Abstract
Purpose
Inhalation therapy is popular to treat lower respiratory tract infections. Azithromycin is effective against some bacteria that cause respiratory tract infections; but it has poor water solubility that may limit its efficacy when administrated as inhalation therapy. In this study, dry powder inhaler formulations were developed by co-spray drying azithromycin with L-leucine with a purpose to improve dissolution.
Methods
The produced powder formulations were characterized regarding particle size, morphology, surface composition and in-vitro aerosolization performance. Effects of L-leucine on the solubility and in-vitro dissolution of azithromycin were also evaluated.
Results
The spray dried azithromycin alone formulation exhibited a satisfactory aerosol performance with a fine particle fraction (FPF) of 62.5 ± 4.1%. Addition of L-leucine in the formulation resulted in no significant change in particle morphology and FPF, which can be attributed to enrichment of azithromycin on the surfaces of composite particles. Importantly, compared with the spray-dried amorphous azithromycin alone powder, the co-spray dried powder formulations of azithromycin and L-leucine demonstrated a substantially enhanced in-vitro dissolution rate. Such enhanced dissolution of azithromycin could be attributed to the formation of composite system and the acidic microenvironment around azithromycin molecules created by the dissolution of acidic L-leucine in the co-spray dried powder. Fourier transform infrared spectroscopic data showed intermolecular interactions between azithromycin and L-leucine in the co-spray dried formulations.
Conclusions
We developed the dry powder formulations with satisfactory aerosol performance and enhanced dissolution for a poorly water soluble weak base, azithromycin, by co-spray drying with an amino acid, L-leucine.
Keywords: aerosol performance, azithromycin, dissolution, dry powder inhaler, L-leucine, poorly water soluble drug, solubility, spray drying
INTRODUCTION
Azithromycin is a broad-spectrum antibiotic with substantial antibacterial activities against Gram-positive and Gram-negative pathogens (1). It has been widely used to treat lower respiratory tract infections (1–3). It also exhibits anti-inflammatory (4), muco-regulatory (5), and anti-biofilm (6) activities. Azithromycin has been approved by the FDA for treatment of community acquired pneumonia and exacerbations of chronic obstructive pulmonary disease (COPD). Furthermore, it is effective against other respiratory tract disorders, like bronchiectasis, nontuberculous mycobacterial pulmonary diseases, and pulmonary nocardia infection (7–10). However, long-term use of systemically administrated azithromycin therapies may lead to adverse effects including gastrointestinal symptoms (11) and hearing loss (12). Local delivery of azithromycin for lower respiratory tract infections may minimize such systemic adverse events while maintain the treatment efficacy (13).
Inhaled formulations are attractive for treatment of respiratory diseases (14). The treatment of respiratory diseases by delivering the therapeutics to the site of diseases offers rapid onset of drug action, high therapeutic efficacy and reduced systemic exposure (15). In-vivo animal and clinical studies have shown superior efficacy and safety profiles of some inhaled antibiotic therapies over traditional systemic routes of drug delivery (16). Among all inhaled devices for antibiotic delivery, dry powder inhalers (DPIs) are becoming a popular form because in general they are easy to use, portable and most of APIs are chemically more stable than in powder form than the liquid counterparts (17).
In pulmonary drug delivery systems, aerosolized fine drug particles (typically aerodynamic diameter 1–5 μm) can be efficiently delivered to the target sites in the lower airways (18). However, such fine powders produced by the traditional jet milling process generally have poor flow and poor aerosolization properties (17,19), which are the main challenges for formulation and manufacturing (20). These are because fine particles tend to stick to each other, forming agglomerates due to the strong inter-particle cohesive forces (21). In practice, fine drug particles are mixed with coarse carriers to improve the flow and ensure the consistency of aerosol performance (22,23). However, such carrier-based DPI formulations may not be suitable for high dose antibiotics because the drug load of a single dose is substantially limited by the inclusion of carriers (24). Particle engineering is of growing interest as an effective approach to improve the flow and aerosolization of the drug particles by reducing intrinsic powder cohesion (25–27). Spray drying is one of the most popular approaches to engineer the inhalable powder formulation to improve aerosol performance (28,29).
Several studies have attempted to develop inhalable powder formulation of azithromycin. Young et al., co-spray dried azithromycin with mannitol (a mucolytic agent) for the treatment of bronchiectasis. The co-sprayed formulation demonstrated a fine particle fraction (≤6.8 μm; over emitted dose) of 82% ± 2.1% through an Orbital DPI device (30). Li et al., reported that higher glass transition temperature (Tg) correlated with a higher fine particle fraction and lower MMAD, in an azithromycin-mannitol co-spray dried formulation (31). Li et al., also reported that spray feed pump rate could significantly affect the aerosol properties of spray dried azithromycin powders (32). An in-vivo study in rats demonstrated that intratracheal administration of azithromycin dry powder achieved significantly higher drug concentrations in lung epithelial lining fluid (ELF) as compared with the intravenous route (33).
The mechanism of antimicrobial activity for azithromycin is that azithromycin binds to the 50S ribosomal subunit of susceptible microorganisms and interferes with bacterial protein synthesis (34). To be effective against bacteria on the airway surfaces, the deposited azithromycin particles should dissolve, which then generate relatively high drug concentrations at the infection sites and drug can enter the bacterial cells. Undissolved drug particles may not be able to enter bacterial cells and be cleared by macrophage uptake or mucociliary clearance (35). Unfortunately azithromycin is poorly water soluble (1,3), and such low water solubility and poor dissolution properties may compromise the efficacy of inhaled azithromycin therapies.
Formulating poorly water soluble drugs into amorphous forms is a recognized approach to improve solubility, dissolution and efficacy (36,37). Amino acids have been investigated for improving the dissolution of poorly soluble drugs by forming co-amorphous dispersions (38–42). It is noted that the weak basic azithromycin exhibits a pH dependent solubility profile (43). Given amino acids’ weakly acidic nature, we hypothesized that formulating azithromycin with L-leucine may improve the solubility and dissolution of azithromycin by providing a more acidic environment. L-leucine has been extensively investigated in DPI formulations to reduce cohesion and improve aerosol performance (44–46) and moisture protection (47); however, its capability to improve dissolution of inhalable poorly water soluble DPI formulations has not been systemically examined. In the present study, we developed co-spray dried inhalable powder formulations consisting of azithromycin and L-leucine. The formulations were characterized to investigate the effects of L-leucine on the physicochemical properties as well as aerosolization, solubility and dissolution of azithromycin. The chemical structures of azithromycin and L-leucine are provided in Appendix 1.
MATERIALS AND METHODS
Materials
Azithromycin dihydrate was purchased from ßetaPharma® (Shanghai) Co., Ltd. (Wujiang City, Jiangsu Province, China). L-leucine was supplied by Sigma–Aldrich (St. Louis, Missouri, USA). Methanol (HPLC grade) was supplied from Merck (Fair Lawn, New Jersey, USA).
Spray Drying
A Büchi 290 spray dryer (Büchi Labortechnik AG, Falwil, Switzerland) was employed to produce the powder formulations. Briefly, formulation components (L-leucine alone, azithromycin alone, combinations with molar ratios of 1:1 and 4:1) were dissolved in the co-solvent of water: ethanol (1:1, v/v). Each solution was spray-dried at the same parameters: inlet temperature 80 ± 2°C; outlet temperature 48 ± 2°C; aspirator 35 m3/h; atomizer setting 700 L/h; feed rate 2 mL/min. Total solid concentration of the feed solutions was kept constant (10 mg/mL) for different formulations. The samples were kept in a desiccator with silica gel at 20 ± 3°C.
