Summary
Cardiac and vascular abnormalities and disease syndromes are major causes of death both during human development and with aging. To identify the cause of congenital defects and to combat this epidemic in the aging population, new models must be created for scientific investigation and new therapies must be developed. Recent advances in pluripotent stem cell biology offer renewed hope for tackling these problems. Of particular importance has been the creation of induced pluripotent (iPS) cells from adult tissues and organs through the forced expression of two to four transcription factors. Moreover, iPS cells, which are phenotypically indistinguishable from embryonic stem (ES) cells, can be generated from any patient. This unique capacity when coupled with samples from patients who have congenital and genetic defects of unknown aetiology should permit the creation of new model systems that foment scientific investigation. Moreover, creation of patient-specific cells should overcome many of the immunological limitations that currently impede therapeutic applications associated with other pluripotent stem cells and their derivatives. The aims of this paper will be to discuss cardiac and vascular diseases and show how iPS cells may be employed to overcome some of the most significant scientific and clinical hurdles facing this field.
Keywords: Induced pluripotent (iPS) stem cells, embryonic stem cells, development and disease, vasculature, heart
Introduction
The vertebrate cardiovascular (CV) system, composed of heart, blood, and blood vessels, is the first organ system to develop and function during embryogenesis. Functional and developmental defects in this system often lead to embryonic lethality, and postnatally, approximately 10 out of 1,000 live infants are born with a CV abnormality (1). In fact, congenital heart defects (CHD) including vascular disorders are the most common cause of premature death, but can result in a variety of outcomes ranging from perinatal lethality to late manifestation defects that are not detected until much later in life (1). Some examples of CHD include syndromes associated with cardiac arrhythmias, hypoplastic left or right heart, cardiomyopathies and numerous vascular problems like aortic arch hypoplasia, aortic valve insufficiency, interrupted aortic arch, persistent truncus arteriosus, double outflow right ventricle, Tetralogy of Fallot (TOF), DiGeorge syndrome, Ehlers-Danlos type IV syndrome, and Marfan syndrome. Although several of these diseases and syndromes have been attributed to specific genes (e.g. hypertrophic cardiomyopathies – contractile proteins like myosin heavy chain; vascular Ehlers-Danlos syndrome – collagen type 3A; Marfan syndrome – fibrillin 1; TOF – NKX2.5 or Jagged 1), many human CHDs and vascular disorders are of unknown aetiology.
In adults, cardiac and vascular diseases are the leading cause of death and reach epidemic proportions with aging (2–5). Although a few of these syndromes are by-products of undiagnosed or unrecognised congenital/genetic defects, the underlying basis for cardiac disorders like ischaemia is vascular in origin. Aging-associated vascular changes are often secondary to pro-inflammatory events and altered endothelial function (6, 7). Normal endothelium, for example, regulates vascular smooth muscle function (relaxation and contraction) by releasing nitric oxide (NO) and endothelium-derived contracting factors (EDCF) in response to a variety of physiological signals. Release of NO and EDCF is, however, altered with aging, diabetes and hypertension. Importantly, senescent arteries may be covered with regenerated endothelium, which lack a pertussis-toxin sensitive pathway for NO-release. This in turn leads to enhanced cellular growth, vasospasms, thrombosis, and an inflammatory reaction. Conversely EDCF-mediated responses are augmented with aging. These two changes thus affect the ability of vascular smooth muscle to respond appropriately to altered haemodynamic conditions and stress (6, 7). The resulting coronary and vascular disorders adversely affect cardiac function and ultimately host survival.
To define the molecular basis of CHDs and to develop novel therapeutic strategies to treat cardiac and vascular disorders, two major hurdles must be overcome. The first requires the generation of suitable model systems that can be employed to define mechanisms underlying CHD of unknown aetiology. The second hurdle requires isolation and expansion of immunologically compatible cells suitable for replacement therapies in senescent adults. Recent progress with pluripotent stem cells and particularly the use of induced pluripotent stem (iPS) cells may represent the scientific leap required to overcome these hurdles.
