Abstract
Previous studies across species have established that the aging process adversely affects certain memory-related brain regions earlier than others. Behavioral tasks targeted at the function of vulnerable regions can provide noninvasive methods for assessing the integrity of particular components of memory throughout the lifespan. The present study modified a previous task designed to separately but concurrently test detailed memory for object identity and spatial location. Memory for objects or items is thought to rely on perirhinal and lateral entorhinal cortices, among the first targets of Alzheimer’s related neurodegeneration. In line with prior work, we split an aged adult sample into “impaired” and “unimpaired” groups on the basis of a standardized word-learning task. The “impaired” group showed widespread difficulty with memory discrimination, whereas the “unimpaired” group showed difficulty with object, but not spatial memory discrimination. These findings support the hypothesized greater age-related impacts on memory for objects or items in older adults, perhaps even with healthy aging.
With a rapidly aging world population, an understanding of neurocognitive aging – both healthy and pathological – is imperative. Of particular importance is the development of behavioral and biological markers that inform us as to one’s current and future neurocognitive status. Previous studies have reported evidence for selective targeting of early age-related (Raz et al., 2004) and Alzheimer’s-related (Braak, Braak, & Bohl, 1993; Braak & Braak, 1995, 1996; Jack et al., 1997) neurodegeneration to particular parts of the brain. In particular, much evidence suggests that the perirhinal and lateral entorhinal cortices (PRC and LEC), thought to compute item/object information, are among the earliest targets (Burke et al., 2011, 2014; Khan et al., 2014; Ryan et al., 2012; Stranahan et al., 2010, 2011; Yassa et al., 2014). It is presently unclear whether dysfunction in these brain regions is exclusive to pathological aging, or is also a feature of healthy aging.
Importantly, these regions are widely held to be dissociable from neighboring regions as a function of information domain (Norman & Eacott, 2005; Eichenbaum, Yonelinas, & Ranganath, 2007; Ranganath & Ritchey, 2012; Hunsaker et al., 2013). It is thus possible to develop and validate behavioral paradigms that can dissociate and provide information about the functional integrity of these underlying systems. Such behavioral paradigms are noninvasive, and also minimize both cost and inconvenience. This makes them powerful and flexible tools for assessing neurocognitive health across the lifespan. We recently used a task concurrently taxing object versus spatial mnemonic discrimination, thought to tax pattern separation (orthogonalization of similar inputs into dissimilar outputs) in the hippocampus. Using this task, we found domain-general hippocampal engagement, but selective engagement of PRC and LEC during object discrimination and engagement of parahippocampal and medial entorhinal cortices (PHC and MEC) during spatial discrimination (Reagh & Yassa, 2014). The present study used a modified version of the task to assess performance in aged adults compared to young adults. In line with prior neurobiological evidence of PRC and LEC vulnerability, we predicted disproportionately greater impairment of object discrimination than spatial discrimination with aging.
Twenty-three young adults (range = 18–27, mean = 21.26, SD = 2.82) and 34 aged adults (range = 60–83, mean = 74.21, SD = 4.58) were recruited from UC Irvine and the greater Orange County community, and were screened for neurological conditions (e.g., history of stroke or mental illness). Subjects gave written informed consent in accordance with the UC Irvine Institutional Review Board, and were compensated for their participation. A brief neuropsychological battery was administered to characterize our sample (Table 1). Of particular note is the Rey Auditory Verbal Learning Task (RAVLT) delayed recall test, which is a 20 minute delayed version of a word-learning task. The RAVLT Delay is thought to be sensitive to general dysfunction of medial temporal lobe structures involved in episodic memory (though it is by no means the only such test one could use to characterize a sample). This was used to split the aged group into aged impaired (AI) and aged unimpaired (AU) subgroups in line with prior work from our group as well as others (Holden et al., 2012; Reagh et al., 2013; Stark, Yassa, & Stark, 2010), reflecting group splits also done in rodent models of aging (Gallagher et al., 2003). The AU/AI group division was done via median split, with RAVLT Delay scores greater than nine designated as AU (n = 19; range = 68–83, mean = 75.16, SD = 3.96) and less than nine designated as AI (n = 15; range = 64–81, mean = 73.00, SD = 5.15). Critically, even the AI individuals were still within their age-based norms on the RAVLT Delay, and thus this represents a subclinical and subtle form of impairment. Furthermore, more general assays of cognitive function (e.g., Mini-Mental State Exam performance or RAVLT Recognition) did not differ among groups. We observed group differences in the Trails A and B tests (sensitive to executive function), but even the lowest performers were within their age-matched norms.