Scanning Electron Microscopy (SEM)
Morphology of the spray-dried particles was examined by a field emission scanning electron microscope (NOVA nanoSEM, FEI Company, Hillsboro, Oregon, USA). Adhesive carbon tape was placed on stainless steel stub and then a small amount of the sample was spread over the tape. Excessive powder was removed by pressurized air. The stubs were then coated with platinum at 40 mA for 1 min using a sputter coater (208 HR, Cressington Sputter Coater, England, UK), corresponding to approximately 10–30 nm coating thickness. The SEM images were captured using an inbuilt software.
Physical Particle Size
The scanning electron microscopy images captured at a fixed magnification were used to determine particle size of the samples (48). Martin’s diameter of approximately 150 randomly-selected particles from three different images was measured for each sample using the ImageJ software (National Institute of Health, Rockville, Maryland, USA). D10 (diameter at 10% undersize), D50 (diameter at 50% undersize), and D90 (diameter at 90% undersize) were calculated according to standard procedures (49).
Powder X-ray Diffraction (PXRD)
Crystallinity was determined by powder X-ray diffraction (Rigaku Smartlab™ diffractometer, Rigaku Americas, Texas, USA). A Cu-Kα radiation source and a D/tex ultra-detector were used. The samples were spread on a glass slide and placed in the measuring chamber. The PXRD scanning was set as 5 to 40° 2θ at 5°/min with a voltage of 40 kV and a current of 44 mA.
X-ray Photoelectron Spectroscopy (XPS)
Surface composition of the composite formulations was evaluated quantitatively by X-ray photoelectron spectroscopy (XPS) (AXIS Ultra DLD spectrometer, Kratos Analytical Inc., Manchester, UK) with monochromic Al Kα radiation (1486.6 eV) using pass energy (PE) of 20 and 160 eV for high-resolution and survey spectra, respectively. A commercial Kratos charge neutralizer was used to avoid non-homogeneous electric charge of non-conducting powder and to achieve better resolution. Typical instrument resolution for PE of 20 eV is ~0.35 eV. Binding energy (BE) values refer to the Fermi edge and the energy scale was calibrated using Au 4f7/2 at 84.0 eV and Cu 2p3/2 at 932.67 eV. Powder samples were placed on a stainless-steel sample holder bar using a double-sided sticking Cu tape. A CasaXPS software (version 2313 Dev64) was applied to process the XPS data. Curve-fitting was performed following a Shirley background subtraction using model peaks obtained from pure compounds. Atomic concentrations of the elements in the near-surface region were estimated after a Shirley background subtraction considering the corresponding Scofield atomic sensitivity factors and inelastic mean free path (IMFP) of photoelectrons using standard procedures in the CasaXPS software assuming homogeneous mixture of the elements within the information depths (~10 nm). The % surface composition was calculated using the C 1 s curve-fitting (50).
Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
Surface composition of the composite formulations was characterized using Time-of-flight secondary ion mass spectrometry (nanoToF instrument, Physical Electronics Inc., Chanhassen, Minnesota, USA) as described elsewhere with slight modifications (51). Data were obtained from 4 areas (75 × 75 μm each) per sample. Characteristic peak fragments for azithromycin and L-leucine were identified. For azithromycin, the peaks at m/z ~98 atomic mass unit (amu) and ~158 amu were selected corresponding to [C6H12N+] and [C8H16NO2+] fragments, respectively. For L-leucine, the fragment at m/z ~ 132 amu corresponding to [C6H14NO2+] was selected as the characteristic peak. A WincadenceN software (Physical Electronics Inc., Chanhassen, Minnesota, USA) was employed to construct high-resolution surface composition overlays.
Solid State Fourier Transform Infrared Spectroscopy (FTIR) Spectroscopy
The raw materials and selected spray-dried formulations were analyzed using a Cary 600 series IR spectrophotometer (Agilent Technologies, Santa Clara, California, USA) equipped with an attenuated total reflectance (ATR) sample stage. Samples were analysed at a resolution of 4 cm−1 in the range on 400–4000 cm−1. A background scan was collected prior to collecting the sample spectra to minimize the interferences of water and CO2 signals (52).
Dynamic Vapor Sorption
Dynamic vapor sorption (DVS-Intrinsic, Surface Measurement Systems Ltd., London, UK) was employed to measure moisture sorption behavior. Each sample was exposed to 0% relative humidity (RH) at the beginning of measurement to provide a baseline. The sorption cycle was set from 0 to 90% RH, and 90 to 0% for the desorption cycle. Equilibrium mass change at each testing RH was determined.
Drug Quantification
Concentration of azithromycin was measured by a validated high performance liquid chromatography (HPLC) method (53). Briefly, the HPLC system consisted of 1260 Quat Pump, 1290 Thermostate, 1260 ALS autosampler, 1260 TCC thermostatic column compartment, 1260 VWD variable wavelength detector and an Eclipse Plus column (C18, 150 × 4.60 mm, 5 μm) (all were supplied by Agilent, Waldbronn, Germany). The mobile phase was composed of: (A) 20 mM potassium dihydrogen phosphate (pH was adjusted to 7 with 10% w/v sodium hydroxide) and methanol (B). The isocratic elution program used for azithromycin detection was 80% A and 20% B v/v for 15 min at the flow rate of 1.0 mL/min at a wavelength of 210 nm. The retention time for azithromycin was 12 mins. A calibration curve of azithromycin was linear (r2 > 0.999) in the required concentration range (0.0125–0.5 mg/mL).
In-Vitro Aerosol Performance
A Multi-Stage Liquid Impinger (MSLI) (Copley Scientific Limited, Nottingham, UK) was used to evaluate In-vitro aerosol performance with a USP induction port (USP throat). Each capsule (size 3 hydroxypropyl methylcellulose capsules, Qualicaps, Whitsett, North Carolina, USA) was loaded with 10 ± 2 mg of powder and dispersed through an RS01 DPI device (Plastiape S.p.A., Osnago, Italy). Aerosol performance was tested using a standard dispersion procedure: 4 L of air was drawn to pass through the inhaler at an airflow of 100 L/min for 2.4 s, with a pressure drop of approximately 4 kPa at 100 L/min across the device (54). The cutoff diameters were 10.4, 4.9, 2.4, and 1.2 μm for Stages 1–4 of the MSLI, respectively. Drug retained in capsule, device, USP throat, Stages 1–4 and filter paper were dissolved with 20 mL of a co-solvent (20 mM potassium dihydrogen phosphate adjusted to pH 5: methanol = 1:1 v/v). Emitted dose (ED) was defined as the collected drug except for those retained in the capsule and device, over the total recovered drug. Fine particle fraction (FPF) represents the fraction of the drugs deposited on Stage 3, Stage 4 and filter paper over the recovered dose.
Equilibrium Solubility
The equilibrium azithromycin solubility was determined. Briefly, an excess of azithromycin (i.e., > 5 mg/mL equivalent of azithromycin of each formulation) was added to 5 ml of dissolution media (phosphate buffer saline, PBS (pH 7.4), potassium chloride buffer (pH 2) and carbonate buffer (pH 11) maintained at 37 ± 1°C. Buffer media were used instead of water to reflect potential buffering effects of lung lining fluid. The resultant suspensions were constantly stirred at 500 rpm (VWR International, Arlington Heights, Illinois, USA) with a magnetic bar (12 mm, VWR, Arlington Heights, Illinois, USA). After 24 h the suspensions were filtered using a 0.45 μm nylon syringe filter (VWR, Arlington Heights, Illinois, USA) and the concentrations of azithromycin was determined by HPLC.
