Abstract
Background
The extent of influence of body mass index (BMIZ) and age on C-peptide at the diagnosis of type 1 diabetes is unknown.
Objective
We studied the influence of BMIZ and age on C-peptide measures at and soon after the diagnosis of type 1 diabetes (T1D).
Subjects
Data from Diabetes Prevention Trial-Type 1 (DPT-1) participants <18.0 years at diagnosis was analyzed.
Methods
Analyses examined associations of C-peptide measures with BMIZ and age in 2 cohorts: oral glucose tolerance tests (OGTT) at diagnosis (n=99); mixed-meal tolerance tests (MMTT) <6 months after diagnosis (n=80). Multivariable linear regression was utilized.
Results
Fasting and area under the curve (AUC) C-peptide from OGTTs (n=99) at diagnosis and MMTTs (n=80) after diagnosis were positively associated with BMIZ and age (p<0.001 for all). Associations persisted when BMIZ and age were included as independent variables in regression models (p<0.001 for all). BMIZ and age explained 31%-47% of the variance of C-peptide measures. In an example, two individuals with identical AUC C-peptide values had an approximate 5-fold difference in values after adjustments for BMIZ and age. The association between fasting glucose and C-peptide decreased markedly when fasting C-peptide values were adjusted (r=0.30, p<0.01 to r=0.07, n.s.).
Conclusions
C-peptide measures are strongly and independently related to BMIZ and age at and soon after the diagnosis of T1D. Adjustments for BMIZ and age cause substantial changes in C-peptide values, and impact the association between glycemia and C-peptide. Such adjustments can improve assessments of β-cell impairment at diagnosis.
Keywords: Type 1 diabetes, body mass index, age, C-peptide, children
INTRODUCTION
C-peptide indices are utilized to assess the loss of insulin secretion prior to and after the diagnosis of type 1 diabetes (T1D) in both clinical and research contexts. Clinically, these measures are used to differentiate T1D from type 2 diabetes and in guiding therapy. In addition, particularly in the United States, insurers have used C-peptide thresholds for determining whether insulin pumps and sensors should be covered. In the research setting, C-peptide measures are used to examine patterns of insulin loss in natural history studies (1), and as entry criteria and endpoints in clinical trials assessing interventions intended to prevent insulin loss (2–7).
In examining β-cell function for these purposes, it is essential to minimize other factors that could influence C-peptide levels apart from the pathology that is specific to T1D. Such factors include adiposity and age. Adiposity is known to be associated with insulin resistance (8,9). Although the direct influence of age on insulin resistance is less certain (10), age could influence insulin secretion by other mechanisms, such as possibly influencing β-cell mass (11). Thus, it is important to characterize the associations of C-peptide measures with indicators of adiposity such as BMI and age at the diagnosis of T1D to avoid a misinterpretation of β-cell function.
Since there is little information available regarding associations of C-peptide with BMI and age at the diagnosis of T1D, we have utilized Diabetes Prevention Trial-Type 1 (DPT-1) data to study the influence of BMI and age on C-peptide at the time of and soon after the diagnosis of T1D. In the DPT-1 study, a number of individuals were diagnosed by oral glucose tolerance testing which included C-peptide determinations. The findings presented below provide information that is relevant to assessments of β-cell function in both clinical and research settings.
METHODS
Subjects
Subjects who participated in the parenteral (12) and oral (13) insulin DPT-1 trials have previously been described in detail. All of the participants had islet cell autoantibodies and all were related to patients with T1D; neither the parenteral nor the oral insulin interventions showed efficacy. All participants included in the analysis were <18.0 years of age at diagnosis. Two analyses were performed from that cohort: a 2-hour oral glucose tolerance test (OGTT) analysis at the time of diagnosis, and a 2-hour mixed meal tolerance test (MMTT) analysis in the post-diagnostic period. For the OGTT analysis, all included were required to have BMI (kg/m2) measurements on the same date as the diagnostic OGTTs. Ninety-nine had BMI measurements on that date. Of those analyzed at diagnosis with OGTTs, 62 were in the parenteral insulin trial (intervention: 36; controls: 26) and 37 were in the oral insulin trial (intervention: 18; controls: 19). Criteria for inclusion in the MMTT analysis were the performance of MMTTs within 6 months after diagnosis and a BMI measurement within ±6 months of the MMTT. Of the 80 included in the MMTT analysis, 52 were in the parenteral insulin trial (intervention: 30; controls: 22) and 28 were in the oral insulin trial (intervention: 11; controls: 17). The mean±SD interval from diagnosis to MMTTs was 0.24±0.10 years. Since each analysis had its own inclusion criteria, the participants could differ between the cohorts; overall, 53 individuals were included in both cohorts. DPT-1 was approved by institutional review boards at all participating sites, and written informed consents or assents as appropriate were obtained.