Table 1.
Demographics and neuropsychological performance.
| Measure | Young Group | AU Group | AI Group | Group Diffs |
|---|---|---|---|---|
| Sample Size | 23 (12F) | 19 (14F) | 15 (8F) | - |
| Age | 21.26 (2.82) | 75.15 (3.96) | 73.00 (5.15) | - |
| MMSE | 29.01 (0.86) | 28.84 (1.16) | 28.11 (1.41) | n.s. |
| RAVLT Imm | 11.39 (2.41) | 12.42 (1.74) | 7.47 (1.81) | * |
| RAVLT Del | 12.52 (1.59) | 12.47 (1.50) | 6.73 (1.62) | * † |
| RAVLT Rec | 14.04 (1.43) | 14.26 (0.81) | 12.27 (2.02) | n.s. |
| Trails A | 25.68 (9.15) | 29.83 (7.89) | 34.08 (14.79) | * |
| Trails B | 45.37 (16.13) | 76.91 (22.28) | 86.87 (39.38) | * # |
| BDI | 2.61 (2.04) | 3.72 (3.30) | 5.53 (8.28) | n.s. |
| Avg Hrs Sleep | 7.23 (1.23) | 7.43 (1.55) | 7.14 (2.19) | n.s. |
Data are presented as mean (SD). MMSE = Mini-Mental State Exam; RAVLT = Rey Auditory Verbal Learning Test (Imm = Immediate, Del = Delay, Rec = Recognition); BDI = Beck Depression Inventory; Avg Hrs Sleep = self-reported typical hours slept per night.
RAVLT Delay scores were used to split aged adults into AU and AI groups. Comparisons were made via one-way ANOVA and post-hoc pairwise comparisons via Tukey’s HSD.
Young > AI (*);
Young > AU (#);
AU > AI (†);
no significant differences (n.s.).
The task consisted of four blocks of study and test, with two blocks testing memory for object identity and the other two testing memory for spatial locations. Stimuli were colored images of common objects appearing on a 7 × 5 grid (not visible to subjects; for further detail, see: Reagh et al., 2013; Reagh & Yassa, 2014). Objects were displayed for 2.5 seconds, with a 0.5 second inter-stimulus interval. Study and test sequences each consisted of 85 trials. During test, each sequence featured 25 exactly repeated target trials and 60 similar lure trials, which were distributed into 20 trials each across high, mid, and low similarity (with respect to the original object). During object test blocks, targets were identical to studied objects whereas lures were objects that were perceptually similar, but not identical to those studied (Figure 1A). Similarity bins for object lures were based on a priori similarity indices validated for use in prior experiments (Yassa et al., 2010; Lacy et al., 2011). During spatial test blocks, targets were studied objects occupying the same grid space whereas lures were studied objects occupying a different grid space than the original location. Similarity bins for spatial lures were based on prior work (Reagh et al., 2013; Reagh & Yassa, 2014), but were matched to object similarity bins resulting in 2, 3, and 4 grid moves for high, mid, and low spatial similarity respectively.
Figure 1.