Pluripotent ES and iPS cells
Simplistically, pluripotent stem cells represent a unique cell type that functions as a unit of embryonic development (8, 9). These cells can be grown indefinitely in culture, can be differentiated into almost any cell type, including cardiac and vascular lineages, and maintain a normal and stable karyotype in vitro (8–11). Moreover, these cells are amenable to genetic manipulation, which can be useful for model generation or to correct genetic defects (8, 12). Although multiple types of pluripotent cells have been described, only two are readily capable of generating an embryo when injected into a blastocyst. These include embryonic stem (ES) cells derived from the inner cell mass (ICM) of pre-implantation embryos or epiblast, and induced pluripotent stem (iPS) cells (9, 11, 13).
Three unique properties distinguish pluripotent stem cells from other types of cells. These include i) a unique transcriptional hierarchy that sustains pluripotentiality during the process of self-renewal; ii) a poised epigenetic state that maintains chromatin in a form ready for rapid cell fate decisions; and iii) a cell cycle characterised by an extremely short gap 1 (G1) phase and the near absence of normal somatic cell checkpoint controls (14). Maintenance of these traits permits the cells to remain pluripotent. During differentiation and as fate decisions are made, these properties are dramatically altered. Moreover, these cells when differentiated can generate almost any cell type, including all cardiac and vascular lineages; consequently, pluripotent cells represent a possible and unlimited source for cardiovascular regeneration in humans (see [8, 9, 15] for references). Although pluripotent human ES cell lines remain mired in ethical controversies, generation of iPS cells have obviated these ethical arguments. IPS cells thus have an unencumbered potential for use as a biological research tool into human cardiovascular development and for clinical applications (16).
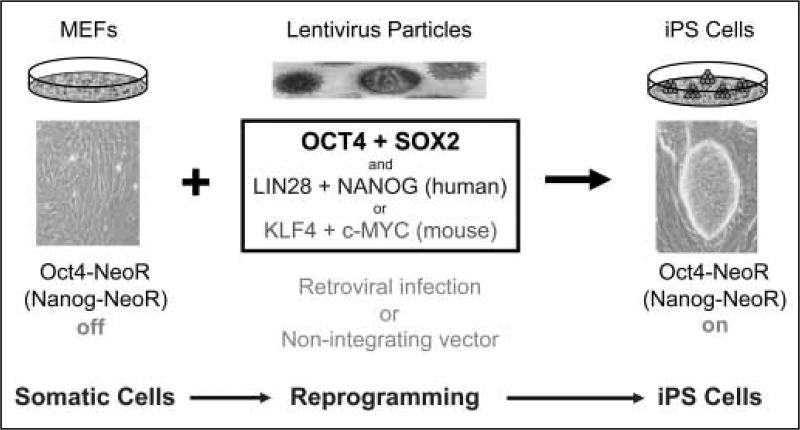
So what exactly are iPS cells and how do these cells offer therapeutic advantages over other pluripotent stem cells? In brief, iPS cells are experimentally-derived cells that are generated in vitro through transcription factor-mediated reprogramming of adult cells using constructs that express OCT4 and SOX2 in conjunction with KLF4 and c-MYC in mouse (10, 11, 17) or histone deactylase inhibitors like valproic acid (18) or with NANOG and LIN28 (19) in human (Fig. 1). Most reprogramming events have been achieved through the use of integrating lentiviral vectors containing these transcription factors, but more recently non-integrating vector systems have been used successfully (20, 21). The reprogramming process from adult mouse cells to iPS cells takes up to two weeks, during which time there is a sequential and time-dependent activation of specific markers like alkaline phosphatase and stage-specific antigens (22, 23). Completion of the reprogramming process is marked by expression of the endogenous genes encoding nanog and pou5f1, and full activation of the pluripotent cell transcriptional hierarchy. Moreover, the epigenetic state of these cells is largely indistinguishable from ES cells (24, 25), and their growth rates are consistent with a robust cell cycle that may lack somatic cell checkpoint controls. IPS and ES cells are, however, distinguished by gene expression signatures (26).
Figure 1. Schematic illustration of how mouse (and human) somatic cells can be reprogrammed to pluripotent iPS cells.
Reprogramming takes place through a series of time-dependent steps. The first is the introduction of pluripotency transcription factors into somatic cells, which lead to the down-regulation of lineage-specific genes. Markers like endogenous alkaline phosphatase activity subsequently increase followed by SSEA1 up-regulation. At the completion of the reprogramming process, endogenous oct4 and nanog are expressed, which, when targeted to activate antibiotic (e.g. NeoR) selection, can be employed to isolate fully reprogrammed cells. MEFs, mouse embryonic fibroblasts; NeoR, neomycin gene cassette that confers resistance to G418.
