Abstract
Activated B-cell-like (ABC) diffuse large B-cell lymphoma (DLBCL) is associated with worse survival after standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) chemoimmunotherapy compared to germinal center B-cell-like (GCB) subtype. Preliminary evidence suggests that benefits from novel agents may vary by subtype. Hypothesizing that treatment stratified by DLBCL subtype could be potentially cost-effective, we developed micro-simulation models to compare three first-line treatment strategies: (1) standard RCHOP for all patients, (2) subtype testing followed by RCHOP for GCB and novel treatment for ABC DLBCL, and (3) novel treatment for all patients. Based on phase 2 evidence, we used lenalidomide+RCHOP as a surrogate novel treatment. The subtype-based approach showed a favorable incremental cost-effectiveness ratio of $15,015/quality-adjusted life year compared with RCHOP. Although our exploratory analyses demonstrated a wide range of conditions where subtype-based treatment remained cost-effective, data from phase 3 trials are needed to validate our models’ findings and draw definitive conclusions.
Keywords: lymphoma, diffuse large B-cell lymphoma, cost-effectiveness, ABC DLBCL, subtype testing
INTRODUCTION
Approximately 24,000 new cases of diffuse large B-cell lymphoma (DLBCL) were diagnosed in the United States in 2016, accounting for approximately one-third of non-Hodgkin lymphomas [1]. Patients with DLBCL experience widely divergent outcomes despite harboring histologically similar tumors. Gene expression profiling (GEP) and immunohistochemistry (IHC) distinguish two major biological subtypes of DLBCL: germinal center B-cell-like (GCB) and activated B-cell-like (ABC, classified as “non-GCB” by some IHC algorithms) [2–8]. These subtypes exhibit substantial differences in survival when treated with standard-of-care therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) [5,8]: ABC DLBCL patients treated with RCHOP exhibit markedly worse outcomes, with a 3-year overall survival (OS) of 69% compared with 85% in GCB DLBCL [9].
Investigational efforts to improve survival for poor-risk DLBCL patients include addition of novel agents to first-line chemoimmunotherapy. One phase 2 study by Nowakowski et al. [10] showed that adding the immunomodulatory drug lenalidomide to RCHOP (R2CHOP) yielded considerably better outcomes in non-GCB DLBCL compared to a historical control arm treated with RCHOP alone, including improved 24-month progression-free survival (PFS; 60% vs. 28%) and 24-month OS (83% vs. 46%); no significant benefits were derived for the GCB subtype. However, randomized trials evaluating the addition of other novel agents to standard frontline therapy thus far have provided insufficient evidence proving that such an approach improves treatment efficacy. For instance, no improvement was observed in a randomized phase 2 study comparing combinatory treatment with the proteasome inhibitor bortezomib and RCHOP to RCHOP alone in ABC subtype [11]. Phase 3 randomized trials examining the addition of novel agents such as lenalidomide [12], ibrutinib (a Btk inhibitor), and others to RCHOP [13–15] are currently accruing but have yet to provide definitive data regarding benefit, subtype-specific or otherwise.
Given the differential survival benefit of novel agents between ABC and GCB DLBCL, we hypothesized that customizing clinical management strategy by subtype could potentially optimize the use of such agents in DLBCL treatment by applying the principles of precision medicine. Indeed, such an approach has been successfully applied in a cost-effective manner in the management of other malignancies, notably breast cancer [16] and lung cancer [17]. However, no modeling study to date has evaluated the costs and clinical outcomes of tailored treatment strategies in DLBCL. In this study, we performed modeling analyses with illustrative examples based on currently available data to demonstrate the potential cost-effectiveness of precision medicine in DLBCL, and to better understand the driving factors the influence the cost-effectiveness outcomes of the precision medicine approach.
METHODS
We hypothesized that a tailored therapeutic approach in DLBCL treatment may be beneficial from a cost-effectiveness standpoint. In this study, our modeling analysis aims to to provide an initial evaluation of the hypothesis based on currently available evidence. It may also serve as illustrative examples to guide comprehensive evaluations as more definitive data become available.
Modeling Framework Overview
We developed micro-simulation models to evaluate the lifetime cost and health outcomes of three different first-line treatment strategies for patients with newly diagnosed DLBCL: (1) administering RCHOP to all patients as the current standard of care; (2) performing subtype testing first, and subsequently administering RCHOP to patients with GCB subtype and the novel treatment to patients with non-GCB subtype, and (3) administering novel treatment to all patients.
To design a model that reflected actual clinical outcomes, our base case analysis specified “novel treatment” in the frontline treatment as R2CHOP based on the Nowakowski study [10]. In subsequent exploratory analysis, we considered “novel treatment” as a hypothetical regimen with a wide range of efficacy. Upon disease progression, we assumed that patients would receive salvage chemotherapy followed by autologous stem cell transplant (ASCT) as the current standard of care for fit individuals with relapsed DLBCL [18,19].
The micro-simulation assigned each patient an age sampled from the age distribution from the Surveillance, Epidemiology, and End Results (SEER) database for DLBCL patients [20], and a specific subtype was randomly generated according to the previously reported prevalence of each subtype derived from a systematic review and meta-analysis [7].