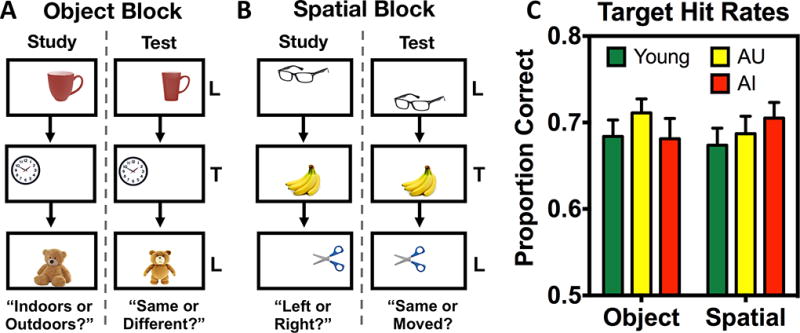
Task schematic and target recognition performance. A) Illustrative diagram of an object block with an “indoor/outdoor” study sequence and a “same/different” test sequence. Two object blocks were completed. B) Illustrative diagram of a spatial block with a “left/right” (of center) study sequence and a “same/different” test sequence. Two spatial blocks were completed. Objects were smaller relative to screen size in the actual task, and presentation order was randomized across runs (i.e., study and test orders were different) and across participants. Stimuli were presented for 2.5 seconds, with a 0.5 second inter-stimulus interval. C) Target hit rates for young, AU, and AI groups across object and spatial discrimination blocks. Data are shown as mean ± standard error.
Subjects were aware of the study-test format, but performed specific non-mnemonic judgments during study to foster attention to stimuli. For object blocks, encoding judgments were “indoor vs. outdoor” and test judgments were “same vs. different” object. For spatial blocks, encoding judgments were “left vs. right” relative to the center of the screen and test judgments were “same vs. different” location. Thus, although judgments varied, object and spatial blocks were perceptually and procedurally similar. Objects were unique to each block, and each space on the grid (excluding corners) was equally likely to be occupied. Subjects encountered either the two object blocks first or the two spatial blocks first, counterbalanced across participants. A task schematic is shown in Figure 1A and 1B. The task was programmed using PsychoPy (Pierce, 2007, 2009).
Analyses were conducted using the R statistical programming language (Version 3.2.2), and figures were generated using GraphPad Prism (Version 6). ANOVAs were corrected for non-sphericity of error using the Greenhouse-Geisser correction, and critical values for pots-hoc contrasts were corrected for multiple comparisons using Scheffé’s method (Fcritical = Finitial / (nconditions − 1)). The significance threshold was set at p < 0.05 (post hoc comparisons using Scheffé’s adjusted threshold are specified as p < 0.05 corrected). We first conducted analyses over raw proportion correct to assess the data in a model-free fashion. Next, given the two responses and two trial types of the task, we used da to compare discriminability of targets vs. lures.
Raw performance indices can be seen in Table 2. We first assessed target recognition across groups by comparing proportions of target hits (p(“same”|target)). We found no differences between object and spatial target hits within any group (all p > 0.05). Furthermore, no across group differences in target recognition were observed (F(1,54) = 0.067, p = 0.796). Thus, our task index of simple recognition memory did not differ across groups or across test formats of information domains (Figure 1C).
Table 2.
Group performance averages on object and spatial mnemonic discrimination tasks.
| Measure | Young Group |
AU Group | AI Group |
|---|---|---|---|
| Object Targets | 0.68 (0.09) | 0.71 (0.07) | 0.68 (0.09) |
| Object High Sim | 0.58 (0.14) | 0.51 (0.13) | 0.46 (0.13) |
| Object Mid Sim | 0.67 (0.11) | 0.56 (0.10) | 0.51 (0.14) |
| Object Low Sim | 0.79 (0.14) | 0.66 (0.09) | 0.64 (0.09) |
| Spatial Targets | 0.67 (0.09) | 0.68 (0.09) | 0.70 (0.07) |
| Spatial High Sim | 0.60 (0.15) | 0.62 (0.10) | 0.54 (0.09) |
| Spatial Mid Sim | 0.70 (0.12) | 0.65 (0.09) | 0.56 (0.13) |
| Spatial Low Sim | 0.78 (0.10) | 0.77 (0.09) | 0.66 (0.09) |
Data are presented as mean (SD). Comparisons were made via one-way ANOVA and post-hoc comparisons were done using contrasts corrected for multiple comparisons with Scheffé’s method.