The major therapeutic advantage of iPS cells over ES cells is related to its source of origin. IPS cells can be derived from almost any somatic tissue, including embryonic and adult tail-tip fibroblasts (11, 27), hepatocytes and gastric epithelial cells (28), pancreatic cells (29), neural stem cells (30, 31) and B lymphocytes (32) in mouse, and skin fibroblasts (17, 19), keratinocytes (33) and peripheral blood cells (16) in human. The ease of generating iPS cells from multiple cell sources has led to the development of patient-derived cells, which unlike most human ES-derived progeny are immunologically compatible with the recipient. These cell lines may therefore prove invaluable for therapeutic interventions and treatment of cardiac and vascular diseases either through cell replacement or through secretion of critical factors (see Therapeutic applications below).
A number of limitations, however, must be overcome before iPS cells will be therapeutically viable. These include i) side effects associated with random DNA integration events, ii) inappropriate lentiviral silencing of the reprogramming transcription factors, and iii) identification of correctly reprogrammed cells. The latter has been overcome in vitro by looking for activation of endogous oct4 or nanog gene activity and activation of a reporter gene targeted to one of these gene loci (11, 34, 35). In the future, non-manipulative techniques (e.g. presence or absence of cell surface markers) in conjunction with morphology will be required to identify these cells before the cells will be suitable for clinical applications (36). Viral-dependent limitations, which were first described in mouse but are also apt for human iPS cells, include incomplete viral silencing and continued expression of the reprogramming transcription factors, which tend to lead to tumour formation. This has been attributed largely to inappropriate c-myc transgene reactivation or silencing (11, 25); however, when iPS cells are produced without the c-myc transgene, the efficiency of iPS generation is markedly reduced, and mouse chimeras and progeny show substantially less tumor formation (27). The generation of iPS cells without integrating vector delivery systems has overcome most of these limitations (20, 21); however, the problem of tumor formation still exists. Transplantation of pluripotent and differentiating ES cells can lead to neoplasm formation (37, 38), and by extension pluripotent iPS cells are likely to do the same. Regrettably, the elimination of undifferentiated cells via lineage selection protocols may prove insufficient to eliminate this neoplastic risk. Additional strategies for the elimination of differentiated iPS cells with tumourigenic potential may need to be developed and tested.
Potential of pluripotent cells to form haematopoietic, cardiac and vascular lineages
To maximise the research and therapeutic potential of iPS cells for cardiovascular regenerative medicine and reduce neoplastic potential, the processes that signal differentiation and mark fate decisions must be better defined. This is important, because the activation of appropriate spatial and developmental cues, and correct cell-cell interactions in vivo permit pluripotent cells to generate normal cells and viable embryos; whereas, inappropriate activation or localisation may cause tumour formation in recipients. It is therefore imperative not to rush iPS cell-derivatives to clinic before more is known about their properties and fate decisions. Encouragingly, many of the developmental markers that have been observed during embryogenesis are also found in differentiating ES and iPS cells, suggesting that these traits will be worked out quickly.