Procedures
As previously described (12,13), in both the parenteral and oral insulin DPT-1 trials, OGTTs were originally performed at 6-month (±3 months) intervals for diagnostic surveillance. After an oral glucose dose of 1.75 g per kilogram (maximum, 75 g of carbohydrate), fasting, 30, 60, 90 and 120 minute samples were obtained for glucose and C-peptide measurements. All of those included in the analysis were diagnosed by American Diabetes Association criteria. When an OGTT was within the diabetic range (fasting glucose values ≥126 mg/dl and/or 2-hr glucose values ≥200 mg/dl), a second OGTT was to be performed within 60 days unless clinically contraindicated. If the second OGTT was confirmatory, the age at the first diabetic OGTT was considered the age at diagnosis. The first diabetic OGTT (i.e., the diagnostic OGTT) was used for the OGTT analysis. For the MMTTs, glucose and C-peptide measurements were obtained before and after the consumption of a liquid formula meal (Sustacal/Boost, Mead Johnson Nutritionals; 6 kcal/kg body weight, maximum 360 kcal). Insulin dosing was held the morning of the OGTT until after test completion. Since children were analyzed, BMI Z-scores (BMIZ) were used for all analyses of BMI. The BMIZ values reflect BMI measures adjusted for age and sex based on Centers for Disease Control reference values (2000 growth charts). BMIZ is used as an indicator of the degree of adiposity.
Laboratory Measures
Plasma glucose levels were measured by the glucose oxidase method. C-peptide levels were measured by radioimmunoassay. Fasting C-peptide values in the undetectable range (<0.2 ng/ml) were assigned a value of 0.1 ng/ml for the analyses. Three C-peptide measures were analyzed: fasting, area under the curve (AUC), and the 30-0 minute difference. The latter was included in the analysis, since it correlates with the first phase insulin response and declines during the latter stages of progression to T1D (14). The same C-peptide measures were used for the OGTT and MMTT analyses.
Data Analysis
Each of the analysis cohorts (OGTT and MMTT) were characterized using summary statistics, and two-sample t-tests and chi-square tests were used to compare factors and measures between groups of interest. Univariate and multivariable generalized linear regression models were used to examine associations of C-peptide variables with BMIZ and age. Models for each of the analysis cohorts were evaluated that adjusted for BMIZ alone, age alone, and for both. Pearson correlations were also utilized. Exploratory analyses supported the use of untransformed C-peptide measures; in addition, associations were similar when C-peptide values were log transformed.
To illustrate and further evaluate the influence of BMIZ and age on AUC C-peptide, coefficients for BMIZ and age for each of these fitted models were used to calculate an adjusted AUC C-peptide estimate from OGTTs at diagnosis and MMTTs after diagnosis. The influence of removing subject-specific BMIZ and age-related effects on AUC C-peptide was assessed by subtracting the subject-specific effects for BMIZ and age, according to the fitted models, from the actual observed AUC C-peptide value. We explored this delineation of effects added to the estimated AUC C-peptide that were specific to BMIZ and/or age for the individual difference from the average subject. In this context, those in the extremes for BMIZ or age in relation to the average cohort subject will have a greater adjustment to their AUC C-peptide than those whose BMIZ or age are near the overall average. An example of the formulaic approach for this functional adjustment is:
where is the predicted model-based estimate of AUC C-peptide for a specific subject and their corresponding BMIZ, is the predicted model-based estimate of AUC C-peptide using the mean BMIZ from the overall analysis cohort, and yi is the actual observed AUC C-peptide for the specific subject of interest. Thus, if our regression coefficient for BMIZ is 0.459, we would calculate the functional component of AUC C-peptide adjusted for BMIZ as . These adjustments were utilized to assess the influence of such a correction and the possible roles BMIZ and age have on AUC C-peptide at or soon after the diagnosis of T1D. The same methodology was used to assess the influence of BMIZ and age on the fasting C-peptide. Pearson correlations were used to assess the impact of associations between glucose and C-peptide variables.
