA1c target, 78% meeting the BP target, 62% meeting the LDL-target, 94% meeting the HDL-C target, 89% meeting the TG target and 69% meeting the BMI target (9). The reasons for the suboptimal goal achievements are likely multifactorial. Scarcity of clinical trials with lipid-lowering and anti-hypertensive medications in adolescents with type 1 diabetes to guide ideal treatment (7), poor medication adherence during adolescence, the physiologic insulin resistance of puberty, and the rising rates of obesity and sedentary behavior among adolescents are all potential causes (33, 34). Perhaps equally important are the limited longitudinal data available on associations between risk factor control and cardiovascular outcomes in adolescents with type 1 diabetes. Therefore, many opportunities remain for further research on improving the medical management of adolescents with type 1 diabetes, and tracking the outcomes of such interventions.
There are limitations to the present study, including the cross-sectional design which prohibits determination of causality and directionality of our results. Therefore, future directions include longitudinal studies and clinical trials targeted at modifying insulin sensitivity and cardiopulmonary fitness to reduce CVD in youth with type 1 diabetes. Another limitation to the study is a that a small number of participants in the EMERALD cohort were unable to reach a RER >1.1 due to the supine ergometer chosen to maximize post-exercise cardiac imaging. However, results between the two cohorts were very similar, irrespective of ergometer protocol, strengthening our findings. Although we adjusted for a variety of important confounding variables, we cannot rule out the presence of unknown risk factors (e.g. underlying inherited factors) that may have biased the present analyses. Our analyses were considered exploratory and hypothesis generating and adjustments for multiple comparisons were not employed. Our cohort is also predominantly non-Hispanic white; representative of the type 1 diabetes population and thus findings may not be generalizable to other youth with type 1 diabetes. Our findings may also not be applicable to pre-pubertal youth with type 1 diabetes. We would also like to acknowledge that the distinction between achievement of 0–3, 4 and 5–6 ISPAD/ADA goals is somewhat arbitrary, and was decided on a priori to ensure adequate number of participants in each group for sufficiently powered analyses. We did obtain similar results when examining ISPAD/ADA goal achievements as a continuous variable. Finally, the ISPAD/ADA goals are made up of interrelated risk factors which may have introduced collinearity in our models. However, no significant collinearities were identified by variance inflation factors in ordinary least squares regression analyses.
In conclusion, suboptimal ISPAD/ADA target control in our cohort was common, and was associated with insulin resistance and lower peak exercise capacity, both markers of increased morbidity and mortality, supporting the importance of achieving ISPAD/ADA clinical targets. Additional efforts and better therapies are required to help adolescents achieve these important goals. Future studies to consider include fitness testing in youth with type 1 diabetes and intensification of risk factor control in those with poor cardiopulmonary fitness. An important future direction in EMERALD is to evaluate the effect of metformin on cardiopulmonary fitness and insulin sensitivity.
Supplementary Material
Supplemental Figure 1 ADA/ISPAD target achievement in RESISTANT/EMERALD
Acknowledgments
Special thanks to the EMERALD and RESISTANT Study Groups and the research participants. This project was supported by the following: NIH T32 DK063687; NCRR K23 RR020038-01; NIH BIRCWH K12 5K12HD057022, R56 DK088971; ADA 7-11-CD-08, ADA 1-11-JF-23; JDRF Award #11-2010-343; K23DK107871; NIH/NCATS Colorado CTSI UL1 TR001082, Center for Women’s Health Research, VA Merit. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views. None of the authors have any conflicts of interest to disclose.
Footnotes
Author Contributions
PB wrote, formulated analytic plan, contributed to discussion and analytic plan, and reviewed/edited the manuscript; MCG researched, contributed to discussion, and reviewed/edited the manuscript; AB researched, contributed to discussion, and reviewed/edited the manuscript; GC researched, contributed to discussion, and reviewed/edited the manuscript; YGR researched, contributed to discussion, and reviewed/edited the manuscript; MS contributed to discussion, and reviewed/edited the manuscript; LP contributed to discussion, and reviewed/edited the manuscript; JGR contributed to discussion, and reviewed/edited the manuscript; JEBR contributed to discussion, and reviewed/edited the manuscript; KN researched, formulated analytic plan, contributed to the discussion and analytic plan, and reviewed/edited the manuscript.