In the subtype-based strategy, treatment selection was based on the patient’s subtyping test result. The GEP test was considered the gold standard with perfect accuracy, while IHC testing using the Hans algorithm exhibited imperfect sensitivity and specificity [6] that could lead to misclassification and suboptimal treatment assignment (Figure 1).
Figure 1. Subtype-based treatment strategy allows misclassification of germinal center B-cell-like (GCB)/activated B-cell-like (ABC) subtype due to imperfect testing by immunohistochemistry.
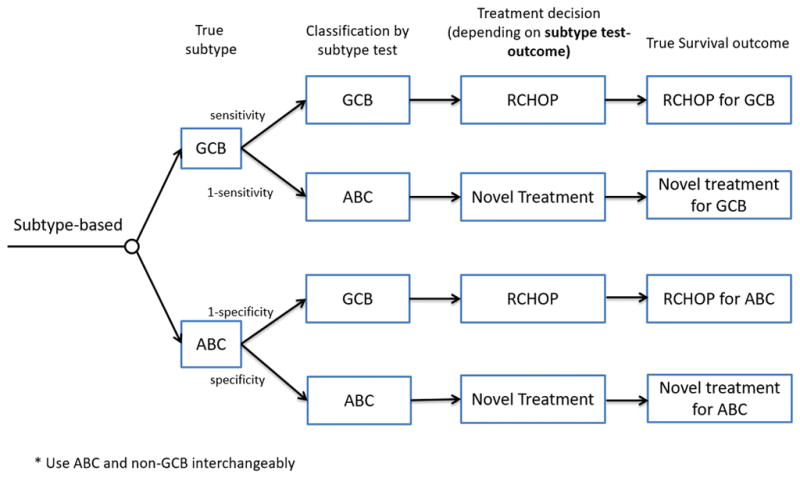
Treatment selection is guided by the subtype identified by the test, whereas survival is determined by the true subtype for a given treatment.
Patients in the model followed a clinical path through three health states: progression-free survival, relapse, and death (Supplement S1), where the likelihood of transition from one health state to another was derived from PFS and OS data dependent on subtype and treatment, and background mortality dependent on age (see Supplement S1 for additional method details). Patients were designated cured if they did not progress by 5 years, and at that point only background mortality was considered. A model cycle constituted three weeks to reflect the typical length of a single cycle of RCHOP chemotherapy for DLBCL. Model outcomes included total life years (LYs), quality-adjusted life years (QALYs), direct costs of each treatment strategy, and incremental cost-effectiveness ratios (ICERs) compared with standard-of-care treatment. All cost estimates were converted to 2016 US dollars based on the Consumer Price Index for medical care [21]. All model outcomes were discounted at an annual rate of 3%.
Treatment Effectiveness
Survival data for patients treated with RCHOP as the standard of care, stratified by true GCB and ABC subtype (as classified by GEP), were derived from seven studies reviewed in our previous meta-analysis [7]. Survival of novel treatment was parameterized by applying certain hazard ratios (HRs; β) to the survival functions of standard RCHOP (i.e., survival function for RCHOP=S(t), and survival for the novel treatment=S(t)β). As an illustrative example, we used R2CHOP as the surrogate for a novel treatment (lenalidomide as an example novel agent for addition to standard RCHOP) and the HR estimates observed from the phase 2 Nowakowski study [10] in our base case analysis. Next, to investigate how variation in treatment efficacy parameters for novel treatment might affect the performance compared to standard RCHOP treatment, we systematically examined various HRs over a wide range to characterize improvements in PFS and OS.
Cost and Utility
We adopted a US payer’s perspective and considered direct medical costs, including costs of drugs, administration, and follow-up (Table I), following our established methodology [22,23]. Average sales prices of drugs were estimated based on payment limits from Centers for Medicare and Medicaid Services (CMS) [24] for drugs covered by Medicare part B (e.g., intravenous agents), and the approach described by Bach [25] for oral drugs covered by Medicare part D (e.g., lenalidomide as the surrogate novel agent). Drug costs for a full treatment cycle were calculated based on standard dose (rounded up to the next full vial). We also included the cost of pegfilgrastim (6mg once per cycle) for patients older than 60 receiving RCHOP and for all patients receiving R2CHOP [10]. Adverse event costs were not considered because no significant differences in adverse event incidences have been observed after introducing lenalidomide to standard RCHOP [10].
Table I.