For the figure, data was categorized according to tertiles; comparisons were made between the highest and lowest groups. OGTT and MMTT AUCs were calculated with the trapezoidal rule. The SAS 9.2 version was used for the analyses. All p-values are two-sided with a significance level set at 0.05.
RESULTS
There were 99 DPT-1 participants with OGTTs at diagnosis (mean±SD age at diagnosis: 11.3±3.3 years; mean±SD BMIZ at diagnosis: 0.42±1.13; 58% male) who were analyzed. Also, 80 DPT-1 participants were analyzed who had MMTTs within 6 months after diagnosis (age at MMTT: 11.8±3.4 years; BMIZ at MMTT: 0.52±1.08; 53% male). Characteristics of the participants are shown in Table 1, and values of C-peptide indices from the OGTTs and MMTTs are shown in Table 2. There were no differences between male and female participants in any of the C-peptide measures among the OGTT (male: n=57; female: n=42; p≥0.78 for differences) and the MMTT (male: n=38; female: n=42; p≥0.28 for differences) cohorts.
Table 1.
Characteristics of the OGTT and MMTT cohorts
| OGTT Cohort (n=99) | MMTT Cohort (n=80) | |
|---|---|---|
| Age at Baseline (years) | 9.0±3.1 | 9.3±3.3 |
| Age at Diagnosis (years) | 11.3±3.3 | 11.6±3.4 |
| Age at MMTTs (years) | -------- | 11.8±3.4 |
| Height (cm) | 149.0±18.2 | 150.0±18.9 |
| Height (Z-value) | 0.55±0.99 | 0.33±0.84 |
| Weight (kg) | 46.0±19.2 | 47.6±19.1 |
| Weight (Z-value) | 0.61±1.04 | 0.55±0.99 |
| BMI (kg/m2) | 19.8±4.5 | 20.2±4.5 |
| BMIZ | 0.42±1.13 | 0.52±1.08 |
| HbA1c (%) | 6.1±0.8 | -------- |
| Gender (% Male) | 57.6 | 52.5 |
mean±SD values shown except for Gender
Table 2.
Mean±SD C-peptide values of OGTTs at diagnosis and MMTTs within 0.5 years after diagnosis
| OGTT (n=99) | MMTT (n=80) | |
|---|---|---|
| Fasting C-peptide (ng/ml) | 1.34±0.89 | 1.02±0.63 |
| 30-0 Minute C-peptide Difference (ng/ml) | 1.31±1.01 | 1.45±1.19 |
| AUC C-peptide (ng/ml)/120 | 2.87±1.54 | 2.39±1.29 |
Regression coefficients for the associations of fasting C-peptide, 30-0 minute C-peptide difference, and AUC C-peptide from the OGTTs and MMTTs with BMIZ and age are shown in Table 3. There were substantial positive associations of the fasting and AUC C-peptide from the OGTTs with BMIZ and with age (p<0.001 for both). However, the 30-0 minute C-peptide difference was not significantly associated with BMIZ or with age.
Table 3.
Regression coefficients and R2 (in parentheses) for univariate associations of C-peptide indices with BMIZ and age for OGTTs at diagnosis and for MMTTs within 0.5 years after diagnosis
| OGTT (n=99) | BMIZ | Age (Years) |
|---|---|---|
| Fasting C-peptide (ng/ml) | 0.41±0.07 (0.27)++ | 0.13±0.02 (0.23)++ |
| 30-0 Minute C-peptide Difference (ng/ml) | 0.07±0.09 (0.01) | 0.05±0.03 (0.03) |
| AUC C-peptide (ng/ml)/120 min | 0.46±0.13 (0.11)++ | 0.23±0.04 (0.24)++ |
| MMTT (n=80) | ||
| Fasting C-peptide (ng/ml) | 0.25±0.06 (0.19)++ | 0.07±0.02 (0.15)++ |
| 30-0 Minute C-peptide Difference (ng/ml) | 0.31±0.12 (0.08)+ | 0.04±0.04 (0.02) |
| AUC C-peptide (ng/ml)/120 min | 0.44±0.13 (0.13)++ | 0.15±0.04 (0.16)++ |
p<0.05;
p<0.001
The associations of the fasting and AUC C-peptide from the MMTTs with BMIZ and with age were also positive and significant (p<0.001 for all). Their magnitudes were similar to those for the OGTT associations. However, in contrast to the OGTTs, there was also a significant association of the 30-0 minute C-peptide difference with BMIZ (p<0.05). There were no significant associations of any of the C-peptide measures from the OGTTs or the MMTTs with gender.