Guarantor Statement
Drs. Petter Bjornstad and Kristen J. Nadeau are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement From the American Heart Association and American Diabetes Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 2.Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular Disease Risk Factors in Youth With Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 3.Krantz JS, Mack WJ, Hodis HN, Liu CR, Liu CH, Kaufman FR. Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J Pediatr. 2004;145(4):452–7. doi: 10.1016/j.jpeds.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Schwab KO, Doerfer J, Hecker W, Grulich-Henn J, Wiemann D, Kordonouri O, et al. Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV) Diabetes Care. 2006;29(2):218–25. doi: 10.2337/diacare.29.02.06.dc05-0724. [DOI] [PubMed] [Google Scholar]
- 5.Margeirsdottir HD, Larsen JR, Brunborg C, Overby NC, Dahl-Jorgensen K. High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population-based study. Diabetologia. 2008;51(4):554–61. doi: 10.1007/s00125-007-0921-8. [DOI] [PubMed] [Google Scholar]
- 6.Kershnar AK, Daniels SR, Imperatore G, Palla SL, Petitti DB, Pettitt DJ, et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. The Journal of pediatrics. 2006;149(3):314–9. doi: 10.1016/j.jpeds.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 7.Bjornstad P, Wadwa RP. Risks and benefits of statin use in young people with type 1 diabetes. Current diabetes reports. 2014;14(7):499. doi: 10.1007/s11892-014-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornstad P, Pyle L, Nguyen N, Snell-Bergeon JK, Bishop FK, Wadwa RP, et al. Achieving International Society for Pediatric and Adolescent Diabetes and American Diabetes Association clinical guidelines offers cardiorenal protection for youth with type 1 diabetes. Pediatr Diabetes. 2015;16(1):22–30. doi: 10.1111/pedi.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood JR, Miller KM, Maahs DM, Beck RW, Dimeglio LA, Libman IM, et al. Most Youth With Type 1 Diabetes in the T1D Exchange Clinic Registry Do Not Meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care. 2013;36(7):2035–7. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, et al. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complications. 2016;30(4):586–90. doi: 10.1016/j.jdiacomp.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornstad P, Snell-Bergeon JK, Nadeau KJ, Maahs DM. Insulin sensitivity and complications in type 1 diabetes: New insights. World J Diabetes. 2015;6(1):8–16. doi: 10.4239/wjd.v6.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornstad P, Maahs DM, Johnson RJ, Rewers M, Snell-Bergeon JK. Estimated insulin sensitivity predicts regression of albuminuria in Type 1 diabetes. Diabet Med. 2015;32(2):257–61. doi: 10.1111/dme.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjornstad P, Snell-Bergeon JK, McFann K, Wadwa RP, Rewers M, Rivard CJ, et al. Serum uric acid and insulin sensitivity in adolescents and adults with and without type 1 diabetes. J Diabetes Complications. 2014;28(3):298–304. doi: 10.1016/j.jdiacomp.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, et al. Early diabetic nephropathy: a complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care. 2013;36(11):3678–83. doi: 10.2337/dc13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–21. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornstad P, Cree-Green M, Baumgartner A, Maahs DM, Cherney DZ, Pyle L, et al. Renal function is associated with peak exercise capacity in adolescents with type 1 diabetes. Diabetes Care. 2015;38(1):126–31. doi: 10.2337/dc14-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CL, Pyle L, Morehead R, Baumgartner A, Cree-Green M, Nadeau KJ. The role of glycemia in insulin resistance in youth with type 1 and type 2 diabetes. Pediatr Diabetes. 2016 doi: 10.1111/pedi.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 19.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–66. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 20.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 21.Acerini C, Craig ME, de Beaufort C, Maahs DM, Hanas R. Introduction to ISPAD Clinical Practice Consensus Guidelines 2014 Compendium. Pediatr Diabetes. 2014;15(Suppl 20):1–3. doi: 10.1111/pedi.12182. [DOI] [PubMed] [Google Scholar]
- 22.Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 23.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin Resistance in Adolescents with Type 2 Diabetes Is Associated with Impaired Exercise Capacity. Journal of Clinical Endocrinology & Metabolism. 2009;94(10):3687–95. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin Resistance in Adolescents with Type 1 Diabetes and Its Relationship to Cardiovascular Function. Journal of Clinical Endocrinology & Metabolism. 2010;95(2):513–21. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quirk H, Blake H, Tennyson R, Randell TL, Glazebrook C. Physical activity interventions in children and young people with Type 1 diabetes mellitus: a systematic review with meta-analysis. Diabet Med. 2014;31(10):1163–73. doi: 10.1111/dme.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitranun W, Deerochanawong C, Tanaka H, Suksom D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand J Med Sci Sports. 2014;24(2):e69–76. doi: 10.1111/sms.12112. [DOI] [PubMed] [Google Scholar]
- 27.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–20. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 28.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985) 2011;111(5):1497–504. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- 29.Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2(6):474–80. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 31.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36(8):2271–9. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjornstad P, Maahs DM, Rewers M, Johnson RJ, Snell-Bergeon JK. ABC goal achievement predicts microvascular but not macrovascular complications over 6-years in adults with type 1 diabetes: The Coronary Artery Calcification in Type 1 Diabetes Study. J Diabetes Complications. 2014 doi: 10.1016/j.jdiacomp.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyons SK, Becker DJ, Helgeson VS. Transfer from pediatric to adult health care: effects on diabetes outcomes. Pediatr Diabetes. 2014;15(1):10–7. doi: 10.1111/pedi.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyons SK, Libman IM, Sperling MA. Diabetes in the adolescent: transitional issues. J Clin Endocrinol Metab. 2013;98(12):4639–45. doi: 10.1210/jc.2013-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 ADA/ISPAD target achievement in RESISTANT/EMERALD