Abstract
Objective
Familial dysautonomia (FD) is an autosomal recessive disorder characterized by impaired development of sensory and afferent autonomic nerves. Untreated sleep-disordered breathing (SDB) has been reported to increase the risk of sudden unexpected death in FD. We aimed to describe the prevalence and characteristics of SDB in FD.
Patients/Methods
Seventy-five patients with FD (20 adults and 55 children) underwent in-lab polysomnography, including peripheral capillary oxygen saturation (SpO2) and end-tidal capnography (EtCO2) measurements. A t-test and Spearman’s correlation analysis were performed to evaluate the impact of age on sleep, occurrence of apneas, SpO2 and EtCO2 levels; and to determine the relationship between apneas and SpO2/EtCO2 measurements during different sleep stages.
Results
Overall, 85% of adults and 91% of pediatric patients had some degree of SDB. Obstructive sleep apneas were more severe in adults (8.5 events/h in adults vs. 3.5 events/h in children, p=0.04), whereas central apneas were more severe (10.8 vs. 2.8 events/h, p=0.04) and frequent (61.8% vs. 45%, p=0.017) in children. Overall, a higher apnea–hypopnea index was associated with increased severity of hypoxia and hypoventilation, although in a significant fraction of patients (67% and 46%), hypoxemia and hypoventilation occurred independent of apneas.
Conclusion
Most adult and pediatric patients with FD suffer from some degree of SDB. There was a differential effect of age in the pattern of SDB observed. In some FD patients, hypoventilation and hypoxia occurred independently of apneas. Therefore, we recommend including EtCO2 monitoring during polysomnography in all patients with FD to detect SDB.
Keywords: Familial dysautonomia, Sleep-disordered breathing, Polysomnography, SpO2, EtCO2
1. INTRODUCTION
Familial dysautonomia (FD, Riley–Day syndrome, hereditary sensory and autonomic neuropathy type III) is a rare autosomal recessive disorder first described in 1949 in children of Jewish Ashkenazi descent.1,2 The disease is caused by a founder mutation in the IkB kinase-associated protein (IKBKAP) gene,3 which impairs the development of mostly sensory (afferent) neurons.4–6 Clinical hallmarks of FD include impaired pain and temperature sensation, absent deep tendon reflexes, gait ataxia7, chronic lung disease,8 and afferent baroreflex failure leading to blood pressure and heart rate instability,4, 9 all of which contribute to morbidity and mortality. Sudden unexpected death during sleep remains a leading cause of death.1,10 We recently reported that untreated sleep-disordered breathing (SDB) increases the risk of sudden unexpected death during sleep in patients with FD.10 Therefore, identification and treatment of SDB is important to prevent premature death in this population.1,6,10
Available reports, some of them published several decades ago, universally indicate that the prevalence of SDB is very high in patients with FD.11–14 These studies have several important limitations to consider, including the very small sample size, inclusion of subjects before genetic testing was available, use of home (instead of in-lab) sleep study, lack of CO2 measurements, selection bias due to inclusion of only symptomatic patients, inclusion of either adult or pediatric patients only, and lack of information about sleep features other than respiratory characteristics.
To overcome all these limitations, we report here the most comprehensive series of in-lab polysomnography (PSG) in consecutive patients with genetic confirmation of the IKBKAP mutation, performed regardless of the presence of symptoms suggesting SDB.
2. MATERIAL AND METHODS
2.1 Subjects
In this prospective study, symptomatic patients with genetically confirmed FD (homozygous for the IKBKAP founder mutation) were consecutively enrolled at the New York University Dysautonomia Center. Patients underwent an in-lab one-night standard diagnostic PSG conducted at the New York University Sleep Center over a 2-year period (from 2013–2015). Given their abnormal respiratory responses to hypoxia and hypercapnia,13,15–18 and their high incidence of sudden unexpected death during sleep, patients were advised to undergo PSG regardless of the presence of clinical symptoms (snoring or pauses in breathing) typically suggestive of SDB. Both children (aged < 18 years) and adults (aged > 18 years) were included in the cohort. Patients using continuous positive airway pressure (CPAP), bi-level airway pressure, or supplementary oxygen during the PSG were excluded. The New York University School of Medicine Institutional Review Board approved this study.