Model parameters
| Variable | Base value | Range | Distribution | References |
|---|---|---|---|---|
| Probability | ||||
| GCB prevalence | ||||
| Trial-based analysis | 0.648 | [10] | ||
| Exploratory analysis | 0.526 | [7] | ||
| Subtype test: sensitivity/specificitya | ||||
| IHC: Hans algorithm | 0.82/0.90 | [6] | ||
| GEP | 1.00/1.00 | |||
| Utility | [28] | |||
| Progression-free | 0.83 | (0.66,1) | Beta(3.42,0.7) | |
| Relapsed | 0.39 | (0.31,0.47) | Beta(14.86,23.24) | |
| Cost ($) | ||||
| Subtype test | ||||
| IHC testb | 288 | (230.4,345.6) | Gamma(25,11.52) | CPT88341, and 88342 |
| GEP test | 4270 | (3416,5124) | Gamma(25,170.8) | [26,43] |
| Drug cost per cycle | ||||
| RCHOPc | 6206 | (4964.8,7447.2) | Gamma(25,248.24) | |
| Lenalidomide | 6870 | (5496,8244) | Gamma(25,274.8) | |
| Supportive care for lenalidomide (pegfilgrastim) | 4041 | (3232.8,4849.2) | Gamma(25,161.64) | |
| Autologous SCT | 114,500 | (91600,137400) | Gamma(25,4580) | [44] |
| Chemo IV infusion 1 hr | 136.41 | (109.13,163.69) | Gamma(25,5.46) | CPT96413 |
| Chemo IV infusion addl hr | 28.64 | (22.91,34.37) | Gamma(25,1.15) | CPT96415 |
| Physician visit | 51.56 | (41.25,61.87) | Gamma(25,2.06) | CPT99213 |
| Routine laboratory blood test | 83 | (66.4,99.6) | Gamma(25,3.32) | |
GCB, germinal center B-cell-like; IHC, immunohistochemistry; GEP, gene expression profiling; SCT, stem cell transplant; IV, intravenous; hr, hour; addl, additional; RCHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
For detection of GCB subtype; because tests were assumed to be binary, the sensitivity for non-GCB subtype is equivalent to specificity for GCB.
Three IHCs are performed, and thus the total test cost is calculated as $107.41 (CPT88342) + $90.23 (CPT88341)*2=$287.87.
We assumed that chemotherapy infusion lasts for 6 hours in the first cycle, and 2 hours in subsequent cycles.
Administration cost (and physician visit, chemotherapy infusion) and IHC test cost were estimated based on the 2016 CMS physician fee schedule [22]. Since GEP test is not commercially available for DLBCL at present, we used the cost of two GEP tests for breast cancer (Mammaprint and Oncotype) [26,27] as a proxy cost estimate for GEP testing for DLBCL in our analysis. Monitoring cost included routine laboratory blood tests and physician visits (see Supplement S2 for details).
Survival was adjusted by health-related quality of life using published utility estimates from the literature. We used a utility of 0.83 for progression-free state and 0.39 for relapsed state with progressive disease, following the utility estimates used in previous economic evaluation studies for DLBCL patients receiving RCHOP [28].
Exploratory Analysis
In exploratory analyses, we relaxed the assumption that novel treatment does not improve survival for GCB patients in the base case analysis. In particular, we evaluated the cost-effectiveness of treatment strategies with hypothetical novel treatment under various HR values for both GCB and ABC DLBCL patients.
To examine joint effects of additional cost and survival benefits introduced by the hypothetical novel treatment, we varied drug cost and subtype-specific HRs for GCB and ABC patients independently, and identified the frontiers for given willingness-to-pay (WTP) thresholds.
Sensitivity Analysis
We performed probabilistic sensitivity analysis (PSA), where the values of cost and utility parameters were sampled simultaneously from statistical distributions [29] for each parameter over 10,000 iterations. We assumed gamma distributions for costs and beta distributions for utility parameters, each with a standard deviation assumed to be 20% of the baseline value (Table I). Extended PSA with greater parameter uncertainties are provided in Supplement S3. We also evaluated the uncertainties in the model’s transition probabilities using different fitted survival distributions, and assessed all treatment strategies using direct survival estimates from the Nowakowski study in an additional trial-based scenario analysis (see Supplement S3).
RESULTS
Base Case Analysis
Examining the three treatment strategies with HRs based on the phase 2 Nowakowski study (i.e., HR=0.35 for PFS and HR=0.24 for OS for non-GCB patients, and HR=1 for both PFS and OS in GCB patients) [10] demonstrated that RCHOP provided 9.85 QALYs (12.19 LYs) at a cost of $53,406; subtype-based treatment guided by GEP testing provided 12.02 QALYs (14.82 LYs) at a cost of $86,104, resulting in an ICER of $15,015/QALY compared with RCHOP. Since novel treatment did not benefit GCB patients as observed in the clinical study [10], providing novel treatment to all patients did not further improve the health outcomes, but increased cost to $111,842, and thus was dominated (i.e., outperformed) by subtype-based treatment.
Exploratory Analyses
To extend our analysis to more general hypothetical settings, we used more conservative HRs for PFS and OS ranging from 0.4 to 0.9 for both subtypes (Table II). We found that with GEP testing, the ICERs of subtype-based treatment compared with RCHOP were influenced primarily by the HR for ABC patients. For instance, the ICER decreased from $118,759/QALY to $35,719/QALY as the HR for ABC patients decreased from 0.9 to 0.7. Conversely, ICERs for universal novel treatment compared with subtype-based treatment were affected predominantly by the HR for GCB patients, with ICERs <$100,000/QALY when HR was <0.8 for GCB patients. When the novel treatment improved survival for GCB patients more than for ABC patients, subtype-based treatment was dominated by universal novel treatment.