In-Vitro Dissolution
Dissolution kinetics of the formulations were evaluated using two methods: beaker method and Franz diffusion cell method using the procedures described previously with minor modifications (55). Briefly, dissolution studies were carried out with aerosolized particles collected on the Stage 4 of the next generation impactor (NGI, Copley Scientific Limited, Nottingham, UK) (55). Two capsules (size 3 HPMC capsules, Qualicaps, Whitsett, North Carolina, USA), with each containing 10 ± 2 mg of the powder formulation, were actuated. For each dispersion, standard dispersion procedure were used: 4 L of air was allowed to pass the inhaler at 100 L/min for 2.4 s (55). The powder in the stage 4 of NGI was collected using a filter disk (Whatman® Grade 2, pore size 5 μm, GE Healthcare, Parramatta, Australia) for dissolution studies. The dissolution media was 20 mL of PBS (pH 7.4) to reflect potential buffering effects of lung lining fluid. An aliquot of 0.2 mL sample was drawn at time intervals of 2, 5, 10, 15, 20, 30, 45, 60, 90, 120, 150 and 180 mins, with a compensation of 0.2 mL fresh medium immediately after sample collection.
In the beaker method, the filter disk was gently placed in 20 mL PBS (pH 7.4 at 37 ± 2°C) held in a 100-mL jacketed beaker (4.7 mm internal diameter × 8.5 cm height, Vineland, New Jersey, USA). The dissolution media was constantly stirred at 500 rpm with the help of magnetic stirrer (VWR, Arlington Heights, Illinois, USA) using a polygon magnetic bar (12 mm, VWR, Arlington Heights, Illinois, USA).
In the Franz cell diffusion method, Franz cell (V6B, PermeGear Inc., Hellertown, Pennsylvania, USA) reservoirs were filled with 20 mL PBS (pH 7.4) and maintained at 37 ± 1°C. The dissolution media was stirred constantly at 600 rpm (6-station Franz Cell stirrer, PermeGear Inc., Hellertown, Pennsylvania, USA) with a magnetic bar (12.5 mm). The stirring speed is fixed at 600 rpm by the manufacturer, which is not adjustable. The filter disk with the aerosolized powder was placed on the top of the Franz cell, being in contact with the dissolution media (55).
Statistical Analysis
The statistical analysis was conducted using one-way analysis of variance (ANOVA) with Tukey–Kramer post-hoc test or independent t-test using a GraphPad Prism software (GraphPad Software, Inc., La Jolla, California, USA).
RESULTS
Scanning Electron Microscopy (SEM)
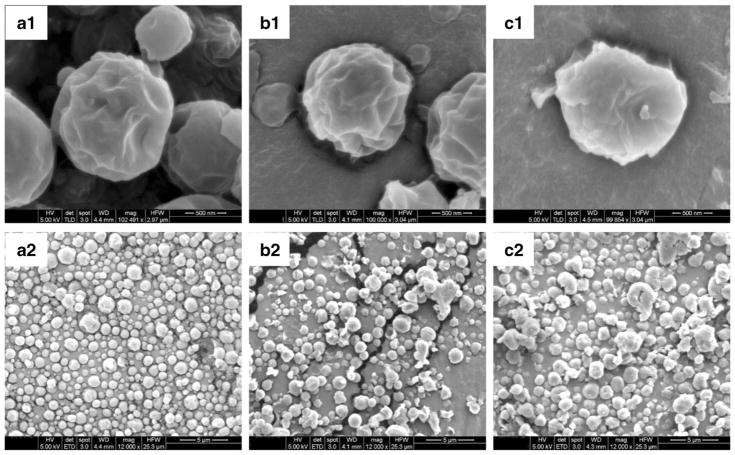
SEM images of the spray-dried formulations are presented in Fig. 1. The Azithromycin-SD particles had a near-spherical shape and corrugated surfaces (Fig. 1a). Composite formulations showed the similar morphology to the Azithromycin-SD particles (Fig. 1b and c) rather than the spray dried L-leucine (47). Previous studies have shown that L-leucine could alter the morphology of some spray-dried particles due to enrichment of L-leucine on the particle surface (56). Our SEM data showed that L-leucine had no apparent effects on the morphology of spray-dried azithromycin particles. Thus, we proposed that L-leucine was not dominant on the surface in these spray-dried composite formulations, which was confirmed by below surface chemical composition analysis.
Fig. 1.
Representative scanning electron microscopy images of: Azithromycin-SD (a1 and a2); L-leucine-Azithromycin_1–1 (b1 and b2); L-leucine-Azithromycin_4–1 (c1 and c2).
Physical Particle Size
Particle sizes of the formulations are presented in Table I. The D50 values of all spray-dried formulations were less than 2 μm. The L-leucine-Azithromycin_4–1 had slightly larger sizes than the Azithromycin-SD and L-leucine-Azithromycin_1–1. The D90 values of all the formulations were less than 3 μm, indicating the fine particle sizes for all formulations.
Table I.
Particle Sizes of the Spray-Dried Powder Formulations
| Formulation | Particle size (μm) | ||
|---|---|---|---|
|
| |||
| D10 | D50 | D90 | |
| Azithromycin-SD | 0.6 ± 0.1 | 0.9 ± 0.1 | 1.4 ± 0.2 |
| L-leucine-Azithromycin_1–1 | 0.9 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.0 |
| L-leucine-Azithromycin_4–1 | 1.4 ± 0.2 | 1.9 ± 0.3 | 2.1 ± 0.2 |
X-ray Photoelectron Spectroscopy (XPS)
The % theoretical composition of L-leucine and azithromycin was calculated by normalizing the molar composition with the number of carbons from each molecule, which gives the contribution of % carbon atom from each molecule. These data can be directly compared with the % surface composition obtained via C 1 s curve-fits from XPS, which represents the surface fraction of carbon atom from L-leucine and azithromycin. In Table II, the XPS data demonstrated that azithromycin concentration on the particle surface was significantly higher than the theoretical ratio, indicating surface enrichment of azithromycin. For example, as for L-leucine-Azithromycin_4–1, the theoretical concentration of azithromycin was 61.3%; while the measured surface concentration was 90.8% (as determined by C1s curvefits).
Table II.
Theoretical and Measured (by XPS) Surface Compositions of the Spray-Dried Formulations
| Formulation | % Theoretical composition | % Surface Composition | ||
|---|---|---|---|---|
|
|
|
|||
| L-leucine | Azithromycin | L-leucine | Azithromycin | |
| L-leucine-Azithromycin _1–1 | 13.6 | 86.4 | 2.4 | 97.6 |
| L-leucine-Azithromycin_4–1 | 38.7 | 61.3 | 9.2 | 90.8 |
Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
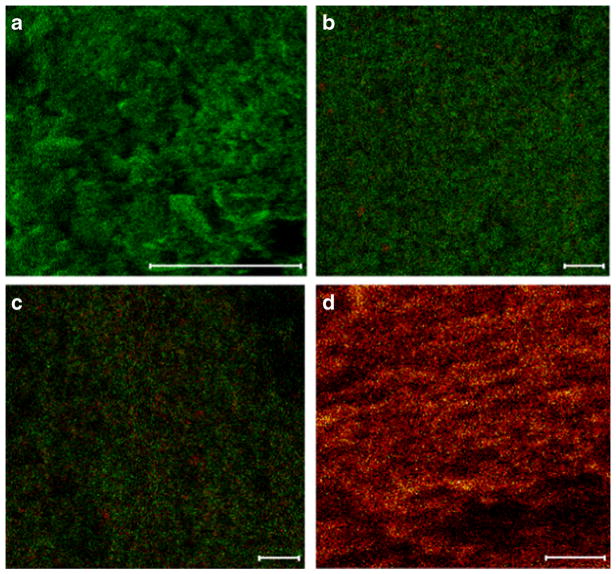
In the ToF-SIMS images (Fig. 2), the green signal represents azithromycin, and the red signal represents L-leucine. For the L-leucine-Azithromycin_1–1, the azithromycin signals are overwhelming on the surface and significantly more enriched on the surface than the L-leucine-Azithromycin_4–1 particles. Such observations are in agreement with the XPS data.