2.2 PSG
PSG studies were conducted and scored by certified sleep technicians and scorers using the 2012 American Academy of Sleep Medicine (AASM) guidelines.19,20 PSG recordings included 10-lead electroencephalography (EEG), electrocardiography (ECG), electrooculography (EOG), submental and tibial electromyography (EMG), recording from an oronasal thermistor, nasal airflow pressure transducer, thoraco-abdominal respiratory inductance plethysmography (RIP) bands for respiratory effort, pulse oximetry (SpO2), and endtidal capnography (EtCO2). SpO2 was measured using Masimo Rad 9 with SET Technology, using a 2-s window. EtCO2 was measured using Nonin system (Plymouth, MN). In addition, video and microphone recordings were included to assess snoring, breathing patterns, and behavior during sleep. PSG was performed using digital polysomnographic equipment (Natus Sleepworks, Natus Medical Incorporated, San Carlos, CA). Bed time cut-off was 20:00 h for children and 22:00 h for adults, and a wake time of 07:00 h for all patients.
Detailed sleep parameters were evaluated, including sleep latency, sleep efficiency, arousal index, periodic limb movement index, oxygen saturation (desaturation of 4% for adults and 3% for children), apnea–hypopnea index (AHI), and average and peak EtCO2. Respiratory events were based on the following criteria: obstructive apnea was defined as cessation of airflow for two respiratory cycles in children and for >10 s in adults, with ≥90% reduction in airflow for ≥90% of the event with ongoing respiratory effort; hypopnea was defined as a decrease in amplitude of airflow signal by >30% lasting two respiratory cycles and accompanied by oxygen desaturation of ≥3% or an arousal. Central events were scored when there was no respiratory effort and the event lasted 20 s in children or 10 s in adults, or at least two breaths with an arousal, awakening, or at least 3% oxygen desaturation.
Apneas and hypopneas (obstructive, central, mixed) were manually scored and classified into mild, moderate, and severe, based on the AHI. In children (age < 18 years), an AHI of 1–5 was classified as mild, 6–10 as moderate, and > 10 as severe. In adults (age > 18 years), AHI of <5 was classified as normal, 5–14 as mild, 15–30 as moderate, and > 30 as severe.
Periodic limb movements of sleep (PLMS) were defined as leg movements with an amplitude increase of 8 μV above the baseline value, duration of 0.5–10 s, a period length between two consecutive movements of 5–90 s, and a minimum of four consecutive movements.21 Five or more movements per hour were considered abnormal in patients < 18 years old; 15 or more movements per hour were considered abnormal in patients > 18 years old.
2.3 Statistical analysis
Descriptive statistics were first performed to characterize the study population. A t-test and Chi-square test (or Fisher’s exact test, where appropriate) was used to compare the demographic and sleep features between the adult and pediatric groups. Spearman’s rank order correlation analysis was performed to assess the relationship between age and the occurrence of apneas, SpO2, and EtCO2. Spearman’s correlation coefficients were also used to assess the relationship between AHI and nadir SpO2, average SpO2, average EtCO2, and maximum EtCO2 during different sleep stages. Statistical significance was set at p < 0.05. Statistical analysis was performed with SAS v. 10.3 (SAS Corp., Cary, NC, USA).
3. RESULTS
3.1 Population characteristics
Complete data, free of artifact, with a minimum of 6 h of total sleep time was obtained in 75 subjects (43 males, 32 females, mean age 14.3±10.5 years (range 2–71 years)). As shown in Table 1, 55/75 (73%) were children (mean age 8.9±5.1 years, range: 2–18 years) and 20/75 (27%) were adults (mean age 28.8±7.3 years range: 19–71 years). All patients had normal BMI (< 25 in adults and < 22 in children).
Table 1.