Table II.
Incremental cost-effectiveness ratios (ICERs) under various survival benefits for GCB and ABC DLBCL patients.
| HR | GEP | IHC using Hans algorithm | |||
|---|---|---|---|---|---|
| GCB | ABC | Subtype-based treatment a | Novel treatment b | Subtype-based treatment | Novel treatment |
| 0.95 | 0.9 | 118,759 | 246,747 | 117,193 | 242,535 |
| 0.8 | 56,017 | 246,747 | 57,487 | 193,286 | |
| 0.7 | 35,719 | 246,747 | 37,235 | 157,201 | |
| 0.4 | 15,478 | 246,747 | 16,373 | 95,938 | |
|
| |||||
| 0.9 | 0.9 | dominated c | 119,725 c | 108,916 | 135,978 |
| 0.8 | 56,017 | 122,254 | 55,387 | 118,923 | |
| 0.7 | 35,719 | 122,254 | 36,327 | 104,081 | |
| 0.4 | 15,478 | 122,254 | 16,186 | 73,051 | |
|
| |||||
| 0.8 | 0.9 | dominated | 81,493 c | dominated | 81,493 c |
| 0.8 | 56,017 | 59,465 | 51,453 | 66,079 | |
| 0.7 | 35,719 | 59,465 | 34,570 | 61,191 | |
| 0.4 | 15,478 | 59,465 | 15,809 | 48,842 | |
|
| |||||
| 0.7 | 0.9 | dominated | 61,067 c | dominated | 61,067 c |
| 0.8 | dominated | 46,375 c | dominated | 46,375 c | |
| 0.7 | 35,719 | 38,542 | 32,878 | 42,722 | |
| 0.4 | 15,478 | 38,542 | 15,425 | 36,259 | |
|
| |||||
| 0.5 | 0.5 | 19,487 | 21,690 | 18,009 | 23,923 |
| 0.4 | 15,478 | 21,690 | 14,692 | 23,078 | |
|
| |||||
| 0.4 | 0.4 | 15,478 | 17,476 | 14,331 | 19,235 |
GCB, germinal center B-cell-like, ABC, activated B-cell-like; IHC, immunohistochemistry; GEP, gene expression profiling.
ICER of subtype-based treatment compared with RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment.
ICER of novel treatment compared with subtype-based treatment.
When subtype-based treatment was dominated, the ICER of novel treatment was calculated by comparing to RCHOP treatment.
Subtype-based treatment using IHC testing became increasingly cost-effective compared with GEP testing as survival benefit for the novel treatment in GCB patients increased. For example, at HR=0.4 for ABC DLBCL and HR=0.9 for GCB DLBCL, the ICER of subtype-based treatment with IHC testing was $16,186/QALY compared with $15,478/QALY with GEP testing; when HR=0.5 for GCB DLBCL, the ICER of subtype-based treatment with IHC testing was $14,692, compared with $15,478/QALY with GEP testing.
Sensitivity Analyses
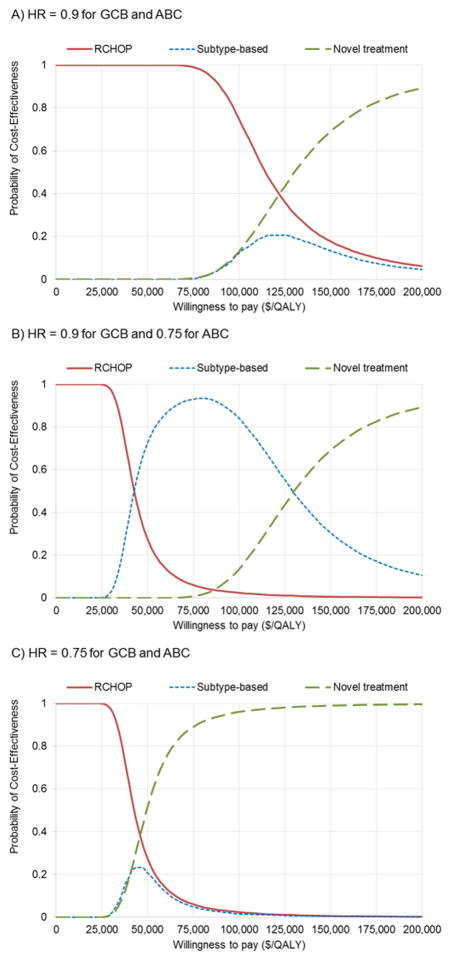
In the PSA, we presented the cost-effectiveness acceptability curves (CEACs) of three treatment strategies. These represent the probability that each strategy was considered cost-effective at any given value of WTP. Commonly used thresholds of WTP values are $50,000/QALY–$100,000/QALY [30]. The CEACs (Figure 2A) demonstrated that when novel treatment produced minimal improvement in survival (HR≥0.9) in both subtypes, subtype-based treatment was unlikely to be cost-effective (with probability <15%) at a WTP below $100,000/QALY, whereas standard RCHOP was cost-effective with high probability in that scenario. When novel treatment produced modest survival improvement for ABC (HR~0.75) but limited improvement for GCB (HR=0.9), subtype-based treatment was cost-effective with high probability (>80%) over a wide range of WTP values (Figure 2B). When novel treatment provided survival improvement in both subtypes (HR≤0.75), universal novel treatment became the most cost-effective approach and demonstrated the highest cost-effectiveness probability for WTP>$50,000/QALY (Figure 2C).