Fig. 2.
Distributions of L-leucine (red) and azithromycin (green) on the particle surfaces measured by ToF-SIMS: (a): Azithromycin-SD; (b): L-leucine-Azithromycin _1–1; (c): L-leucine-Azithromycin_4–1; and (d): L-leucine (scale bar represents 10 μm).
Solid State Fourier Transform Infrared Spectroscopy (FTIR)
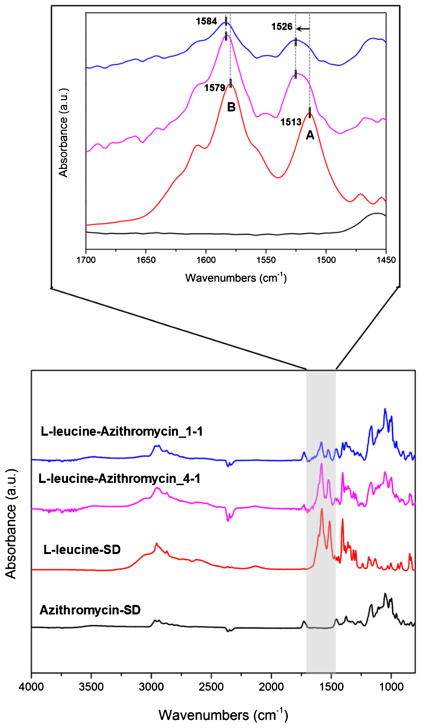
FTIR was employed to investigate the potential interactions between L-leucine and azithromycin at the molecular level in the co-spray dried formulations (57). First, IR spectra of the Azithromycin-SD and the raw azithromycin (dihydrate) were compared (Fig. 3). In the spectrum of raw azithromycin dihydrate, sharp peaks at 3492 and 3559 cm−1 (gray area) were clearly observed. These distinct peaks can be assigned to the O–H stretching modes attribute to the presence of ‘tightly bound’ water in the crystal lattice (58). Interestingly, these peaks disappeared in the Azithromycin-SD indicating conversion of azithromycin dihydrate to an anhydrous form (58). The amorphous nature of this anhydrous form of azithromycin was further confirmed by the PXRD data in the following section. Because azithromycin maintained its amorphous form in the co-spray dried formulation with L-leucine, the IR spectrum of the Azithromycin-SD was used as the reference in the following context.
Fig. 3.
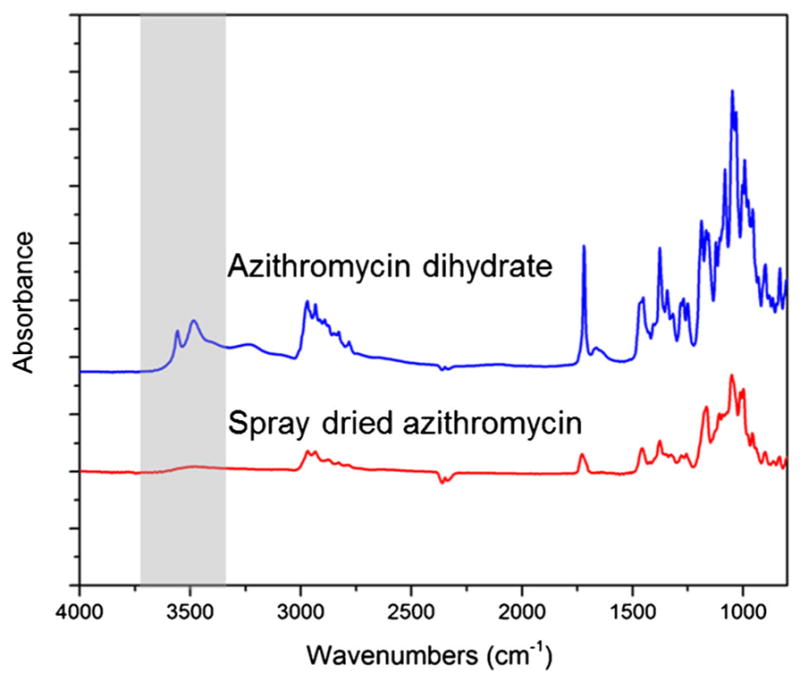
FTIR spectra of the raw azithromycin (dihydrate) and Azithromycin-SD (amorphous).
In Fig. 4, we observed that azithromycin is largely infrared inactive in the 1500–1700 cm−1 spectral region, while L-leucine is infrared active. To be specific, the L-leucine-SD demonstrates two distinct peaks at 1513 and 1579 cm−1, which can be assigned to the carbonyl stretching on the amino acid. It has been reported that these two carbonyl peaks in a lower wavenumber were attributed to the formation of intramolecular hydrogen bonding between NH2 moiety and carbonyl oxygen (59). Hence, breaking such intramolecular interactions can lead to the carbonyl peaks shifting towards higher wavenumber. Interestingly, hypsochromic shifts of Peak A (from 1513 to 1526 cm−1) and Peak B (from 1579 to 1584 cm−1) in the IR spectra of the co-spray dried formulation are noted (52,60). These blue shifts indicate the new molecular interactions are formed during the process of co-spray drying azithromycin with L-leucine. By breaking the intramolecular interactions discussed above, carboxylic acid group can work as a strong hydrogen bond donor to interact with the hydrogen bond accepting group of L-leucine. There are many possible hydrogen bond acceptors located at the azithromycin to potentially form intermolecular interactions with L-leucine. However, it is very challenging to confirm the interaction center on azithromycin due to its complex chemical structure and the limited spectral information. Further investigation of the molecular interactions in the azithromycin-L-leucine complex will be warranted.
Fig. 4.
FTIR spectra of the spray-dried formulations.
Dynamic Water Vapor Sorption (DVS)
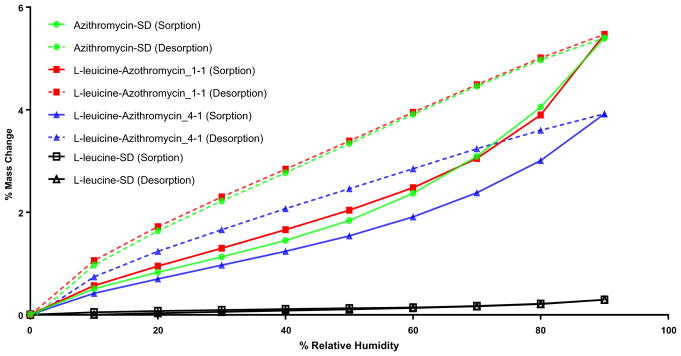
DVS data showed that L-leucine-SD absorbed negligible water (<1% w/w) even at very high relative humidity (Fig. 5). Azithromycin-SD absorbed relatively higher amounts of water (~5% w/w), which was reversible during desorption. This indicates that water molecule was not associated chemically with azithromycin and no crystallization occurred during the measurement. L-leucine-Azithromycin_1–1 showed similar moisture sorption and desorption profiles as the Azithromycin-SD. L-leucine-Azithromycin_4–1 demonstrated less water sorption than Azithromycin-SD, but higher than L-leucine-SD. This reduction in hygroscopicity of L-leucine-Azithromycin_4–1 as compared with Azithromycin-SD is attributed to higher concentration of non-hygroscopic L-leucine.