Patients’ demographic and sleep characteristics.
| Mean* | SD | Median | |
|---|---|---|---|
| Females, N (%) | 30 (43%) | — | — |
| Age (years) | 14.3 | 10.5 | 12 |
| Sleep efficiency (%) | 75.7 | 15.9 | 80 |
| Wake % | 16.9 | 12.3 | 15.4 |
| Stage 1 % | 6.1 | 6 | 4.5 |
| Stage 2 % | 42.5 | 19.3 | 42.9 |
| Stage 3 % | 14.7 | 12.9 | 13.6 |
| Stage 4 % | 14.5 | 18.4 | 11.05 |
| REM % | 20 | 10 | 18.3 |
| Sleep latency (min) | 41.7 | 50.7 | 22 |
| Latency to REM (min) | 128.7 | 85.3 | 105.5 |
| Latency to stage 2 (min) | 14 | 18.8 | 5.5 |
| AHI (events/h) | 12.2 | 13.4 | 7.7 |
| Obstructive apnea events per hour | 5 | 10 | 1 |
| Central apnea events per hour | 9 | 24 | 2 |
| Mixed apnea events per hour | 1 | 3 | 0 |
| Hypopnea events per hour | 59 | 62 | 41 |
| Obstructive sleep apneas | 35/75 (57%) | ||
| Mild | 21/35 (60%) | ||
| Moderate | 9/35 (26%) | ||
| Severe | 5/35 (14%) | ||
| Central sleep apneas | 43/75 (57%) | ||
| Mild | 30/43 (70%) | ||
| Moderate | 5/43 (12%) | ||
| Severe | 8/43 (19%) | ||
| Mixed apneas | 6/75 (8%) | ||
| Mild | 4/6 (67%) | ||
| Moderate | 2/6 (33%) | ||
| Severe | 0/6 (0%) | ||
| Nadir SpO2 (%) | 78 | 13 | 81 |
| Average SpO2 (%) | 94 | 4 | 95 |
| Average EtCO2 awake (mmHg) | 41 | 4 | 40 |
| Average EtCO2 NREM sleep (mmHg) | 44 | 4 | 45 |
| Average EtCO2 REM sleep (mmHg) | 47 | 4 | 48 |
| Maximum EtCO2 (mmHg) | 54 | 6 | 54 |
| Average HR (bpm) | 86 | 17.9 | 86 |
| Minimum HR (bpm) | 60 | 14.3 | 61 |
| Max HR (bpm) | 129 | 22.7 | 128 |
AHI, apnea–hypopnea index; EtCO2, end-tidal capnography; HR, heart rate; SD, standard deviation.
Mean, unless otherwise stated.
Some patients had multiple comorbidities, which could potentially contribute to the severity of sleep disorders. This included 66% (42/75) of patients with chronic respiratory disease (eg, laryngo-tracheo-bronchomalacia, atelectasis, bronchiectasis), 31% (22/75) had craniofacial morphological abnormalities, and 6% (4/75) had congenital heart defects (eg, ventricular septal defects) (Table 1).
3.2 Prevalence of SDB
Eighty-five percent (17/20) of adult patients and 91% (50/55) of pediatric patients had some degree of SDB. Specifically, obstructive sleep apneas (OSAs) occurred in 47% of patients (35/75) overall, in 45% of children (25/55) and in 50% of adults (10/20). Central apneas (CAs) occurred in 57% of patients (43/75) overall, in 62% of children (34/55) and in 45% of adults (9/20). Mixed apneas occurred in 8% of patients (6/75) overall, in 5.5% of children (3/55) and in 15% of adults (3/20).
Of the 35 patients with OSA, 60% (21/35) had mild, 26% (9/35) had moderate, and 14% (5/35) had severe OSA. Of the 43 patients with CAs, 70% (30/43) had mild, 12% (5/43) had moderate, and 19% (8/43) had severe CAs. Of the six patients with mixed apnea events, 67% (4/6) had mild, and 33% (2/6) had severe mixed apnea. All central apneas occurred in all stages of sleep, independent of any fragmented sleep or arousals.
Severity breakdown based on age of obstructive, central, and mixed apneas is shown in Table 2.
Table 2.