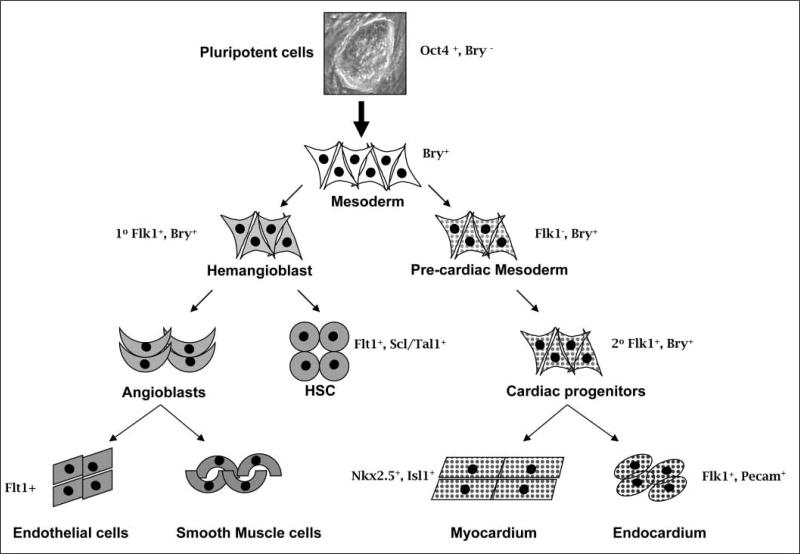
For illustrative purposes, the gene brachyury, an early primitive streak marker, is expressed in all nascent mesoderm and is down-regulated during patterning and specification in mouse. In normal development, haematopoietic, vascular, and cardiac cell lineages develop from subpopulations of Brachyury-positive mesoderm that are induced in a defined temporal pattern. The use of markers restricted to haematopoietic, endothelial and cardiac lineages has facilitated the identification of these progenitors. In vitro studies of pluripotent cells have demonstrated that Brachyury can be employed with these markers to identify early committed mesoderm and its sublineages (39–42). These analyses led to the isolation of two sub-populations of mesoderm that differ in their expression of the fetal liver kinase-1 (Flk-1) receptor (VEGFR-2 in human), a developmental marker of haematopoietic and endothelial lineages (43). The developmental potential of these two populations indicate that pre-mesoderm (Brachyury−Flk1−), prehaemangioblast mesoderm (Brachyury+Flk1−), and haemangioblast mesoderm (Brachyury+Flk1+), the precursor cells of primitive and definitive haematopoiesis and endothelium (40), form sequentially during embryoid body-mediated differentiation of ES cells. Kouskoff et al. showed that the cardiac potential of these two mesodermal populations resides almost exclusively in the Brachyury+Flk1− cells (44), demonstrating that both sets of mesodermal precursors (haematopoietic and cardiac) are sequentially and independently generated from epiblast-like cells formed in the ES cell-derived embryoid body. Importantly, the developmental pattern of marker expression that is recapitulated during differentiation of mouse pluripotent ES cells is highly conserved in humans (45). VEGFR-2 positive progenitors from human ES retain the capacity to generate blast colonies that display haematopoietic, cardiac and vascular potential, and there have been reports (unpublished) that human iPS cells can do the same. A non-comprehensive example of how these markers can be employed to identify cardiac, vascular, endothelial and blood cell lineages in ES cells is shown in Figure 2.
Figure 2. Developmental markers in the selection of subsets of mesodermally-derived cells.
This schema illustrates the use of developmental markers in the identification of subsets of mesodermally derived cells (smooth muscle cells, cardiac muscle cells, haematopoietic cells, endothelial cells) that can be isolated from differentiating pluripotent cells. Selected developmental markers are indicated and additional lineage specificity is as follows: haematopoietic (Flt1, Scl/Tal1 positive), endothelial (Flk-1-, Flt-1-, Tie-2-positive) and cardiac lineages (Nkx2.5 and Isl1-positive).
Pluripotent cell derivatives for therapeutic applications
Multiple studies have already established that ES cells and their progeny can be used for repair of heart and growth of vasculature. As representative examples, Kofidis et al. showed that ES cells can prevent myocardial wall thinning and improve contractility after implantation into injured myocardium (46). Swijnenburg et al. subsequently found that the immunogenicity of ES cells increases upon differentiation after transplantation into ischaemic myocardium, suggesting that immunosuppressive therapies may be required for ES cell-based therapies (47). Kolossov et al., however, demonstrated that engraftment of purified ES cell-derived cardiomyocytes could restore contractile function in ischaemic hearts (48). Other groups have confirmed the potential of human ES cell-derived cardiomyocytes to improve heart function following an infarct (49). For the vasculature, Li et al. demonstrated that ES cell-derived endothelial cells could lead to functional improvements in ischaemic hearts and that these cells could promote the formation of small capillaries and venules (50). Moreover, Wang et al. showed that human ES-derived endothelial cells are functional and that blood vessels isolated from hES cells could integrate into the host circulatory system of SCID mice and serve as blood conduits (51). These results demonstrate the potential of pluripotent cells for therapeutic purposes.