Young > AI (*);
Young > AU (#);
AU > AI (†);
no significant differences (n.s.).
We next analyzed lure rejection rates via proportion correct. In the first step, we probed for an interaction between group and domain to test the prediction of disproportionate task impairment with aging. We began with a 2×2×3 ANOVA with group (between subjects), domain (within subjects), and similarity (within subjects) as factors. Where this group by domain interaction was observed, we planned to further explore the data with more specific contrasts within and across groups. For raw lure rejections, we found significant effects of domain (F(1,54) = 8.671, p = 0.005, η2p = 0.138), group (F(2,54) = 15.361, p < 0.001, η2p =0.363), and similarity (F(2,108) = 142.853, p < 0.001, η2p = 0.726). Furthermore, we did indeed observe a significant interaction between group and domain (F(2,54) = 3.614, p = 0.034, η2p = 0.114).
To further unpack the data, we next analyzed performance across domain and similarity via a 2×3 (domain by similarity) ANOVA for each group. Significant effects of similarity were observed in all groups: young (F(2,44) = 60.846, p = 0.004, η2p = 0.734), AU (F(2,36) = 67.341, p < 0.001, η2p = 0.721), and AI (F(2,28) = 34.932, p < 0.001, η2p = 0.577). We observed a significant effect of domain in the AU group (F(1,18) = 13.611, p = 0.002, η2p = 0.198), but not in the young or AI groups. Post-hoc contrasts revealed greater object discrimination compared to spatial discrimination at all similarity levels in the AU group (all p < 0.05 corrected). Importantly, we did not observe a global floor effect in the AI group: despite marked impairment, chance performance was exceeded for both object (t(14) = 5.243, p < 0.001) and spatial (t(14) = 5.198, p < 0.001) lures at low similarity.
We next compared performance across groups on object and spatial lure rejection rates separately via a 3×3 (group by similarity) ANOVA for each domain. For object lures, we found significant effects of similarity (F(2,108) = 105.013, p < 0.001, η2p = 0.660) and group (F(1,54) = 9.476, p < 0.001, η2p = 0.260), but no interaction (F(4,108) = 1.428, p = 0.232, η2p = 0.05) (Figure 2A). Post-hoc contrasts revealed that the young group outperformed the AI group at the high similarity level, and both the AI and AU groups at mid and low similarity (all p < 0.05 corrected). For rejection of spatial lures, we observed significant effects of similarity (F(2,108) = 48.808, p < 0.001, η2p = 0.459) and group (F(1,54) = 8.376, p = 0.001, η2p = 0.237), but no interaction (F(4,108) = 1.800, p = 0.137, η2p = 0.062) (Figure 2B). Post-hoc contrasts revealed lower performance in the AI group compared to young and AU groups at mid and low similarity (all p < 0.05 corrected). Unlike with object lures, young and AU groups did not differ for spatial lures.
Figure 2.