Sleep disordered-breathing differences according to age.
| Pediatric N=55 (mean ± SD; median) |
Adult N=20 (mean ± SD; median) |
P | Spearmann’s correlation coefficient |
Spearmann’s P-value |
|
|---|---|---|---|---|---|
| Obstructive sleep apnea | 25/55 (45.5%) | 10/20 (50%) | 0.52* | — | — |
| Mild | 16/25 (64%) | 5/10 (50%) | |||
| Moderate | 5/25 (20%) | 4/10 (40%) | — | — | — |
| Severe | 4/25 (16%) | 1/10 (10%) | — | — | — |
| Central sleep apnea | 34/55 (61.8%) | 9/20 (45%) | 0.017* | — | — |
| Mild | 22/34 (64.7%) | 8/9 (88.9%) | — | — | — |
| Moderate | 4/34 (11.8%) | 1/9 (11.1%) | — | — | — |
| Severe | 8/34 (23.5%) | 0/9 (0%) | — | — | — |
| Mixed apneas | 3/55 (5.5%) | 3/20 (15%) | 0.90† | — | — |
| Mild | 1/3 (33.3%) | 3/3 (100%) | — | — | — |
| Moderate | 0/3 (0%) | 0/3 (0%) | — | — | — |
| Severe | 2/3 (66.7%) | 0/3 (0%) | — | — | — |
| Apnea-hypopnea index (events/h) | 11.2 ± 11.1,8.6 | 15.5 ± 18.4, 5.5 | 0.21‡ | −0.10 | 0.39§ |
| Obstructive apnea (events/h) | 3.5 ± 6.4, 1 | 8.5 ± 13.5, 1 | 0.04‡ | 0.11 | 0.39§ |
| Central apnea (events/h) | 10.8 ± 27.9, 2 | 2.8 ± 6.2, 1 | 0.04‡ | −0.39 | 0.002§ |
| Mixed apnea (events/h) | 0.8 ± 3.2, 0 | 1.1 ± 2.4, 0 | 0.76‡ | 0.10 | 0.5§ |
| Hypopnea (events/h) | 57.9 ± 55.8, 47 | 62.7 ± 79.6, 28 | 0.77‡ | −0.17 | 0.16§ |
| Nadir SpO2 (%) | 78.2 ± 12.1, 81 | 76.9 ± 15.7, 82 | 0.69‡ | 0.02 | 0.84§ |
| Average SpO2 (%) | 94.7 ± 4.3, 96 | 93.1 ± 3.4, 93 | 0.17‡ | −0.3 | 0.008§ |
| Average EtCO2 awake (mmHg) | 39.8 ± 3.6, 40 | 42.5 ± 2.8, 43 | 0.004‡ | 0.47 | <0.0001§ |
| Average EtCO2 NREM (mmHg) | 43.2 ± 3.9, 44 | 46.2 ± 3.0, 46.5 | 0.003‡ | 0.5 | <0.0001§ |
| Average EtCO2 REM (mmHg) | 46.7 ± 4.3, 47 | 49.5 ± 3.2, 50.5 | 0.009‡ | 0.52 | <0.0001§ |
| Maximum EtCO2 (mmHg) | 52.6 ± 5.5, 52 | 57 ± 5.8, 57.5 | 0.003‡ | 0.47 | <0.0001§ |
EtCO2, end-tidal capnography.
Chi-square test p-value.
Fisher’s exact test p-value.
Two-sample t-test p-value.
Spearman’s rank order correlation test p-value.
In the population as a whole, the average AHI was 12.3±13.5 events/h (range: 0.2–58 events/h). The mean AHI was 11.2±11.1 events/h in children (range 0.2–54.3), and 15.5±18.4 events/h in adults (range 2.90–58).
Duration of apneas ranged from 10 to 30 s in all cases.
The mean SpO2 as a group was 94.2±4.1% (range 70–99%). In children, the mean SpO2 was 94.6±4.3% (range 70–99%), whereas in adults it was 93.1±3.3% (range 84.6–98.7%). Nadir SpO2 was 77.9±13.1% (range 37–93%). In children, the nadir SpO2 was 78.2±12.1% (range 40–93%), whereas in adults it was 76.9±15.7% (range 37–93%).
SpO2 desaturations (average O2 levels < 92% in children and < 90% in adults) occurred in 11% (6/55) of children and 15% (3/20) of adults, a total of nine patients. Out of these nine patients with SpO2 desaturations, there were six patients (ie, 67%) (four children, two adults) who experienced SpO2 desaturations with no accompanying significant sleep apnea. (Fig. 1)
Fig. 1.