Figure 2. Cost-effectiveness acceptability curves at various survival benefit levels for germinal center B-cell-like (GCB) and activated B-cell-like (ABC) subtypes.
Subtype-based treatment is based on gene expression profiling (GEP) assay. (A) Minimal survival improvement for both subtypes by novel treatment, (B) modest survival improvement only for ABC subtype by novel treatment, (C) modest survival improvement for both subtypes by novel treatment.
The same general patterns were also observed in the CEACs that used IHC testing for the subtype-based strategy (Supplement S3). Notably, when both subtypes had similar survival benefits with novel treatment, subtype-based treatment with IHC testing showed higher likelihood of cost-effectiveness than GEP testing.
We found that our model outcomes were also robust across various fitted survival distributions, and yielded similar findings in the trial-based scenario analysis using survival data from Nowakowski’s study (see Supplement S3 for details). In addition, we identified the threshold frontiers of drug cost (i.e., the per-cycle cost of adding a novel agent to RCHOP) and survival improvement of the novel treatment at different WTP thresholds (Supplement S4). Given the current cost of our surrogate novel agent lenalidomide, subtype-based treatment was expected to be cost-effective at WTP thresholds of $100,000/QALY (cost-effective) and $50,000/QALY (highly cost-effective) when ABC-specific HRs were <0.9 and <0.8, respectively. We translated these results to project the corresponding 2-year PFS for novel treatment (Supplement S5), which can serve as reference points to determine the clinical benefit and cost-effectiveness of subtype-based treatment approaches as new data emerge.
DISCUSSION
As emerging agents show promise for improving outcomes in certain DLBCL subtypes, efforts to optimize treatment strategy in this heterogeneous patient population will become ever more important. We hypothesized that tailoring treatment based on DLBCL subtype could potentially improve the cost-effectiveness of DLBCL treatment strategies. To examine this issue, our study utilized a modeling approach and currently available clinical trial data to compare the cost-effectiveness of three strategies for utilizing novel agents in the first-line treatment of DLBCL patients: standard-of-care treatment with RCHOP, tailored treatment based on subtype that assigned RCHOP to GCB patients and novel therapy to ABC patients, and novel treatment for all patients regardless of subtype.
We utilized a general analytical model framework and performed analyses with a wide range of parameters and assumptions to evaluate subtype-based therapy. In particular, based on the best available observations from a phase 2 study, subtype-based treatment showed a favorable cost-effectiveness profile. In exploratory analyses, we examined joint effects of multiple factors under various conditions, including how variations in treatment cost, survival benefit, and subtype testing methods influence cost-effectiveness of subtype-based treatment. Furthermore, our models may help define survival endpoints for examining cost-effectiveness of subtype-based treatment approaches in DLBCL.
Precision medicine aims to deliver care customized to the needs of an individual patient. Thus, we can expect that, in the case of DLBCL treatment, a subtype-based treatment strategy would show its maximum advantages in the settings with an unbalanced survival benefit introduced by the novel treatment across different subtypes. This hypothesis is in line with our initial analysis: we found that subtype-based treatment was shown to be highly cost-effective when benefit for ABC patients significantly outweighed that for GCB, such as in the scenario based on the outcomes from Nowakowski’s study. Similar findings resulted when the HR of novel treatment was >0.8 for GCB and <0.8 for ABC in our exploratory analysis. Conversely, subtype-based treatment was less cost-effective than or even dominated by universal novel treatment when the novel therapy improved survival for both subtypes. Although novel agents such as bortezomib, carfilzomib, and ibrutinib have been evaluated predominantly in ABC DLBCL [12–15], our findings suggest that understanding the differential efficacy in GCB subtype will also be important to guide the efficient use of these therapeutic resources in the frontline setting.
An important trade-off in precision medicine lies between the accessibility (e.g., clinical availability, costs) and the accuracy (i.e., sensitivity and specificity) of testing. By comparing the cost-effectiveness of subtype-based treatment using (imperfect) IHC and (perfect) GEP testing, we found that when the disparity between subtype-specific survival outcomes was substantial (e.g., HR>0.9 for GCB and HR <0.5 for ABC subtype), more accurate testing was preferred, even at a higher per-test cost. On the other hand, if the novel treatment showed comparable improvements for both subtypes, subtype-based treatment with IHC testing became more favorable than using GEP testing. Thus, the improved ICER resulted from the IHC test’s lower cost coupled with the fact that the decreased accuracy of such testing did not result in significant loss of health outcomes given similar survival benefits for both subtypes, despite risk of possible misclassifications.