Figure 1 shows C-peptide values for each OGTT time point according to BMIZ (A) and age (B) tertiles. The C-peptide values were significantly greater for the highest BMIZ tertile than for the lowest tertile at all OGTT time points (p<0.01 for all). The differences were even greater between the highest and lowest age tertiles (p<0.001 at all of the OGTT time points). For BMIZ, the values of the middle tertile were slightly higher than the values of the lowest tertile, whereas for age the values of the middle tertile were closer to the highest tertile.
Figure 1.
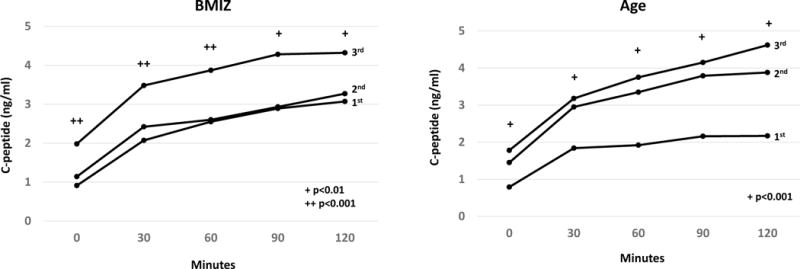
Shown are mean AUC C-peptide levels during OGTTs according to BMIZ and age tertiles. Those in the highest tertiles of BMIZ and age had markedly higher AUC C-peptide levels than those in the lowest tertiles. For BMIZ, the values of the middle tertile were slightly higher than the values of the lowest tertile, whereas for age the values of the middle tertile were closer to the highest tertile. The ages of those in the middle BMIZ tertile (10.6±3.2 years) tended to be lower than the ages of those in the lowest 11.4±3.6 and highest 11.8±3.1 tertiles. After an adjustment for age, the AUC C-peptide values of the middle BMIZ tertile moved further from the lowest tertile (from 13% of the difference between the highest and lowest tertiles to 28% of the distance).
Table 4 shows data from regression models with the C-peptide indices of the OGTTs and MMTTs as dependent variables, and BMIZ and age included together as independent variables for each model. Fasting and AUC C-peptide levels were significantly related to BMIZ and age in each model for both OGTTs and MMTTs. The R2 (the proportion of the variance explained by BMIZ and age together in the models) for the associations of the fasting and AUC C-peptide with BMIZ and age varied from 0.31 to 0.47 for the OGTTs and MMTTs. Notable were the similarities of the multivariable coefficients in Table 4 to the univariate coefficients in Table 3. These findings indicate that the associations with BMIZ and age were largely independent of each other.
Table 4.
Regression coefficients from models that included C-peptide indices as dependent variables with BMIZ and age as independent variables for OGTTs at diagnosis and for MMTTs within 0.5 years after diagnosis
| OGTT (n=99) | MMTT (n=80) | |||||
|---|---|---|---|---|---|---|
| BMIZ | Age (Years) | R2 | BMIZ | Age (Years) | R2 | |
| Fasting C-peptide (ng/ml) | 0.38±0.06++ | 0.12±0.02++ | 0.47 | 0.27±0.05++ | 0.07±0.02++ | 0.36 |
| 30-0 Minute C-peptide Difference (ng/ml) | 0.06±0.09 | 0.05±0.03 | 0.03 | 0.31±0.12+ | 0.05±0.04 | 0.10 |
| AUC C-peptide (ng/ml)/120 min | 0.42±0.11++ | 0.22±0.04++ | 0.33 | 0.46±0.11++ | 0.16±0.04++ | 0.31 |
p<0.05;
p<0.001
To demonstrate the extent of influence of BMIZ and age on C-peptide levels at diagnosis, we adjusted AUC C-peptide values to mean BMIZ and mean age values of the 99 children in the OGTT cohort and the 80 children in the MMTT cohort (Table 5). In the example shown, a 2.87 ng/ml/120 AUC C-peptide value of a 5 year-old child at the 10th BMIZ percentile of the OGTT cohort (BMIZ value=−1.13) would increase to an adjusted AUC C-peptide value of 4.91 ng/ml/120. Conversely, a 2.87 ng/ml/120 AUC C-peptide value in a 17 year-old adolescent at the 90th BMIZ percentile would decrease to an adjusted AUC C-peptide value of 0.97 ng/ml/120. Thus, although the actual AUC C-peptide values were the same for each child, the adjusted AUC C-peptide value (i.e., after removal of the influence of BMIZ and age) was approximately 5-fold higher in the younger and thinner child. A large divergence was similarly evident for the adjusted AUC C-peptide from the MMTTs.