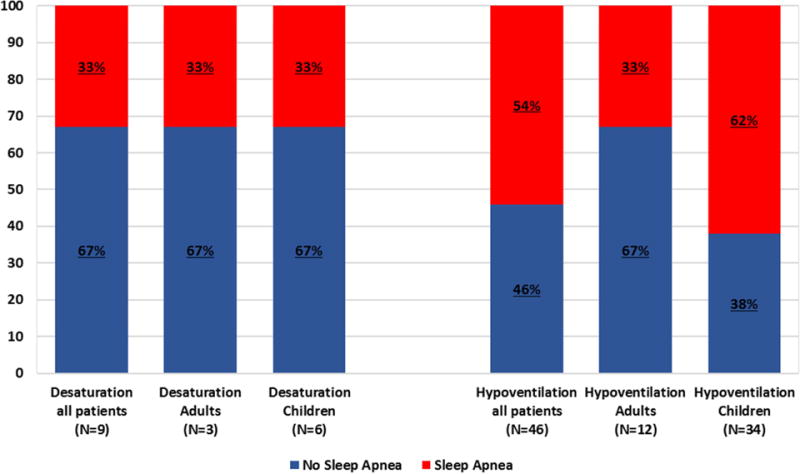
Discordance between sleep apneas and hypoxemia/hypoventilation in familial dysautonomia. Although in many patients (both adults and children), sleep apneas were accompanied by oxygen desaturation (average SpO2 levels < 92% in children and < 90% in adults) and/or hypoventilation (maximum EtCO2 levels > 50% in children and > 55% in adults), in a significant number of them that was not the case. The y-axis depicts percentage of patients.
Hypoventilation (maximum EtCO2 levels > 50 mmHg in children and > 55 mmHg in adults) occurred in 62% (34/55) of children and in 60% (12/20) of adults, a total of 46 patients. Out of these 46 patients with hypoventilation, there were 21 patients (ie, 46%) (13 children, eight adults) who experienced hypoventilation with no accompanying significant sleep apnea (Fig. 1).
The majority of respiratory events occurred in the supine position (80%) and during REM sleep (65%).
3.3 Prevalence of movement abnormalities during sleep
PLM index was < 5 in all patients. No patient had muscle atonia or abnormal behavior during REM sleep suggesting REM behavior disorder.
3.4 Relationship between age and SDB
OSA events were more frequent (8.5 vs 3.5 events/h, p=0.04) in adult patients, whereas CA events were more severe (10.8 vs 2.8 events/h, p=0.04) and more frequent in children (61.8% of pediatric vs. 45% of adults, p=0.017). Spearman’s correlation analysis revealed that the number of CA per hour declined with age (Spearman correlation coefficient R= −0.39, p=0.002). In contrast, hypoventilation significantly worsened with age as reflected by EtCO2 during sleep and EtCO2 while awake (R=0.47, p < 0.0001), during REM sleep (R=0.52, p < 0.0001), non-REM sleep (R=0.50, p < 0.0001), and maximal EtCO2 during any sleep stage (R=0.47, p < 0.0001). The remaining parameters (eg, AHI, nadir or average SpO2) did not have any association with age (Table 2).
3.5 Relationship between apneas, oxygenation and hypoventilation
As expected, the frequency of obstructive apneas overall (AHI, number of obstructive apnea events and hypopnea events) was negatively correlated to SpO2 levels (p < 0.05), and positively correlated to EtCO2 levels (average EtCO2 during REM and non-REM sleep, as well as the maximum EtCO2 during any sleep stage) (p < 0.05). In contrast, central or mixed apneas were not significantly correlated with SpO2 or EtCO2 values (Table 3).
Table 3.