Until recently, it was unclear whether iPS cells were also therapeutically viable. As a proof-of-principle, Hanna et al. demonstrated that transgenic mice suffering from human sickle cell anaemia could be treated with haematopoietic cell precursors produced from autologous iPS cells (32, 52). Basically, mouse iPS cells were generated from affected mice and the genetic defect was repaired in vitro through gene targeting of the affected gene locus. The corrected iPS cells were then differentiated and haematopoietic progenitor cells isolated, using lineage-restricted selection techniques. When transplanted back into the diseased host mice, the cells corrected the disease phenotype and all haematological symptoms associated with sickle cell anaemia were absent. This same group subsequently reported that iPS cells differentiated into neuronal and glial cell types in vitro could be employed to treat a model of Parkinsonism in rats (53). IPS generated dopaminergic neurons that were transplanted into adult brain could restore function and improve many of the behavioral symptoms associated with Parkinson's disease. Since these initial studies, numerous groups have published reports demonstrating how iPS cells could be employed to generate therapeutically relevant cell derivatives, including iPS cells differentiating into multiple cardiovascular lineages (54), functional cardiomyocytes (55, 56), insulin-secreting cells (57), and mouse endothelial cells, which were employed to treat a murine model of haemophilia A (58). Importantly, iPS cells have been generated directly from patients with diabetes mellitus, Parkinsonism, amyotrophic lateral sclerosis, Duchennes and Beckers muscular dystrophy and Downs syndrome (59, 60), and major efforts are underway to generate iPS cells from patients with cardiovascular diseases and congenital heart and vascular defects.
Given these proof-of-principle experiments, immunologically compatible patient-derived iPS cells have the potential to treat damaged vascular endothelium associated with aging and other cardiovascular diseases (see Introduction). Moreover, when these cells are generated from individuals with CHD, the resulting lines have the potential to revolutionise our understanding of the molecular mechanisms responsible for CHDs of unknown aetiology (see Fig. 3). To date, no therapies have yet been attempted to address aging-associated vascular diseases, but numerous groups are actively preparing iPS cells from patients with diseases of unknown aetiology. As these new lines become available, it is likely that the mechanisms underlying these disease processes will be uncovered, which should lead directly to the development of new stem cell-based therapies based on previously established criteria (61).
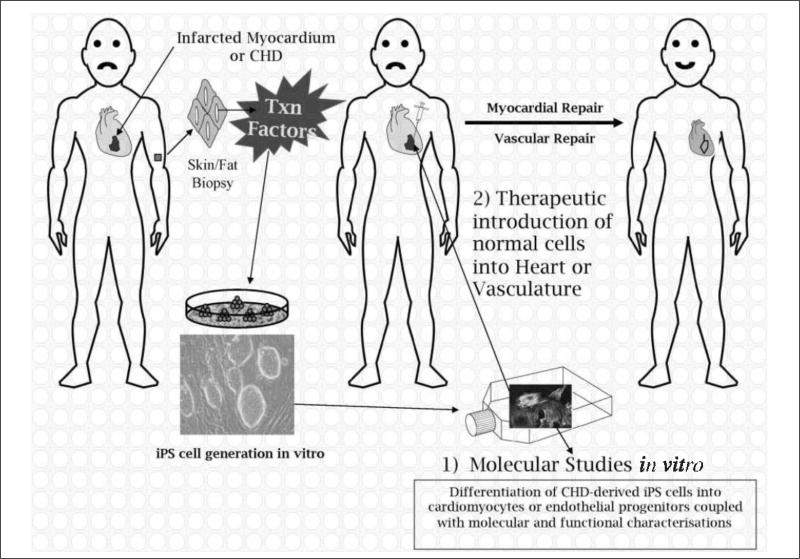
Figure 3. Schematic illustration of the generation of iPS cells and how these cells can theoretically be employed to study 1) molecular mechanisms in vitro or 2) used for therapeutic interventions in humans.
In the case of myocardial infarctions (or vascular defects), iPS cells could be generated from a patient, differentiated to cardiomyocytes (or endothelial cells) and reintroduced into the damaged tissue to elicit repair. Where iPS cells are produced from patients with CHDs of unknown aetiology, the aim would be to determine the cause of the disease in vitro, and not to produce iPS cells for repair, unless the genetic defect could be repaired in vitro prior to introduction of corrected cells back into the host (see Therapeutic applications for details). CHD, congenital heart defect; Txn factors, transcription factors (Oct4, Sox2, Nanog, Lin28).
Mechanisms of action
Assuming that iPS cells isolated directly from patients with cardiac or vascular syndromes are in fact therapeutically viable, the question thus arises as to how these cells might function following re-introduction into a host. For haematopoietic disorders, in theory, complete replacement of defective haematopoietic stem cells with functional iPS derivatives should cure any blood-based disease; however, for organ- or tissue-based syndromes, it is unclear how the introduction of large numbers of individual cells (mature or immature) will act to functionally improve a pre-existing human cardiac or vascular condition. In some cases, the rescue of a congenital defect may be non-cell autonomous; whereas in others, large-scale replacement of defective cells may be required.