Fig. 5.
Dynamic water sorption behavior of the spray-dried formulations.
Powder X-ray Diffraction (PXRD)
PXRD diffractograms of the raw azithromycin and raw L-leucine showed sharp peaks indicating they were crystalline (Fig. 6a). L-leucine-SD showed certain degrees of crystallinity that reflect their fast crystallization behavior right after spray drying. In contrast, Azithromycin-SD did not exhibit any crystalline peaks, indicating it was amorphous (Fig. 6b). Interestingly, the L-leucine crystalline peaks were also evident in the diffractograms of composite formulations with a broader shape and the intensity of these peaks decreased with an increase in azithromycin proportion (Fig. 6b). The physical stability of the spray-dried formulations stored at 55% RH and 75% RH was also tested using PXRD (Fig. 6c and d). There was no significant changes in crystalline peaks indicating that spray-dried formulations were physically stable upon short-term exposure to the elevated humidity.
Fig. 6.
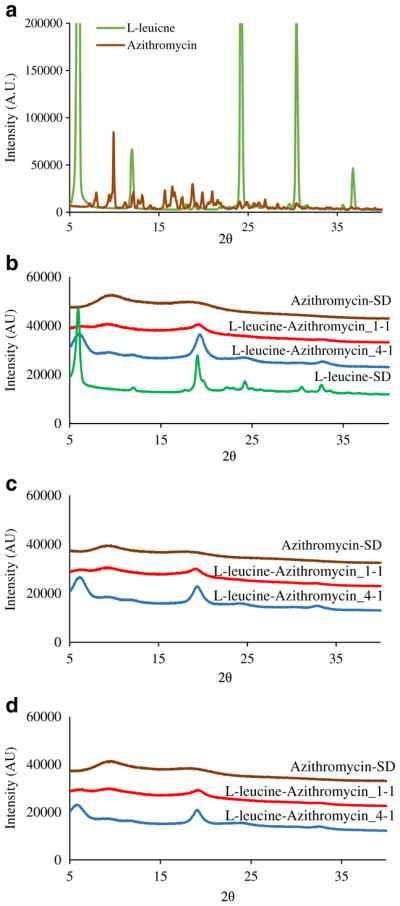
Power X-ray diffraction patterns of: (a) raw azithromycin and L-leucine; (b) freshly spray-dried formulations; (c) spray-dried formulations stored at 55% RH for a week; (d) spray-dried formulations stored at 75% RH for a week.
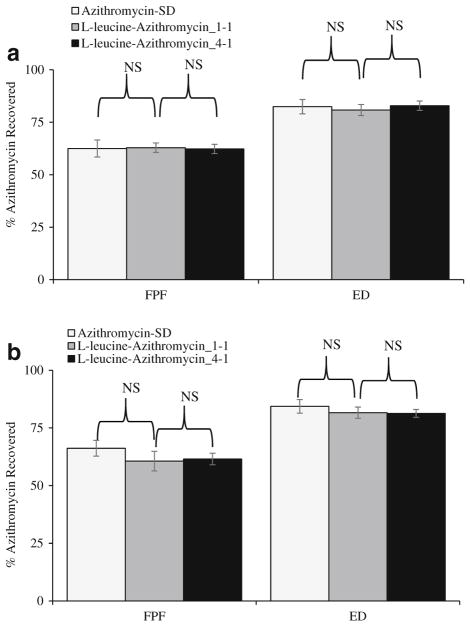
In-Vitro Aerosol Performance
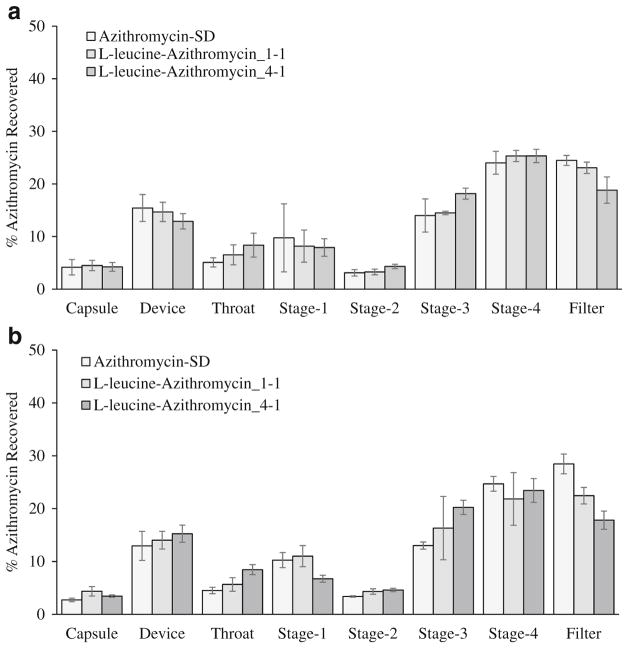
All formulations demonstrated similar ED around 80% and FPF around 62%. The Azithromycin-SD showed a fine particle fraction of 62.5 ± 4.1% (Fig. 7a). Composite formulations of L-leucine-Azithromycin_1–1 and L-leucine-Azithromycin_4–1 showed fine particle fractions of 62.9 ± 2.3% and 62.3 ± 2.2%, respectively, (Fig. 7a) suggesting that L-leucine had no effect on the aerosolization of azithromycin. Previous studies have shown that L-leucine can act as the force control agent to reduce the cohesion and improve the aerosolization upon co-spraying (61,62). The capability of L-leucine to reduce cohesion is believed to be associated with its enrichment on the particle surface, which allows it to produce rougher particles with lower surface energy (56). Our results showed that L-leucine did not enrich on the surfaces of the composite formulations and have no significant impact in aerosolization. This suggests that surface enrichment of L-leucine is pivotal to aerosolization enhancing capability. We also investigated the effect of elevated humidity on the aerosolization of the spray-dried formulations. The results indicated short-term exposure to elevated humidity caused no significant change in the ED and FPF (Fig. 7b). A detailed deposition profiles of different formulations is provided in Appendix 2. The aerodynamic size seems larger for the spray dried formulations with higher L-leucine concentration than the Azithromycin-SD. For instance, there was an increase in azithromycin deposition in Stage 3 and a decrease in Filter when the L-leucine concentration was increased. This is in agreement with the marginal increases in physical size of the spray dried formulations with L-leucine (Table I). However, such minor difference in aerodynamic size distribution should not contribute to different dissolution behavior because the particles used for dissolution were collected from the same aerodynamic size fraction in Stage 4 of NGI.
Fig. 7.
Fine particle fraction (FPF) (%) and emitted dose (ED) (%) of the spray-dried formulations stored at: (a) 55% RH; and (b) 75% RH. The data are presented as mean ± SD (n = 4). NS denotes not significant (p > 0.05).
Equilibrium Solubility
The solubility of raw azithromycin was 1.0 mg/mL (Table III). The Azithromycin-SD demonstrated a similar solubility (1.0 mg/mL) compared with the raw azithromycin (p > 0.05). It is not surprising that Azithromycin-SD has a similar equilibrium solubility to the raw crystalline drug as the crystallization likely occurs during the 24-h measurement. The formulations spray-dried with L-leucine showed greater solubility than the Azithromycin-SD (p < 0.05). A trend was observed that the solubility increased with increasing L-leucine concentration. L-leucine-Azithromycin_1–1_Physical Mixture has a similar solubility to the raw azithromycin and L-leucine-Azithromycin_4–1_Physical Mixture only showed a minor increase. Solubility of the spray-dried composite formulations was higher than the corresponding physical mixtures (p < 0.05).