Our hypothesis-generating study should be interpreted as a proof of concept that showcases the promising cost-effectiveness profile of subtype-based treatment, a precision medicine approach, for DLBCL primarily based on data from a phase 2 study. However, we acknowledge that phase 2 studies are prone to overestimate benefits and underrepresent toxicity. To draw more definitive conclusions, further validations will be needed, with more mature data from phase 3 trials comparing subtype-specific outcomes after novel and standard treatments. Such trials already in progress include a phase 3 study comparing R2CHOP with RCHOP for ABC DLBCL [12] and a phase 3 study comparing first-line ibrutinib and RCHOP vs. RCHOP for non-GCB patients [13]. In particular, three recent unpublished randomized controlled trials have demonstrated no benefit for novel therapy over RCHOP [31–33].
Examination of the cost-effectiveness of precision medicine treatment strategies for DLBCL should continue in a broader scope as new molecular data about this heterogeneous disease emerge. Many clinical studies have proposed new predictive biomarkers and testing techniques that could be used for tailoring the management of DLBCL patients. For example, a blood-based non-invasive test utilizing circulating tumor DNA to predict cell-of-origin classification [34] has been shown to successfully stratify poor-risk groups. Another study found that concurrent MYC, BCL2 and/or BCL6 chromosomal translocations (in so-called double- or triple-hit lymphomas) were also predictive of clinical outcomes when combined with the cell-of-origin classification [35]. A recent study combining whole-exome and transcriptome analysis as well as single nucleotide polymorphism (SNP) arrays in >1000 DLBCL patients [36] has identified other mutations associated with poor survival that could be targeted with new therapies.
In addition to molecular biomarkers, clinical factors may also influence treatment options for certain patient groups. For example, the phase 3 REMARC trial [37] found that 2-year lenalidomide maintenance in elderly patients who responded to first-line RCHOP significantly improved PFS (HR=0.708 with 95% CI 0.538–0.932). Such results suggest that patient-level factors such as age and treatment responses can be utilized to determine the best use of available therapeutic options. As our knowledge of this disease becomes more extensive, we expect a more comprehensive approach that includes cell-of-origin subtype, genetic abnormalities, and patient factors will be crucial in informing decisions about tailored therapy, and improving the cost-effectiveness of healthcare resource utilization.
Our study has some limitations. First, the utility estimates were obtained from previously published cost-effectiveness studies in DLBCL treatment, which are not specific to treatment and do not differentiate cured patients from those who have not yet progressed. Second, we did not consider adverse event-related treatment discontinuation or dose reduction, or the cost of adverse events associated with novel treatment. In the Nowakowski study, most patients completed full cycles of treatment. Only 13% of total treatment cycles underwent lenalidomide dose reduction, and no significantly different adverse event incidences were observed between treatment groups [10]. Thus, we believed that incorporating these events would not substantially change our findings. To address these concerns, we performed sensitivity analyses to examine uncertainty in cost estimates and other model variables. These analyses indicated that changes in these parameters had little influence on model results.
To the best of our knowledge, this study is the first to assess the potential cost-effectiveness of a precision medicine treatment strategy for DLBCL. Customizing treatment based on biological factors of individual patients has been studied for other malignancies. For example, in metastatic non-small-cell lung cancer, first-line treatment guided by testing for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) gene rearrangements is considered cost-effective[38] and recommended in practice [17]. In breast cancer, trastuzumab treatment based on human epidermal growth factor receptor-2 (HER2) testing [39] and adjuvant chemotherapy decisions guided by GEP testing (e.g., OncotypeDX 21-gene assay) [16] are both considered cost-effective. However, unlike the growing utilization of GEP in breast cancer [40], GEP assays to ascertain subtype of DLBCL are not yet widely available in the clinical setting. Tests that accurately assign subtype using formalin-fixed paraffin-embedded tissue samples are in development [41,42] and represent a promising tool that could guide DLBCL treatment in the future.