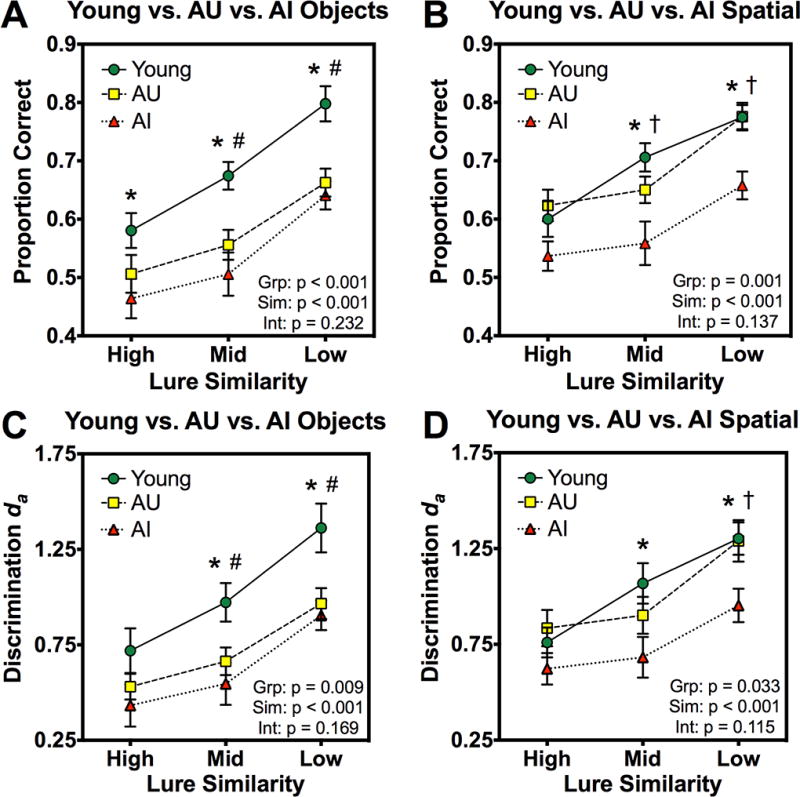
Object and spatial mnemonic discrimination performance across young, AU, and AI groups. Across groups and as a function of lure similarity: A) correct rejection rates of object lures (proportion correct), B) correct rejection rates of spatial lures (proportion correct), C) discrimination of object lures with respect to targets (da), D) discrimination of spatial lures with respect to targets (da). Results from each 3×3 (group by similarity) ANOVA are included in the lower-right corner of each corresponding panel. Post-hoc comparisons were carried out using contrasts corrected for multiple comparisons with Scheffé’s method. Data are shown as mean ± standard error. Young > AI (*); Young > AU (#); AU > AI (†).
We next analyzed the data using signal detection analyses. Though not targeted at memory representations per se, this decision-based framework can provide insight as to the evidence upon which subjects base their mnemonic decisions. Specifically, we used da across participants calculated as the distance between two discrete distribution means (here, targets and lures in a given information domain) divided by the root-mean square of the two distributions’ standard deviations (for more detail, see Mickes, Wixted, and Wais, 2007). Conceptually, this model-based analysis supplements prior model-free analyses by assessing the discriminability of “signal” from “noise” (though it bears mentioning that, to an extent, lures are a mixture of “signal” and “noise”). In brief, converging results across both sets of related but distinct analyses would bolster the findings both empirically and conceptually. All analytical steps and ANOVAs were conducted as with raw correct rejection rates.
In agreement with the prior results, analysis of da values revealed significance of all effects and a significant interaction between group and domain in the full 2×2×3 ANOVA (F(2,54) = 3.409, p = 0.040, η2p = 0.097). Additionally, the abbreviated within-group analyses revealed effects of similarity in all groups: young (F(2,44) = 48.802, p < 0.001, η2p = 0.689), AU (F(2,36) = 66.411, p < 0.001, η2p = 0.722), and AI (F(2,28) = 34.053, p < 0.001, η2p = 0.545). We found a significant effect of domain in the AU group (F(1,18) = 13.606, p = 0.002, η2p = 0. 183), but not in the young or AI groups. Post-hoc contrasts revealed greater object discrimination compared to spatial discrimination at all similarity levels in the AU group (all p < 0.05 corrected).
We next separately assessed object and spatial lure discrimination across groups via da values. For object lures, we found significant effects of similarity (F(2,108) = 84.035, p < 0.001, η2p = 0.609) and group (F(1,54) = 5.139, p = 0.009, η2p = 0.161), but no interaction (F(4,108) = 1.682, p = 0.169, η2p = 0.059) (Figure 2C). Post-hoc contrasts revealed that both the young and AU groups outperformed the AI group at mid and low similarity (all p < 0.05 corrected). For rejection of spatial lures, we observed significant effects of similarity (F(2,108) = 44.525, p < 0.001, η2p = 0.452) and group (F(1,54) = 3.651, p = 0.033, η2p = 0.119), as well as a marginal but non-significant interaction (F(4,108) = 1.927, p = 0.115, η2p = 0.067) (Figure 2D). Post-hoc contrasts revealed lower performance in the AI than the young group at mid similarity, and compared to both the young and AU groups at low similarity (all p < 0.05 corrected). Young and AU groups did not differ for spatial lures.