The most illuminating experiments into how these cells may function in vivo have come through mutant mouse blastocysts that have incorporated normal ES cells. An example of a non-cell autonomous rescue was shown using the Id1/Id3 double knockout embryos to look at heart development (62). Loss of both of these transcription factor inhibitors normally led to embryonic lethality and the presence of a “thin myocardial syndrome“. When wild-type ES cells were introduced into mouse blastocysts lacking both Id1 and Id3, some chimeric embryos survived to birth and a subset of these animals survived to adulthood. Remarkably, only ~20% or more of the heart had to be composed of wild-type ES cell-derived cardiomyocytes to rescue this phenotype. Two plausible explanations can explain these findings. The first was that only wild-type cells are functional and are present in sufficient numbers to improve the innate phenotype. The second was that wild-type cells produced factors (paracrine or through direct cell-to-cell interactions) that could promote “normal“ function in Id1/Id3-deficient cells. Based on results showing that the wild-type cells released IGF-1 and that its injection into the pregnant mother could partially correct this defect, it appears that the latter possibility was correct. Moreover, the authors went on to find that Wnt5a is also involved and is necessary for normal cardiac function. Thus, for some congenital defects or aging-associated syndromes, successful therapeutic interventions may be possible either after enough cells have been incorporated into an organ system or alternatively through the identification of secreted factors that may correct a syndrome. Based on what we currently know about the endothelial cell contribution to atheroschlerosis, this is a very intriguing possibility and one worthy of future investigation.
Therapeutic treatments of cell autonomous defects may, however, be more challenging, as large numbers of cells with a high degree of tissue incorporation may be required. In those instances where scar tissue or inflammatory cells are present, it is unclear how well the cells will take up residence or even survive. Treatment of infarcted and scarred hearts therefore may be particularly challenging due to the very low turnover rate of endogenous cells. For vascular disorders, however, use of iPS derivatives to replace defective cells may be easier. This is because much of the vasculature (i.e. the endothelial lining) will be in direct contact with cells injected into the blood stream. Theoretically, iPS cell-derived endothelial progenitor cells (EPCs) would take up residence in the appropriate vascular niche through attachment and migration, and given enough time, these cells should be capable of slowly replacing defective ones. While speculative at this time, this type of experiment is easily tested in large animal models. The results could lead to a novel therapeutic paradigm for treatment of aging vasculature, which may ultimately reduce the incidence of cardiac infarctions – a major goal of cardiovascular aging research.
Conclusions
In conclusion, iPS cells, when produced directly from patients with cardiovascular diseases of unknown aetiologies, will lead to the development of new model systems that can be employed to investigate cardiac and vascular developmental defects. These cells are also likely to lead to novel replacement therapies designed to improve cardiac and vascular function in compromised individuals. Such cell-based therapies may eventually lead to decreased patient morbidity and mortality with aging. Perhaps most significantly, iPS cells, because of immunologic compatibility and the lack of ethical controversies, may have already rendered the therapeutic potential of ES cells obsolete. Both types of pluripotent cells are likely, however, to be very important for continued analysis of pathways critical to normal human development.
Finally, major challenges remain for researchers and clinicians, before novel treatment paradigm can be envisaged. Numerous mechanistic questions regarding the molecular process of transcription factor-induced reprogramming remain unanswered, as do questions concerning mechanisms governing the epigenetic remodelling and cell cycle de-control that occurs during reprogramming. Moreover, efforts must be made to identify fully reprogrammed iPS cells, as opposed to partially reprogrammed cells, with cell surface markers alone. Routes of cell introduction into humans must also be better elucidated, as this seems to be a major technical limitation for many organ systems. Irrespective of these perceived problems, these cells are likely to reveal new principles underlying normal development and disease. More importantly, iPS cells represent a novel and promising system that offers therapeutic hope for numerous diseases (Parkinsonism, diabetes, spinal injuries, heart failure, CHDs), which have thus far been unresponsive to classical therapeutic approaches.