Association between hypoxia, hypoventilation and sleep apnea.
| Spearman’s correlation coefficient, p | |||||
|---|---|---|---|---|---|
| AHI (events/h) | Hypopneas (N*) |
Obstructive apneas (N*) |
Central apneas (N*) |
Mixed apneas (N*) |
|
| Nadir SpO2 | −0.4, 0.0004 | −0.37, 0.001 | −0.34, 0.006 | −0.23, 0.07 | 0.11, 0.48 |
| Average SpO2 | 0.03, 0.78 | 0.07, 0.58 | −0.06, 0.64 | 0.23, 0.07 | 0.23, 0.12 |
| Average EtCO2 NREM (mmHg) | 0.35, 0.002 | 0.3, 0.01 | 0.17, 0.17 | −0.12, 0.33 | 0.01, 0.93 |
| Average EtCO2 REM (mmHg) | 0.37, 0.0009 | 0.31, 0.008 | 0.25, 0.04 | −0.13, 0.29 | 0.06, 0.67 |
| Maximum EtCO2 (mmHg) | 0.39, 0.0006 | 0.33, 0.005 | 0.31, 0.01 | −0.1, 0.44 | 0.006, 0.96 |
| Average HR (bpm) | 0.27, 0.03 | 0.3, 0.02 | 0.12, 0.36 | 0.4, 0.002 | −0.29, 0.05 |
| Minimum HR (bpm) | 0.25, 0.06 | 0.18, 0.19 | 0.06, 0.69 | 0.19, 0.2 | −0.19, 0.22 |
| Maximum HR (bpm) | 0.06, 0.63 | 0.04, 0.75 | −0.12, 0.41 | 0.46, 0.009 | −0.27, 0.08 |
AHI, apnea–hypopnea index; EtCO2, end-tidal capnography; HR, heart rate.
Total number of events recorded during sleep.
4. DISCUSSION
In this study, we report the largest series of PSG findings in a sample of consecutive patients with genetically confirmed FD. Unlike previous studies, our sample cohort consisted of both children and adults, symptomatic and asymptomatic, allowing an accurate cross-sectional analysis of the entire age spectrum. Many of our results differ from those shown previously.
Overall, a large majority of adult and pediatric patients had some degree of SDB. OSA events were more frequent in adult patients, whereas CA events were more severe and frequent in children. While the number of CA events decreased with advancing age, the, severity of hypoventilation progressively worsened with age. Overall, a higher AHI was associated with increased severity of hypoxia and hypoventilation. In addition, hypoventilation and desaturation was seen independent of sleep apnea in a significant fraction of patients. Surprisingly, none of our study patients had PLMI > 5; this may be due to the peculiar nature of FD and perhaps FD patients are less prone to PLMS than the general population.
In normal subjects, the respiratory center is sensitive to the CO2 concentration in blood. A progressive increase in the fractional concentration of inspired CO2 induces a progressive increase in ventilatory frequency, thus preventing the excessive accumulation of CO2 in the arterial blood. In a seminal work published in 1965, Filler and colleagues18 showed that the ventilatory response in patients with FD, however, was drastically different: when the concentration of CO2 was increased in inspired air, the ventilatory frequency failed to increase as much as in normal subjects and, consequently, the amount of ventilation was insufficient to prevent the development of respiratory acidosis. Similarly, inhalation of air with reduced O2 concentration, which causes a small drop in SpO2 and increase in ventilatory frequency in normal subjects, induced a sharp drop in SpO2 and severe hypotension in the patients with FD. Edelman and colleagues16 confirmed and expanded these results by showing that sudden relief of hypoxemia in FD caused a severe reduction in ventilatory frequency (ie, apnea); that hypoxia in FD induced bradycardia; and that hypoxia and hypercapnia induced both bradycardia and hypotension. Bernardi and colleagues confirmed these results.15 Together, these observations indicate that chemoreflex failure results in abnormal ventilator and hemodynamic responses to hypoxia and hypercapnea in FD.
In the 1980s, Guilleminault and colleagues17 and Gadoth and colleagues12 first documented, a high frequency of sleep apnea in patients with FD. This latter study also reported abnormal sleep structure, with decreased REM sleep and increased REM latency, which may have been due to poor sleep quality due to a “first night effect.” Indeed, in our study, we found no major sleep architectural abnormalities.
In the first report including patients with genetically confirmed FD, Weese-Mayer and colleagues11 performed in-home sleep studies (inductance plethysmography, heart rate, SpO2, and pulse waveform) in 25 pediatric patients and 25 age-matched controls. Children with FD had more frequent, prolonged, and severe episodes of hypoxemia than controls.