The present study sought to compare mnemonic discrimination across two information domains – object identity and spatial location – in young and aged adults with and without subclinical memory impairments (defined via the RAVLT Delay). The advantage of this multimodal approach lies in the widely supported idea that related, but distinct neural circuits compute memory for items/objects and memory for contexts/space. Guided by studies suggesting selective vulnerability of PRC and LEC in aging (Stranahan et al., 2010, 2011; Burke et al., 2011, 2014; Khan et al., 2014), we tested the hypothesis that the capacity to overcome mnemonic interference with respect to objects or items would be selectively diminished in aged adults compared to young adults.
This hypothesis was supported by our data, particularly in the case of a subsample of our aged participants whose neuropsychological profile was comparable to the younger group. This raises the possibility is that even apparently healthy aging elicits functional decline (distinct from abnormal pathology per se) in the neural circuitry underlying memory for items or objects, namely PRC and LEC. Conversely, other neighboring cortices thought to process spatial or contextual information (such as PHC and MEC) may be relatively spared in the absence of age-related neuropathology. Additionally, as PRC and LEC project to the hippocampus, it is likely that disruptions of these input regions also disrupt hippocampal computations (such as pattern separation) during object discrimination, but less so during spatial discrimination. Relatedly, impaired performance on both aspects of the task in the AI group suggests that both the item/object and context/spatial neural circuits may be negatively impacted. Such individuals may be on the decline towards mild cognitive impairment and eventual Alzheimer’s disease, though this cannot be addressed in the present study. These issues can be further elucidated by future studies employing this task in conjunction with data such as biomarkers and longitudinal assessments. Recent work has suggested that age-related pathology features a pre-cortical phase, affecting areas of the brainstem and midbrain (notably the locus coeruleus of the pons) before even the “transentorhinal region” (Stratmann et al., 2015; Theofilas et al., 2015). Thus, although our task may indeed be capable of detecting preclinical age-related memory deficits, even earlier methods of detection may be possible through continued development of neurobiologically-validated behavioral tasks.
It warrants reiterating that the present study analyzed our dataset in two different ways: first with proportion correct, and second with signal detection analyses. Though these analyses may seem somewhat redundant, they complement one another to demonstrate that both model-agnostic and model-based assessments of the data converge on the same conclusions. Indeed, the overall structure of the data and relationships observed are wholly in agreement across raw proportion correct and adjusted da values.
We also note that in these data, encoding questions for object and spatial blocks differed. Our goal was to foster encoding of object and spatial properties for each respective block. Consequently, a difference in the circumstances of encoding cannot be entirely ruled out as accounting for differences in memory performance. However, we do not believe this to be the case for these data. Notably, the “indoor/outdoor” judgment can require greater deliberation and “deeper” processing than the somewhat simpler “left/right” judgment. As “deeper” encoding judgments are found to be associated with better memory performance (Craik & Tulving, 1975), one might thus expect better memory for objects than for spatial locations. However, when any object vs. spatial task differences were observed for any group, they reflected the opposite pattern.
To summarize, this study used an object vs. spatial mnemonic discrimination task to demonstrate novel evidence for age-related memory deficits that are disproportionate across information domains. These results are consistent with recent findings suggesting selective vulnerability of PRC and LEC in age-related pathology. Our findings furthermore raise the interesting possibility that this circuitry comprising the “transentorhinal region” may be adversely affected even with cognitively normal aging (though the trajectory of “normal” aging is itself a complex issue; see Jagust 2013). Relative performance deficits on different aspects of a multi-domain task such as this can provide valuable insight to the overall functioning of memory networks in the brain, and may offer clues about the functional integrity of particular brain regions with dissociable roles.