Table 5.
Examples of AUC C-peptide values adjusted for BMIZ, age, or both from regression coefficients for 2 individuals at opposite ends of BMIZ and age distributions with same AUC C-peptide at diagnosis
| AUC C-peptide=2.87 from OGTT at Diagnosis | AUC C-peptide=2.39 from MMTT after Diagnosis | |||||
|---|---|---|---|---|---|---|
| Adjusted for BMIZ | Adjusted for Age | Adjusted for Both | Adjusted for BMIZ | Adjusted for Age | Adjusted for Both | |
| Age=5 Years; BMIZ=10th %tile | 3.58 | 4.32 | 4.91 | 2.98 | 3.43 | 4.10 |
| Age=17 Years; BMIZ=90th %tile | 2.17 | 1.56 | 0.97 | 1.76 | 1.60 | 0.90 |
We examined the extent to which BMIZ and age influenced the impact of C-peptide on glycemia at diagnosis. Whereas there was a significant association of fasting glucose values (log transformed) with unadjusted fasting C-peptide levels (r=0.30, p<0.01), there was little association when fasting C-peptide levels were adjusted for BMIZ and age (r=-0.07, n.s.). There were significant associations of AUC glucose with both unadjusted and adjusted AUC-C-peptide levels; however, the association tended to be stronger (negatively) with adjusted AUC C-peptide levels (r=−0.37, p<0.001 for adjusted; r=−0.30, p<0.01 for unadjusted).
DISCUSSION
The findings showed that at the diagnosis of T1D, an appreciable proportion of the variance of the fasting and AUC C-peptide from OGTTs was explained by BMIZ and age. Notably, the associations of the C-peptide indices with BMIZ and age were independent of each other in multivariable models. When the AUC C-peptide was adjusted for BMIZ and/or age, the C-peptide values differed markedly from the actual values.
There is an appreciable loss of C-peptide after diagnosis (15,16). Since MMTTs occurred on average about 3 months after the OGTTs, it appears that despite the loss of C-peptide after diagnosis, the magnitude of the association of C-peptide with BMIZ and age persists.
The independence of BMIZ and age in their associations with the C-peptide indices suggests that the basis for the associations differs between BMIZ and age. Evidence from prior studies are consistent with this finding. Whereas studies have consistently shown associations between insulin resistance and indicators of the degree of adiposity such as BMI in other populations (8,9), the relationship of insulin resistance with age is less certain (10). Since some β-cell characteristics are associated with age (11,17), it is possible that insulin secretion might be better sustained in older individuals who develop T1D. Age has been shown to be a mitigating factor for the risk of T1D (18) and for the loss of C-peptide levels soon after diagnosis (16).
The associations of C-peptide with BMIZ and age are clinically relevant, since C-peptide measurements are sometimes used to help differentiate the types of diabetes at diagnosis and to guide therapy. Also, low C-peptide values have been used as a criterion for providing insurance coverage of insulin pumps and glucose sensors. Thus, overweight and older children recently diagnosed with T1D could be less likely to obtain coverage than thinner and younger children.
These findings also have implications with regard to clinical trials evaluating interventions for preserving β-cell function in recently diagnosed T1D patients. Such trials have used the AUC C-peptide from MMTTs as endpoints (2–7). Adjustments of the AUC C-peptide have been made for age in those trials, but not for the degree of BMI. Moreover, the findings are also relevant to the selection of subjects for trials, since C-peptide levels below a minimum threshold are used as an exclusion criterion. Without appropriate adjustments for BMI and age, younger and thinner children would more likely be excluded from clinical trials than overweight adolescents.