Because data from randomized clinical trials evaluating subtype-specific therapy in DLBCL is currently limited, our study is not definitive, but it does support the idea that a subtype-based treatment strategy for DLBCL patients can be cost-effective. We evaluated this concept under various conditions of cost and survival benefit associated with hypothetical novel treatments. Through our illustrative example based on currently available phase 2 evidence, we found that subtype-based treatment could be highly cost-effective when the novel treatment showed significant survival benefit for ABC DLBCL but limited effects for GCB subtype. By exploring a wide range of parameters and model assumptions, we also characterized thresholds for survival benefit and drug cost of novel agents that would likely result in cost-effective subtype-based treatment approaches. Our analyses demonstrated the feasibility of establishing precision medicine strategies for DLBCL that are both practical and cost-effective. Additional data from larger randomized trials will be needed to confirm our findings and guide the best use of standard and novel therapies for DLBCL patients in clinical practice.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by a Burroughs Wellcome Fund Innovation in Regulatory Science Award, a Healthcare Innovations pilot award through the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number UL1TR000454, and by National Cancer Institute award number K24CA208132 to Dr. Flowers and award number T32 CA160040 supporting Dr. Staton. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA. J Clin. 2016 doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 5.Lenz G, Wright G, Dave S, et al. Gene expression signatures predict survival in diffuse large B cell lymphoma following rituximab and CHOP-like chemotherapy. Annals of Oncology. 2008;19:93–93. [Google Scholar]
- 6.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. Journal of Clinical Oncology. 2010 doi: 10.1200/JCO.2010.30.0368. JCO. 2010.2030. 0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read JA, Koff JL, Nastoupil LJ, Williams JN, Cohen JB, Flowers CR. Evaluating Cell-of-Origin Subtype Methods for Predicting Diffuse Large B-Cell Lymphoma Survival: A Meta-Analysis of Gene Expression Profiling and Immunohistochemistry Algorithms. Clin Lymphoma Myeloma Leuk. 2014 doi: 10.1016/j.clml.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 9.Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–4594. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 10.Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide Combined With R-CHOP Overcomes Negative Prognostic Impact of Non–Germinal Center B-Cell Phenotype in Newly Diagnosed Diffuse Large B-Cell Lymphoma: A Phase II Study. Journal of Clinical Oncology. 2015;33:251–257. doi: 10.1200/JCO.2014.55.5714. [DOI] [PubMed] [Google Scholar]
- 11.Leonard JP, Kolibaba K, Reeves JA, et al. Randomized Phase 2 Open-Label Study of R-CHOP±Bortezomib in Patients (Pts) with Untreated Non-Germinal Center B-Cell-like (Non-GCB) Subtype Diffuse Large Cell Lymphoma (DLBCL): Results from the Pyramid Trial ( NCT00931918) Blood. 2015;126:811–811. [PubMed] [Google Scholar]
- 12.Celgene Corporation. Efficacy and Safety Study of Lenalidomide Plus R-CHOP Chemotherapy Versus Placebo Plus R-CHOP Chemotherapy in Untreated ABC Type Diffuse Large B-cell Lymphoma (ROBUST) [Accessed 2016 1/28/2016];ClinicalTrials.gov. 2016 Jan 28; 2014. < https://clinicaltrials.gov/ct2/show/NCT02285062?term=lenalidomide+ROBUST&rank=1>.
- 13.Janssen Research & Development. A Study of the Bruton’s Tyrosine Kinase Inhibitor, PCI-32765 (Ibrutinib), in Combination With Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Patients With Newly Diagnosed Non-Germinal Center B-Cell Subtype of Diffuse Large B-Cell Lymphoma. [Accessed 2016 2/16/2016];ClinicalTrials.gov. 2016 Feb 16; 2013. < https://clinicaltrials.gov/ct2/show/NCT01855750>.
- 14.Case Comprehensive Cancer Center. ClinicalTrials.gov. Case Comprehensive Cancer Center; 2014. Jun 11, [Accessed 2014 6/11/2014]. Carfilzomib, Rituximab, and Combination Chemotherapy in Treating Patients With Diffuse Large B-Cell Lymphoma. < http://clinicaltrials.gov/ct2/show/NCT02073097?term=carfilzomib+R-CHOP&rank=1>. [Google Scholar]
- 15.Millennium Pharmaceuticals. Study to Assess the Effectiveness of RCHOP With or Without VELCADE in Previously Untreated Non-Germinal Center B-Cell-like Diffuse Large B-Cell Lymphoma Patients. [Accessed 2016 2/16/2016];ClinicalTrials.gov. Feb 16; 2014. 2016. < https://clinicaltrials.gov/ct2/show/NCT00931918>.
- 16.Elkin EB, Marshall DA, Kulin NA, et al. Economic evaluation of targeted cancer interventions: critical review and recommendations. Genetics in Medicine. 2011;13:853–860. doi: 10.1097/GIM.0b013e31821f3e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. The Journal of molecular diagnostics. 2013;15:415–453. doi: 10.1016/j.jmoldx.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Kenkre VP, Smith SM. Management of relapsed diffuse large B-cell lymphoma. Current oncology reports. 2008;10:393–403. doi: 10.1007/s11912-008-0061-4. [DOI] [PubMed] [Google Scholar]
- 19.Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. Journal of Clinical Oncology. 2011;29:4079–4087. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- 20.SEER; Surveillance Research Program SSB, editor. Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS; 2014. ( www.seer.cancer.gov) Research Data (1973–2011), based on the November 2013 submission. [Google Scholar]
- 21.The Bureau of Labor Statistics. [Accessed 2015 July 1];Archived Consumer Price Index detailed report information. 2015 Jul 1; < http://www.bls.gov/cpi/cpi_dr.htm>.
- 22.Tumeh JW, Moore SG, Shapiro R, Flowers CR. Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. 2005 doi: 10.1586/14737167.5.2.153. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DA, Chen Q, Ayer T, et al. First-and Second-Line Bevacizumab in Addition to Chemotherapy for Metastatic Colorectal Cancer: A United States–Based Cost-Effectiveness Analysis. Journal of Clinical Oncology. 2015;33:1112–1118. doi: 10.1200/JCO.2014.58.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services. [Accessed 2016 Feb];2016 ASP Drug Pricing Files. 2016 Feb; < https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2016ASPFiles.html>.