More recently, Hilz and colleagues, performed in-hospital PSG in 11 adults, mildly affected patients with FD without symptoms of SDB and 13 age-matched controls.14 Sleep structure was similar in patients with FD and controls, and obstructive, rather than central apneas, predominated in patients with FD. In keeping with this, our study also found higher frequency of OSA events, rather than CA, in adult patients. However, in pediatric patients, CA events were more frequent than OSA events. Also, obstructive apneas and hypoventilation (average and maximum EtCO2 levels) tended to worsen with advancing age. These results suggest that the mechanisms driving CA tended to have less influence as the brain matured. The amygdala and the hippocampal head are specifically involved in breathing control and the genesis of CA.22 Because the IKBPAP gene is required for the normal development of the CNS and it is highly expressed in amygdala and hippocampus,23 it is conceivable that abnormal development and maturation of these regions may underlie the high frequency of central apneas during the pediatric age in patients with FD. As these regions mature with age, CAs are less frequent. Conversely, the mechanisms driving obstructive apneas tended to worsen with age.
By monitoring EtCO2, we were able to find that the overall severity (in terms of number of events per hour) of apneas/hypopneas was associated with increased severity of hypoxia and hypoventilation. EtCO2 monitoring also allowed us to demonstrate that, in a significant subset of patients, hypoventilation and hypoxia occurred independently of sleep apnea events.
Had we not used EtCO2 monitoring, these episodes of hypercapnia not associated with apneas would have gone undetected. This finding prompts us to strongly recommend the use of EtCO2 monitoring during PSG in patients with FD.
Our study has several strengths: it comprises the largest sample size of genetically confirmed patients with FD so far, consisting of both adults and children, which allowed us to assess the effect of age on apneas, oxygen desaturation, and hypoventilation. We also used EtCO2 monitoring, which had never been done before. We also included consecutive patients, regardless of their symptoms or complaints related to SDB, which avoided selection bias and provided an accurate value of the actual prevalence of SDB in this population.
Our study has three main limitations. The first is the lack of a control group; the second is the fact that standard one-night PSGs were performed, thus the “first-night effect” could be present in some of our study patients; and the third is the lack of prospective follow-up to determine the prognosis of patients with different patterns of SDB. A prospective longitudinal follow-up study is ongoing.
5. CONCLUSIONS
In conclusion, our findings show that a majority of adult and pediatric patients with FD suffer from some degree of SDB. There is a differential effect of age in the SDB pattern in patients with FD. Central apneas were more frequent in childhood and improved with age. Conversely, hypoventilation and obstructive apneas were mild in childhood but worsened in adulthood. Some patients had hypoventilation and hypoxia independently of sleep apnea events. Therefore, measuring apnea events only without EtCO2 monitoring may not be enough to detect the full spectrum of SDB in patients with FD. Our findings support the use of EtCO2 monitoring during PSG in all patients with FD. Early identification of sleep abnormalities in patients with FD is key as treatment of SDB with non-invasive ventilation has been reported to reduce the risk of sudden unexpected death during sleep in this population.10
HIGHLIGHTS.
Untreated sleep-disordered breathing can increase the risk of sudden death in familial dysautonomia (FD).
We describe the prevalence and characteristics of sleep-disordered breathing in FD.
In-lab polysomnography including SpO2 and EtCO2 measurements were used.
There is a differential impact of age in the pattern of sleep-disordered breathing in FD.
Hypoventilation and hypoxemia can occur independent of sleep apneas in FD.
Acknowledgments
This study was supported by the Dysautonomia Foundation, Inc. and NIH (U54NS065736).
This was not an industry-supported study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have indicated no financial support or conflicts of interest.