Since adjustments of C-peptide for BMIZ and age essentially exclude the portion of C-peptide that is attributable to those characteristics, we examined differences between unadjusted and adjusted C-peptide values with regard to their associations with glucose values. The analysis showed that the positive association between the fasting glucose and fasting C-peptide was especially impacted by the adjustment, indicating that the association was almost fully attributable to BMIZ and age. The adjustment of the AUC C-peptide resulted in a stronger inverse association with the AUC glucose, but the impact was smaller.
Despite the substantial impact of BMIZ and age on C-peptide values, adjustments might not always be warranted. For example, adjustments for age could obscure the reasonable possibility that β-cell compromise is less severe at the diagnosis of T1D in older individuals. Thus, for natural history studies of β-cell decline, age stratification might be more appropriate than age adjustment. As more is learned about the bases for the associations of C-peptide with BMIZ and age, decisions regarding the need for adjustment can be made with more certainty.
The adjustments demonstrating the extent to which BMIZ and age influenced C-peptide levels do not necessarily generalize to other populations, since the coefficients for the associations could differ among populations. However, similar modeling procedures for adjustments could be utilized.
One of the limitations of the study was the lack of assessment of insulin resistance. We chose not to use indirect measures such as HOMA-IR, as they have not been validated in newly diagnosed T1D patients. In addition, since pubertal status was not ascertained at the time of diagnosis, we could not determine the extent of association between C-peptide indices and puberty. Insulin resistance has been shown to be associated with puberty (8). As a result of OGTT surveillance, it is likely that the diagnosis in DPT-1 occurred earlier than the typical diagnosis made clinically. Thus, the associations are not necessarily indicative of those at the time of a clinical diagnosis. Finally, since the analyses were cross-sectional, inferences could not be made on β-cell decline.
No prior studies have examined the impact of BMIZ and age on C-peptide indices from OGTTs at diagnosis, including their impact on associations between glucose and C-peptide. Recent studies (19,20) found that random C-peptide levels at diagnosis were higher in children with a greater degree of adiposity, and in older children. In a DPT-1 analysis, a correlation was observed between the AUC C-peptide from MMTTs and age in individuals at risk for T1D (21). A study of children and adult T1D patients within 3 months of their diagnosis found positive associations of the AUC C-peptide from MMTTs with BMIZ and age (16).
In conclusion, the findings indicate that BMIZ and age substantially and independently influence C-peptide levels at and soon after the diagnosis of T1D. These associations should be considered in both clinical and research settings. Although adjustments for BMIZ and age can add clarity to assessments of β-cell function, they should only be undertaken after determining their appropriateness for a particular objective.
Supplementary Material
Acknowledgments
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, and the Juvenile Diabetes Research Foundation International (JDRF). The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF. Jay Sosenko is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups (see Online-Only Supplement)
AUTHOR CONTRIBUTIONS
Jay Sosenko analyzed data and wrote the manuscript. Susan Geyer provided statistical guidance and reviewed the manuscript. Jay Skyler conducted the study and reviewed the manuscript. Lisa Rafkin conducted the study and reviewed the manuscript. Heba Ismail reviewed the manuscript. Ingrid Libman reviewed the manuscript. Linda DiMeglio reviewed the manuscript. Carmella Evans-Molina reviewed the manuscript. Jerry Palmer conducted the study, reviewed the manuscript, and assisted in writing the manuscript.
There was no conflict of interest for any of the authors.
Contributor Information
Jay M. Sosenko, Division of Endocrinology; University of Miami; Miami, FL 33101.
Susan Geyer, Health Informatics Institute; University of South Florida; Tampa, Florida 33612.
Jay S. Skyler, Division of Endocrinology; University of Miami; Miami, Florida 33101.
Lisa E. Rafkin, Division of Endocrinology; University of Miami; Miami, Florida 33101.
Heba M. Ismail, Division of Endocrinology, Diabetes and Metabolism, University of Pittsburgh and Children’s Hospital of Pittsburgh of UPMC; Pittsburgh, PA 15224.
Ingrid M. Libman, Division of Endocrinology, Diabetes and Metabolism, University of Pittsburgh and Children’s Hospital of Pittsburgh of UPMC; Pittsburgh, PA 15224.
Yuk-Fun Liu, Immunobiology, Kings College; London, UK.
Linda A. DiMeglio, Section of Pediatric Endocrinology/Diabetology, Indiana University; Indianapolis, Indiana 46202.