Table III.
Equilibrium Solubility of Azithromycin in Various Formulations (Mean ± SD, n = 4)
| Formulation | Azithromycin solubility (mg/mL) at 37°C |
|---|---|
| Azithromycin-Raw | 1.0 ± 0.1 |
| L-leucine-Azithromycin_1–1_Physical Mixture | 1.1 ± 0.1 |
| L-leucine-Azithromycin_4–1_Physical Mixture | 1.3 ± 0.1 |
| Azithromycin-SD | 1.0 ± 0.1 |
| L-leucine-Azithromycin_1–1 | 1.3 ± 0.1 |
| L-leucine-Azithromycin_4–1 | 1.8 ± 0.2 |
In-Vitro Dissolution
Beaker Method
The results measured using the beaker method indicated that the composite formulations had faster dissolution rate compared with the Azithromycin-SD (Fig. 8a). As for the Azithromycin-SD, only 62.0 ± 5.0% of azithromycin was dissolved in 30 min and the maximum drug release obtained was >90% after 180 min. Addition of L-leucine in the formulation was shown to increase the release of azithromycin with >75% and >85% of drug released within 30 min for L-leucine-Azithromycin_1–1 and L-leucine-Azithromycin_4–1, respectively. More than 90% of the drug was released within 60 min for the composite formulations.
Fig. 8.
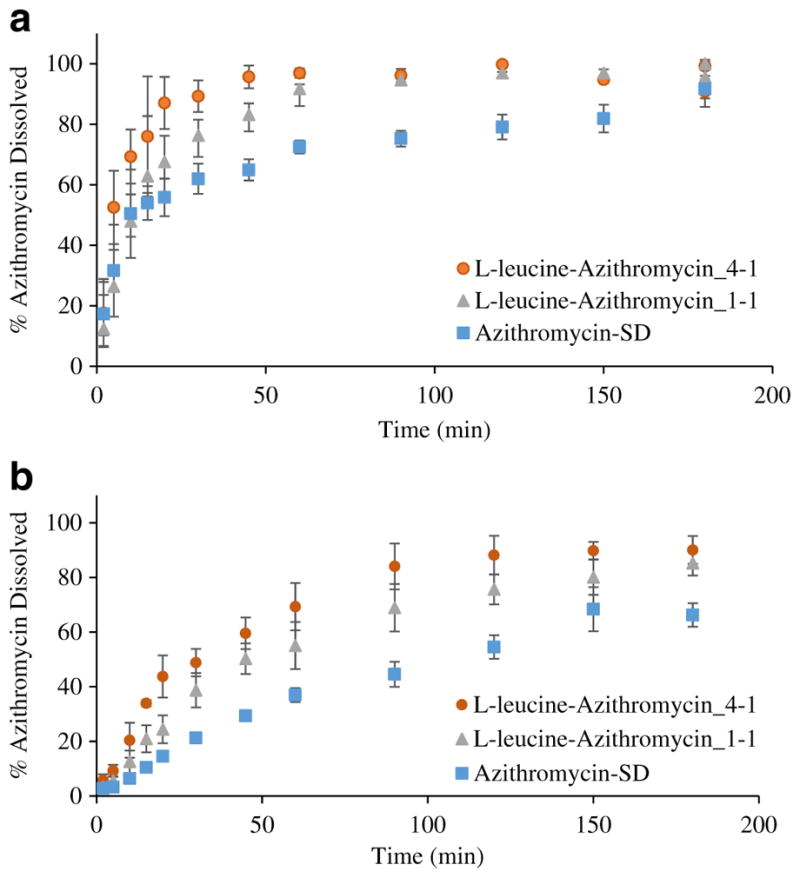
In-vitro dissolution of azithromycin from the spray-dried formulations as determined by: (a) beaker method; and (b) Franz cell method. Data are presented as mean ± SD (n = 4).
Franz Cell Method
The Franz cell dissolution data demonstrated that for the Azithromycin-SD, only 55.5 ± 4.3% of the drug was dissolved after 120 min (Fig. 8b). However, the azithromycin released after 90 min was >60% for the L-leucine-Azithromycin_1–1 and >80% for the L-leucine-Azithromycin_4–1. At 180 min, the total drug released was 66.2 ± 4.3% for the Azithromycin-SD, whereas >85% of azithromycin was released for both composite formulations. The results indicate that addition of L-leucine in the spray drying formulations substantially improved the dissolution rate of azithromycin, which are in agreement with the beaker method data.
DISCUSSION
Drug concentration in the ELF is vital to the effective treatment of lower respiratory tract infections (16). However, for many antibiotics oral or parenteral administrations only result in low and suboptimal drug concentrations on the airway surfaces (16). Inhalation therapy enables direct delivery of drugs to the infection sites on the lower airway surfaces. Recent studies have attempted to develop inhaled azithromycin formulations (13,30–33).
For dry powder formulations, dissolution of the drug aerosol particles deposited on the airway surfaces is critical to the antimicrobial efficacy. For some drugs with high water solubility and fast systemic absorption such as ciprofloxacin HCl, there may be a need to develop controlled released formulations to achieve high local drug concentrations for longer time (63). However, for poorly water soluble drugs, there is a need to improve the dissolution and maintain drug concentrations in the lung lining fluid above minimum inhibitory concentrations (16,64,65), particularly for azithromycin which has to enter the bacterial cells to be effective (34). Given lung surface is not an ideal place for drug dissolution due to the low fluid volume and particle clearance mechanisms (66), dissolution behavior is crucial for the efficacy of inhaled azithromycin DPIs. However, there is a scarce in the literature that examines the dissolution behavior of inhalation formulations of azithromycin, a poorly water-soluble drug. Thus, the main objective of this study was to develop composite dry powder inhaler formulations of azithromycin with L-leucine, and characterize the formulations regarding aerosolization and dissolution.
L-leucine is commonly employed as a force control agent to improve the aerosolization of spray dried fine powders (61,62). It is believed that the addition of L-leucine increases the surface roughness and reduces surface energy of the spray-dried particles as consequences of L-leucine enrichment on the particle surface (56,67–69). However, in this study, co-spray drying with L-leucine had no significant effects on the FPF of azithromycin. Our results indicated that co-spray drying with L-leucine resulted no apparent change in the morphology compared with the Azithromycin-SD particles. This suggests that surface enrichment of L-leucine is essential to alter particle morphology and improve the aerosolization of co-sprayed dried powder formulations, which is associated with the self-assembly behavior of L-leucine on the surfaces of spray-dried formulations (67,68). Interestingly, our results indicated that surfaces concentration of L-leucine was lower than the theoretical value. This can be attributed to rapid evaporation of ethanol or low solubility of azithromycin in the co-solvent system, resulting in super-saturation of azithromycin in aqueous phase of the droplet. Because of low diffusion rate of azithromycin towards to the core of the droplet and rapid precipitation in the drying phase, particle surfaces are enriched with azithromycin (53,55,70). All the spray-dried formulations exhibited satisfactory physical stability even upon short-term storage at the elevated humidity, which was attributed to the relative hydrophobic nature of azithromycin as indicated by the DVS data.
Spray-drying with L-leucine was shown to accelerate the dissolution of azithromycin as measured by two different testing methods, in comparison to the Azithromycin-SD powder. We would highlight that while there was an increase in the particle size with increasing L-leucine feed concentration, such size differences are unlikely to affect the dissolution kinetics of the resultant formulations. This is because the dissolution studies were carried out with the particles in the aerodynamically classified fraction i.e., the particles deposited in Stage 4 of the NGI. Thus, any change in the dissolution kinetics are not due to the change in particle size.