References
- 1.Norcliffe-Kaufmann L, Slaugenhaupt SA, Kaufmann H. Familial dysautonomia: History, genotype, phenotype and translational research. Prog Neurobiol. 2017;152:131–48. doi: 10.1016/j.pneurobio.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Riley CM, Day RL, Greeley DM, Langford WS. Central autonomic dysfunction with defective lacrimation; report of five cases. Pediatrics. 1949;3:468–78. [PubMed] [Google Scholar]
- 3.Slaugenhaupt SA, Blumenfeld A, Gill SP, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. American journal of human genetics. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–11. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez JV, Norcliffe-Kaufmann L, Kaufmann H. Brainstem reflexes in patients with familial dysautonomia. Clin Neurophysiol. 2015;126:626–33. doi: 10.1016/j.clinph.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma JA, Norcliffe-Kaufmann L, Fuente-Mora C, Percival L, Mendoza-Santiesteban C, Kaufmann H. Current treatments in familial dysautonomia. Exp Opin Pharmacother. 2014;15:2653–71. doi: 10.1517/14656566.2014.970530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macefield VG, Norcliffe-Kaufmann L, Gutierrez J, Axelrod FB, Kaufmann H. Can loss of muscle spindle afferents explain the ataxic gait in Riley-Day syndrome? Brain. 2011;134:3198–208. doi: 10.1093/brain/awr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maayan HC. Respiratory aspects of Riley-Day Syndrome: familial dysautonomia. Paediatr Resp Rev. 2006;7(Suppl 1):S258–9. doi: 10.1016/j.prrv.2006.04.184. [DOI] [PubMed] [Google Scholar]
- 9.Norcliffe-Kaufmann L, Palma JA, Kaufmann H. Mother-induced hypertension in familial dysautonomia. Clin Autonom Res. 2016;26:79–81. doi: 10.1007/s10286-015-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palma JA, Norcliffe-Kaufmann L, Perez MA, Spalink CL, Kaufmann H. Sudden unexpected death during sleep in familial dysautonomia: a case-control study. Sleep. 2017 doi: 10.1093/sleep/zsx083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weese-Mayer DE, Kenny AS, Bennett HL, Ramirez JM, Leurgans SE. Familial dysautonomia: frequent, prolonged and severe hypoxemia during wakefulness and sleep. Pediatr Pulmonol. 2008;43:251–60. doi: 10.1002/ppul.20764. [DOI] [PubMed] [Google Scholar]
- 12.Gadoth N, Sokol J, Lavie P. Sleep structure and nocturnal disordered breathing in familial dysautonomia. J Neurol Sci. 1983;60:117–25. doi: 10.1016/0022-510x(83)90131-4. [DOI] [PubMed] [Google Scholar]
- 13.McNicholas WT, Rutherford R, Grossman R, Moldofsky H, Zamel N, Phillipson EA. Abnormal respiratory pattern generation during sleep in patients with autonomic dysfunction. Am Rev Respir Dis. 1983;128:429–33. doi: 10.1164/arrd.1983.128.3.429. [DOI] [PubMed] [Google Scholar]
- 14.Hilz MJ, Moeller S, Buechner S, et al. Obstructive Sleep-Disordered Breathing Is More Common than Central in Mild Familial Dysautonomia. J Clin Sleep Med. 2016;12:1607–14. doi: 10.5664/jcsm.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardi L, Hilz M, Stemper B, Passino C, Welsch G, Axelrod FB. Respiratory and cerebrovascular responses to hypoxia and hypercapnia in familial dysautonomia. Am J Resp Crit Care Med. 2003;167:141–9. doi: 10.1164/rccm.200207-677OC. [DOI] [PubMed] [Google Scholar]
- 16.Edelman NH, Cherniack NS, Lahiri S, Richards E, Fishman AP. The effects of abnormal sympathetic nervous function upon the ventilatory response to hypoxia. The Journal of clinical investigation. 1970;49:1153–65. doi: 10.1172/JCI106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilleminault C, Mondini S, Greenfield M. Abnormal respiratory pattern generation during sleep in patients with autonomic dysfunction. Am Rev Respir Dis. 1984;129:512–3. doi: 10.1164/arrd.1984.129.3.512a. [DOI] [PubMed] [Google Scholar]
- 18.Filler J, Smith AA, Stone S, Dancis J. Respiratory Control in Familial Dysautonomia. J Pediatr. 1965;66:509–16. doi: 10.1016/s0022-3476(65)80115-9. [DOI] [PubMed] [Google Scholar]
- 19.The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester: AASM; 2007. [Google Scholar]
- 20.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology. 2017;88:701–05. doi: 10.1212/WNL.0000000000003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaverra M, George L, Mergy M, et al. The Familial Dysautonomia disease gene, Ikbkap/Elp1, is required in the developing and adult central nervous system. Dis Model Mech. 2017 doi: 10.1242/dmm.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]