Carmella Evans-Molina, Divison of Endocrinology, Indiana University; Indianapolis, Indiana 46202.
Jerry P. Palmer, VA Puget Sound Health Care System; Division of Endocrinology, Metabolism, and Nutrition; University of Washington; Seattle, Washington 98108.
References
- 1.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. Type 1 Diabetes TrialNet Study Group TheTrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 2.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 3.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS, The Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, Schatz DA, Moran AM, Lachin JM, Skyler J, the Type 1 Diabetes TrialNet MMF/DZB Study Group Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new onset type 1 diabetes. Diabetes Care. 2010;33:826–832. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, Monzavi R, Moran A, Orban T, Raskin P, Rodriguez H, Russell WE, Schatz D, Wilson DM, Skyler JS, The Type 1 Diabetes TrialNet GAD Study Group Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gitelman SE, Fisher LK, Gottlieb PA, Gottschalk M, Moore WV, Moran A, Rigby MR, Willi SM, Keyes-Elstein L, Pinckney A, Ding L, Ehlers MR, START Study Team Effect of anti-thymocyte globulin (ATG) on preserving beta cell function in new-onset type 1 diabetes. Diabetologia. 2012;55(Suppl 1):S191–192. [Google Scholar]
- 7.Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, Sanda S, Schatz D, Wherrett D, Wilson DM, Skyler JS, The Type 1 Diabetes TrialNet Canakinumab Study Group. Pickersgill L, de Koning E, Ziegler A-G, Böehm B, Badenhoop K, Schloot N, Bak JF, Pozzilli P, Mauricio D, Donath MY, Castaño L, Wägner A, Lervang HH, Perrild H, Mandrup-Poulsen T, The AIDA Study Group Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicenter, randomized double-masked, placebo-controlled trials. Lancet. 2013;381(9881):1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran A, Jacobs DR, Jr, Steinberger J, Ching-Ping Hong, Prineas R, Luepker R, Siaaiko AR. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 9.Riserus U, Arnlov J, Berglund L. Long-term predictors of insulin resistance: role of lifestyle and metabolic factors in middle-aged men. Diabetes Care. 2007;30:2928–2933. doi: 10.2337/dc07-0360. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U, European Group for Study of Insulin Resistance Insulin action and age. Diabetes. 1996;45:947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- 11.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. α-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of oral insulin in relatives of patients with type 1 diabetes. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 14.Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Greenbaum CJ, Eisenbarth G, Skyler JS, Diabetes Prevention Trial-Type 1 Study Group Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in Diabetes Prevention Trial–Type 1 participants. Diabetes Care. 2010;33:620–625. doi: 10.2337/dc09-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosenko JMMD, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Matheson D, Skyler JS, Diabetes Prevention Trial -Type 1 Study Group Glucose and C-peptide changes in the perionset period of type 1 diabetes in The Diabetes Prevention Trial-Type 1. Diabetes Care. 2008;31:2188–2192. doi: 10.2337/dc08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, Lachin JM, McGee P, Palmer JP, Pescovitz MD, Krause-Steinrauf H, Skyler JS, Sosenko JM, on behalf of the Type 1 Diabetes TrialNet Study Group Fall in C-Peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 diabetes TrialNet data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. α-cell mass and Turnover in Humans. Diabetes Care. 2013;36:111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Mahon J, Greenbaum CJ, Cowie CC, Skyler JS, the Diabetes Prevention Trial–Type 1 Study Group Incident dysglycemia and the progression to type 1 diabetes among participants in the Diabetes Prevention Trial–Type 1. Diabetes Care. 2009;32:1603–1607. doi: 10.2337/dc08-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redondo MJ, Rodriguez LM, Escalante M, O’Brian Smith E, Balasubramanyam A, Haymond MW. Beta cell function and BMI in ethnically diverse children with newly diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2012;13:564–571. doi: 10.1111/j.1399-5448.2012.00875.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu HW, Lee YJ, Cho WI, Lee YA, Shin CH, Yang SW. Preserved C-peptide levels in overweight or obese compared with underweight children upon diagnosis of type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab. 2015;20:92–97. doi: 10.6065/apem.2015.20.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherry N, Tsai EB, Herold KC. Natural history of β-cell function in type 1 diabetes. Diabetes. 2005;54(Supplement 2):S32–S39. doi: 10.2337/diabetes.54.suppl_2.s32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