The solubility (Table III) for the L-leucine-containing formulations is much higher than the amorphous azithromycin only formulation, and there is an increase in solubility when the L-leucine concentration increases. These data have shown that improvements in solubility and dissolution are not only the consequence of the amorphous form. We have also measured the equilibrium solubility of azithromycin with presence of mannitol (31), but no change was observed (1 mg/mL). A few factors can contribute to the enhanced dissolution. First, azithromycin and L-leucine likely formed a complex as supported by intermolecular interactions between the two components in the composite formulation (60,71). To be specific, the peaks assigned to the carboxylic acid group of L-leucine were hypsochromically shifted suggesting that the formation of hydrogen bond with azithromycin changed the stretching mode of carbonyl group (Fig. 4). Moreover, L-leucine has much higher aqueous solubility than azithromycin. In the composite formulations, each particle contains both azithromycin and L-leucine as evident by the ToF-SIMS data. Mixing of azithromycin with water-soluble L-leucine at the sub-micron or even molecular level could enhance the dissolution behavior of poorly water-soluble azithromycin (72).
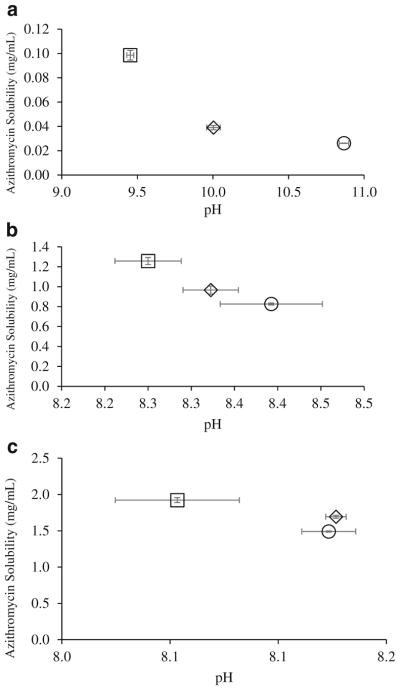
We conducted solubility experiments for Azithromycin-SD and composite formulations in dissolution media with acidic (potassium chloride buffer, pH 2), neutral (PBS, pH 7.4) and basic pH (carbonate buffer, pH 11). Solubility of azithromycin reduced with the increased pH; addition of L-leucine was shown to increase the solubility of azithromycin (Fig. 9). Due to its weakly basic nature, azithromycin tends to increase the pH of the media upon dissolving in potassium chloride buffer and PBS at the end of solubility experiments (24 h); the changes in pH at pH 7.4 are marginal and unlikely have safety concerns considering the buffering capacity of airway lining fluids (73). In the media of pH 11, the dissolving of L-leucine-containing formulations decreased the media pH. These data indicate a minor change in environmental pH can alter the solubility of azithromycin substantially (33). Thus, it is likely that due to the acidic nature of L-leucine, L-leucine decreases the pH of the dissolution media and creates an acidic microenvironment surrounding the azithromycin, although majority of the surfaces are azithromycin. Further investigations are warranted to fully understand the mechanisms of such dissolution enhancement by co-spray drying.
Fig. 9.
Azithromycin solubility at 37°C in: (a) carbonate buffer at pH 11, (b) PBS at pH 7.4 and (c) potassium chloride buffer (pH 2): (□) L-leucine-Azithromycin_4–1; (⋄) L-leucine-Azithromycin_1–1; (○) Azithromycin-SD. Data are presented as mean ± SD (n = 4).
CONCLUSIONS
Traditionally, L-leucine has been widely used to reduce the cohesion and improve the aerosolization of spray dried inhalable powder formulations. Interestingly, in the present study L-leucine did not enrich on the surface of the composite particles containing hydrophobic azithromycin and had no apparent effects on the aerosolization performance. This suggests that improvement of aerosolization by spray drying drugs with L-leucine is dependent on whether L-leucine can be dominant on the particle surface.
The composite formulations exhibited excellent aerosol performance and physical and aerosolization stability against short-term exposure to elevated humidity. Our results showed that co-spray drying with L-leucine improved the dissolution of poorly water-soluble azithromycin. Such enhanced dissolution behavior is attributed to the formation of composite system of azithromycin with water-soluble L-leucine, and the creation of acidic dissolution microenvironment around azithromycin (a weak base) during dissolution. Such increased dissolution of poorly water-soluble azithromycin by co-spray drying with L-leucine may be transferred to the enhanced antimicrobial activities, which deserve further investigations.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURES
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI132681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Qi (Tony) Zhou is a recipient of the Ralph W. and Grace M. Showalter Research Trust Award. The authors are grateful for the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Future Industries Institute, University of South Australia. Kind donations of RS01 DPI device from Plastiape S.p.A. and HPMC capsules from Qualicaps, Inc. are acknowledged.
Appendix 1
Fig. 10.
Chemical structures of: (a) azithromycin; (b) L-leucine.
Appendix 2
Fig. 11.
Deposition profiles of spray-dried formulations at various stages of MSLI after storage for 1 week at 55% RH (a) and 75% RH (b).
References
- 1.Wagner T, Soong G, Sokol S, Saiman L, Prince A. Effects of azithromycin on clinical isolates of pseudomonas aeruginosa from cystic fibrosis patients. Chest. 2005;128(2):912–9. doi: 10.1378/chest.128.2.912. [DOI] [PubMed] [Google Scholar]
- 2.Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24(5):834–8. doi: 10.1183/09031936.04.00084304. [DOI] [PubMed] [Google Scholar]
- 3.Wilms EB, Touw DJ, Heijerman HGM, van der Ent CK. Azithromycin maintenance therapy in patients with cystic fibrosis: a dose advice based on a review of pharmacokinetics, efficacy, and side effects. Pediatr Pulmonol. 2012;47(7):658–65. doi: 10.1002/ppul.21620. [DOI] [PubMed] [Google Scholar]
- 4.Amsden GW. Anti-inflammatory effects of macrolides–an under-appreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(1):10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]
- 5.Gotfried MH. Macrolides for the treatment of chronic sinusitis, asthma, and COPD. Chest. 2004;125(2, Supplement):52S–61S. doi: 10.1378/chest.125.2_suppl.52s. [DOI] [PubMed] [Google Scholar]
- 6.Favre-Bonté S, Köhler T, Van Delden C. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J Antimicrob Chemother. 2003;52(4):598–604. doi: 10.1093/jac/dkg397. [DOI] [PubMed] [Google Scholar]
- 7.Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189(12):1503–8. doi: 10.1164/rccm.201402-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catherinot E, Roux AL, Vibet MA, Bellis G, Lemonnier L, Le Roux E, et al. Inhaled therapies, azithromycin and Mycobacterium abscessus in cystic fibrosis patients. Eur Respir J. 2013;41(5):1101–6. doi: 10.1183/09031936.00065612. [DOI] [PubMed] [Google Scholar]
- 9.Chang AB, Grimwood K, White AV, Maclennan C, Sloots TP, Sive A, et al. Randomized placebo-controlled trial on azithromycin to reduce the morbidity of bronchiolitis in Indigenous Australian infants: rationale and protocol. Trials. 2011;12:94. doi: 10.1186/1745-6215-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekici A, Ekici M, Erdemoglu AK. Effect of azithromycin on the severity of bronchial hyperresponsiveness in patients with mild asthma. J Asthma Off J Assoc Care Asthma. 2002;39(2):181–5. doi: 10.1081/jas-120002199. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins S. Clinical toleration and safety of azithromycin. Am J Med. 1991;91(3):S40–5. doi: 10.1016/0002-9343(91)90401-i. [DOI] [PubMed] [Google Scholar]