- 25.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. New England Journal of Medicine. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 26.Slodkowska EA, Ross JS. MammaPrint™ 70-gene signature: another milestone in personalized medical care for breast cancer patients. 2009 doi: 10.1586/erm.09.32. [DOI] [PubMed] [Google Scholar]
- 27.Roberts MC, Dusetzina SB. Use and Costs for Tumor Gene Expression Profiling Panels in the Management of Breast Cancer From 2006 to 2012: Implications for Genomic Test Adoption Among Private Payers. Journal of Oncology Practice. 2015;11:273–277. doi: 10.1200/JOP.2015.003624. [DOI] [PubMed] [Google Scholar]
- 28.Best JH, Hornberger J, Proctor SJ, Omnes LF, Jost F. Cost - Effectiveness Analysis of Rituximab Combined with CHOP for Treatment of Diffuse Large B - Cell Lymphoma. Value in health. 2005;8:462–470. doi: 10.1111/j.1524-4733.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 29.Briggs A, Claxton C, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford University Press; 2006. [Google Scholar]
- 30.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. New England Journal of Medicine. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 31.Davies AJ, Caddy J, Maishman T, et al. A Prospective Randomised Trial of Targeted Therapy for Diffuse Large B-Cell Lymphoma (DLBCL) Based upon Real-Time Gene Expression Profiling: The Remodl-B Study of the UK NCRI and SAKK Lymphoma Groups (<a href=“pending:yes” l:ref-type=“ISRCTN” l:ref=“51837425”>ISRCTN51837425</a>) Blood. 2015;126:812–812. [Google Scholar]
- 32.Leonard JP, Kolibaba K, Reeves JA, et al. Randomized Phase 2 Open-Label Study of R-CHOP ± Bortezomib in Patients (Pts) with Untreated Non-Germinal Center B-Cell-like (Non-GCB) Subtype Diffuse Large Cell Lymphoma (DLBCL): Results from the Pyramid Trial (<a href=“pending:yes” l:ref-type=“CLINTRIALGOV” l:ref=“ NCT00931918”> NCT00931918</a>) Blood. 2015;126:811–811. [Google Scholar]
- 33.Wilson WH, sin-Ho J, Pitcher BN, et al. Phase III Randomized Study of R-CHOP Versus DA-EPOCH-R and Molecular Analysis of Untreated Diffuse Large B-Cell Lymphoma: CALGB/Alliance 50303. Blood. 2016;128:469–469. [Google Scholar]
- 34.Scherer F, Kurtz DM, Newman AM, et al. Development and Validation of Biopsy-Free Genotyping for Molecular Subtyping of Diffuse Large B-Cell Lymphoma. Am Soc Hematology. 2016 [Google Scholar]
- 35.Staiger AM, Ziepert M, Horn H, et al. The Clinical Impact of the Cell-of-Origin Classification and the MYC+/BCL2+ Double Expresser Status in DLBCL Treated within Prospective Clinical Trials of the Dshnhl. Blood. 2016;128:151–151. [Google Scholar]
- 36.Zhang J, Reddy A, Love C, et al. Integrative Genetic and Clinical Analysis through Whole Exome Sequencing in 1001 Diffuse Large B Cell Lymphoma (DLBCL) Patients Reveals Novel Disease Drivers and Risk Groups. Am Soc Hematology. 2016 [Google Scholar]
- 37.Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide Maintenance Compared With Placebo in Responding Elderly Patients With Diffuse Large B-Cell Lymphoma Treated With First-Line Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. Journal of Clinical Oncology. 2017 doi: 10.1200/JCO.2017.72.6984. JCO. 2017.2072. 6984. [DOI] [PubMed] [Google Scholar]
- 38.Romanus D, Cardarella S, Cutler D, Landrum MB, Lindeman NI, Gazelle GS. Cost-Effectiveness of Multiplexed Predictive Biomarker Screening in Non-Small-Cell Lung Cancer. Journal of Thoracic Oncology. 2015;10:586–594. doi: 10.1097/JTO.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2–positive breast cancer. Journal of Clinical Oncology. 2007;25:625–633. doi: 10.1200/JCO.2006.06.4220. [DOI] [PubMed] [Google Scholar]
- 40.Roberts MC, Dusetzina SB. Use and Costs for Tumor Gene Expression Profiling Panels in the Management of Breast Cancer From 2006 to 2012: Implications for Genomic Test Adoption Among Private Payers. Journal of Oncology Practice. 2015 doi: 10.1200/JOP.2015.003624. JOP. 2015.003624. [DOI] [PubMed] [Google Scholar]
- 41.Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123:1214–1217. doi: 10.1182/blood-2013-11-536433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. Journal of Clinical Oncology. 2015;33:2848–2856. doi: 10.1200/JCO.2014.60.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang M, Rajan S, Issa AM. Cost effectiveness of gene expression profiling for early stage breast cancer. Cancer. 2012;118:5163–5170. doi: 10.1002/cncr.27443. [DOI] [PubMed] [Google Scholar]
- 44.Majhail NS, Mau L-W, Denzen EM, Arneson TJ. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. Bone marrow transplantation. 2013;48:294–300. doi: 10.1038/bmt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.