Acknowledgments
We thank our participants for volunteering to take part in our research. We also thank Rebecca Stevenson, Maria Montchal, and Craig Stark for helpful discussions. This research was supported by US National Institute on Aging P50 AG05146 and NIA R21 AG049220, as well as support for Z.M.R. provided by US National Science Foundation Graduate Research Fellowship DGE-1232825 and a fellowship from the ARCS and Roche Foundations.
References
- 1.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;9(Suppl 1):257–261. doi: 10.1159/000116984. discussion 269–272. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 4.Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-associated deficits in pattern separation functions of the perirhinal cortex: a cross-species consensus. Behav Neurosci. 2011;125:836–847. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke SN, Maurer AP, Nematollahi S, Uprety A, Wallace JL, Barnes CA. Advanced age dissociates dual functions of the perirhinal cortex. J Neurosci. 2014;34:467–480. doi: 10.1523/JNEUROSCI.2875-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craik FIM, Tulving E. Depth of processing and the retention of words in episodic memory. J Exp Psychol Gen. 1975;104:268–294. [Google Scholar]
- 7.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallahger M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp Geront. 2003;38(1–2):71–77. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 9.Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22(9):1826–1832. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunsaker MR, Chen V, Tran GT, Kesner RP. The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: a test of the binding of items and context model. Hippocampus. 2013;23:380–391. doi: 10.1002/hipo.22097. [DOI] [PubMed] [Google Scholar]
- 11.Jack CR, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49(3):786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77(2):219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan U, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Isolating the anatomical site and molecular drivers of entorhinal dysfunction in preclinical Alzheimer’s disease and mapping a pattern of cortical spread. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mickes L, Wixted JT, Wais PE. A direct test of the unequal-variance signal detection model of recognition memory. Psychon Bull Rev. 2007;14(5):858–865. doi: 10.3758/bf03194112. [DOI] [PubMed] [Google Scholar]
- 16.Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and postrhinal cortex on memory for context and objects in rats. Behav Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- 17.Pierce JW. PsychoPy – Psychophysics software in Python. J Neurosci Methods. 2007;162(1–2):8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce JW. Generating stimuli for neuroscience using PsychoPy. Front Neuroinform. 2009;2:10. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranganath C, Ritchey M. Two cortical systems for memory-guided behavior. Nat Reviews Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 20.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe. Neurology. 2004;62(3):433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 21.Reagh ZM, Roberts JM, Ly M, DiProspero N, Murray E, Yassa MA. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2013;24(3):303–314. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reagh ZM, Yassa MA. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc Natl Acad Sci. 2014;111(40):E4264–4273. doi: 10.1073/pnas.1411250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan L, Cardoza JA, Barense MD, Kawa KH, Wallentin-Flores J, Arnold WT, Alexander GE. Age-related impairment in a complex object discrimination task that engages perirhinal cortex. Hippocampus. 2012;22:1978–1989. doi: 10.1002/hipo.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17(6):284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stranahan AM, Mattson MP. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s Disease. Neural Plast. 2010;2010:108190. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stranahan AM, Haberman RP, Gallagher M. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cereb Cortex. 2011;21:392–400. doi: 10.1093/cercor/bhq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stratmann K, Heinsen H, Korf HW, Del Turco D, Ghebremedhin E, Siedel K, Bouzrou M, Grinberg LT, Bohl J, Wharton SB, den Dunnen W, Rüb U. Precortical phase of Alzheimer’s disease (AD)-related tau cytoskeletal pathology. Brain Pathology. 2015 doi: 10.1111/bpa.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theofilas P, Dunlop S, Heinsen H, Grinberg LT. Turning on the light within: Subcortical nuclei of the isodendritic core and their role in Alzheimer’s disease pathogenesis. J Alz Disease. 2015;46(1):17–34. doi: 10.3233/JAD-142682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010;21(9):968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yassa MA. Ground zero in Alzheimer’s disease. Nat Neurosci. 2014;17:146–147. doi: 10.1038/nn.3631. [DOI] [PubMed] [Google Scholar]