Acknowledgments
Financial support:
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Pierpont ME, Basson CT, Benson DW, et al. Genetic basis for congenital heart defects: Current knowledge – A scientific statement from the American heart association congenital cardiac defects committee, council on cardiovascular disease in the young. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 2.Najjar SS, Scuteri A, Lakatta EG. Arterial aging – Is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Wang MY, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604. doi: 10.1016/j.mcna.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102:240–247. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 6.Vanhoutte PM, Shimokawa H, Tang EHC, et al. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 7.Vanhoutte PM. Endothelial dysfunction – The first step toward coronary arteriosclerosis. Circ J. 2009;73:595–601. doi: 10.1253/circj.cj-08-1169. [DOI] [PubMed] [Google Scholar]
- 8.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka S, Li JL, Kania G, et al. Pluripotency of embryonic stem cells. Cell Tissue Res. 2008;331:5–22. doi: 10.1007/s00441-007-0520-5. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 12.Zou JZ, Maeder ML, Mali P, et al. Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell. 2009;5:97–110. doi: 10.1016/j.stem.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 14.Boheler KR. Stem cell pluripotency: A cellular trait that depends on transcription factors, chromatin state and a checkpoint deficient cell cycle. J Cell Physiol. 2009;221:10–17. doi: 10.1002/jcp.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perino MG, Yamanaka S, Li JL, et al. Cardiomyogenic stem and progenitor cell plasticity and the dissection of cardiopoiesis. J Mol Cell Cardiol. 2008;45:475–494. doi: 10.1016/j.yjmcc.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka S. A Fresh Look at iPS Cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Huangfu DW, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Stadtfeld M, Hochedlinger K. Without a trace? PiggyBacing toward pluripotency. Nat Methods. 2009;6:329–330. doi: 10.1038/nmeth0509-329. [DOI] [PubMed] [Google Scholar]
- 21.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced Pluripotent Stem Cells Generated Without Viral Integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadtfeld M, Maherali N, Breault DT, et al. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 26.Chin MH, Mason MJ, Xie W, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 28.Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 29.Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Current Biol. 2008;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JB, Zaehres H, Wu GM, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 31.Eminli S, Utikal J, Arnold K, et al. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 32.Hanna J, Markoulaki S, Schorderet P, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 34.Maherali N, Ahfeldt T, Rigamonti A, et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. 2008;3:340–345. doi: 10.1016/j.stem.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3:595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Gundry RL, Boheler KR, Van Eyk JE, et al. A novel role for proteomics in the discovery of cell-surface markers on stem cells: Scratching the surface. Proteom Clin Appl. 2008;2:892–903. doi: 10.1002/prca.200780122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Auerbach JM, Rodriguez-Gomez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 38.Blyszczuk P, Czyz J, Kania G, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi K, Kennedy M, Kazarov A, et al. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 40.Fehling HJ, Lacaud G, Kubo A, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 41.Huber TL, Kouskoff V, Fehling HJ, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 42.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Shalaby F, Ho J, Stanford WL, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 44.Kouskoff V, Lacaud G, Schwantz S, et al. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy M, D'Souza SL, Lynch-Kattman M, et al. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kofidis T, de Bruin JL, Hoyt G, et al. Myocardial restoration with embryonic stem cell bioartificial tissue transplantation. J Heart Lung Transpl. 2005;24:737–744. doi: 10.1016/j.healun.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Swijnenburg RJ, Tanaka M, Vogel H, et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–I172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 48.Kolossov E, Bostani T, Roell W, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Wu JC, Sheikh AY, et al. Differentiation, survival, and function of embryonic stem cell-derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–I54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 52.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 53.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of Acute Myocardial Infarction by Human Stemness Factors Induced Pluripotent Stem Cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narazaki G, Uosaki H, Teranishi M, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JH, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:E30–E41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tateishi K, He J, Taranova O, et al. Generation of Insulin-secreting Islet-like Clusters from Human Skin Fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 58.Xu D, Alipio Z, Fink LM, et al. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci USA. 2009;106:808–813. doi: 10.1073/pnas.0812090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 60.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menasche P. Stem cells for clinical use in cardiovascular medicine – Current limitations and future perspectives. Thromb Haemost. 2005;94:697–701. doi: 10.1160/TH05-03-0218. [DOI] [PubMed] [Google Scholar]
- 62.Fraidenraich D, Stillwell E, Romero E, et al. Rescue of cardiac defects in Id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]