- 12.Wallace MR, Miller LK, Nguyen MT, Shields AR. Ototoxicity with azithromycin. Lancet (Lond Engl) 1994;343(8891):241. doi: 10.1016/s0140-6736(94)91030-8. [DOI] [PubMed] [Google Scholar]
- 13.Hickey AJ, Lu D, Ashley ED, Stout J. Inhaled azithromycin therapy. J Aerosol Med. 2006;19(1):54–60. doi: 10.1089/jam.2006.19.54. [DOI] [PubMed] [Google Scholar]
- 14.Frijlink H, De Boer A. Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deliv. 2004;1(1):67–86. doi: 10.1517/17425247.1.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Hickey A, Durham P, Dharmadhikari A, Nardell E. Inhaled drug treatment for tuberculosis: past progress and future prospects. J Control Release. 2016;240:127–34. doi: 10.1016/j.jconrel.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Velkov T, Abdul Rahim N, Zhou Q, Chan H-K, Li J. Inhaled anti-infective chemotherapy for respiratory tract infections: successes, challenges and the road ahead. Adv Drug Deliv Rev. 2015;85:65–82. doi: 10.1016/j.addr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou QT, Tang P, Leung SSY, Chan JGY, Chan H-K. Emerging inhalation aerosol devices and strategies: where are we headed? Adv Drug Deliv Rev. 2014;75:3–17. doi: 10.1016/j.addr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Timsina MP, Martin GP, Marriott C, Ganderton D, Yianneskis M. Drug-delivery to the respiratory-tract using dry powder inhalers. Int J Pharm. 1994;101(1–2):1–13. [Google Scholar]
- 19.Weers J. Inhaled antimicrobial therapy–barriers to effective treatment. Adv Drug Deliv Rev. 2015;85:24–43. doi: 10.1016/j.addr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Buttini F, Colombo P, Rossi A, Sonvico F, Colombo G. Particles and powders: tools of innovation for non-invasive drug administration. J Control Release. 2012;161(2):693–702. doi: 10.1016/j.jconrel.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Chan HK, Chew NYK. Novel alternative methods for the delivery of drugs for the treatment of asthma. Adv Drug Deliv Rev. 2003;55(7):793–805. doi: 10.1016/s0169-409x(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 22.de Boer AH, Chan HK, Price R. A critical view on lactose-based drug formulation and device studies for dry powder inhalation: which are relevant and what interactions to expect? Adv Drug Deliv Rev. 2012;64(3):257–74. doi: 10.1016/j.addr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Smyth HD, Hickey AJ. Carriers in drug powder delivery. Am J Drug Deliv. 2005;3(2):117–32. [Google Scholar]
- 24.Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y-W, Wong J, Qu L, Chan H-K, Zhou QT. Powder production and particle engineering for dry powder inhaler formulations. Curr Pharm Des. 2015;21(27):3902–16. doi: 10.2174/1381612821666150820111134. [DOI] [PubMed] [Google Scholar]
- 26.Thakkar SG, Fathe K, Smyth HD. Amorphous or crystalline? A comparison of particle engineering methods and selection. Curr Pharm Des. 2015;21(40):5789–801. doi: 10.2174/1381612821666151008150457. [DOI] [PubMed] [Google Scholar]
- 27.Cun D, Wan F, Yang M. Formulation strategies and particle engineering technologies for pulmonary delivery of biopharmaceuticals. Curr Pharm Des. 2015;21(19):2599–610. doi: 10.2174/1381612821666150416100800. [DOI] [PubMed] [Google Scholar]
- 28.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohr AP, Boetker J, Rades T, Rantanen J, Yang M. Application of spray-drying and electrospraying/electospinning for poorly watersoluble drugs: a particle engineering approach. Curr Pharm Des. 2014;20(3):325–48. doi: 10.2174/13816128113199990399. [DOI] [PubMed] [Google Scholar]
- 30.Young PM, Salama RO, Zhu B, Phillips G, Crapper J, Chan HK, et al. Multi-breath dry powder inhaler for delivery of cohesive powders in the treatment of bronchiectasis. Drug Dev Ind Pharm. 2015;41(5):859–65. doi: 10.3109/03639045.2014.909841. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Vogt FG, Hayes D, Mansour HM. Design, characterization, and aerosol dispersion performance modeling of advanced co-spray dried antibiotics with mannitol as respirable microparticles/nanoparticles for targeted pulmonary delivery as dry powder inhalers. J Pharm Sci. 2014;103(9):2937–49. doi: 10.1002/jps.23955. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Vogt FG, Hayes D, Jr, Mansour HM. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried microparticulate/nanoparticulate antibiotic dry powders of tobramycin and azithromycin for pulmonary inhalation aerosol delivery. Eur J Pharm Sci. 2014;52:191–205. doi: 10.1016/j.ejps.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Wang X, Lin X, Liu X, Tian B, Tang X. High azithromycin loading powders for inhalation and their in vivo evaluation in rats. Int J Pharm. 2010;395(1–2):205–14. doi: 10.1016/j.ijpharm.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 34. [Accessed 10 Dec 2017];Highlights of prescribing information for ZITHROMAX. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050710s043,050711s040,050784s027lbl.pdf.
- 35.Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv Drug Deliv Rev. 2016;100:27–50. doi: 10.1016/j.addr.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Okuda T, Lu X-Y, Chan H-K. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016;100(Supplement C):102–15. doi: 10.1016/j.addr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Laitinen R, Lobmann K, Grohganz H, Strachan C, Rades T. Amino acids as co-amorphous excipients for simvastatin and glibenclamide: physical properties and stability. Mol Pharm. 2014;11(7):2381–9. doi: 10.1021/mp500107s. [DOI] [PubMed] [Google Scholar]
- 39.Chavan RB, Thipparaboina R, Kumar D, Shastri NR. Co amorphous systems: a product development perspective. Int J Pharm. 2016;515(1–2):403–15. doi: 10.1016/j.ijpharm.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 40.Lenz E, Jensen KT, Blaabjerg LI, Knop K, Grohganz H, Löbmann K, et al. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin–arginine. Eur J Pharm Biopharm. 2015;96:44–52. doi: 10.1016/j.ejpb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Jensen KT, Larsen FH, Cornett C, Löbmann K, Grohganz H, Rades T. Formation mechanism of coamorphous drug-amino acid mixtures. Mol Pharm. 2015;12(7):2484–92. doi: 10.1021/acs.molpharmaceut.5b00295. [DOI] [PubMed] [Google Scholar]
- 42.Löbmann K, Laitinen R, Strachan C, Rades T, Grohganz H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs – part 2: molecular interactions. Eur J Pharm Biopharm. 2013;85(3 Part B):882–8. doi: 10.1016/j.ejpb.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Aucamp M, Odendaal R, Liebenberg W, Hamman J. Amorphous azithromycin with improved aqueous solubility and intestinal membrane permeability. Drug Dev Ind Pharm. 2015;41(7):1100–8. doi: 10.3109/03639045.2014.931967. [DOI] [PubMed] [Google Scholar]
- 44.Arora S, Haghi M, Young PM, Kappl M, Traini D, Jain S. Highly respirable dry powder inhalable formulation of voriconazole with enhanced pulmonary bioavailability. Expert Opin Drug Deliv. 2016;13(2):183–93. doi: 10.1517/17425247.2016.1114603. [DOI] [PubMed] [Google Scholar]
- 45.Rabbani NR, Seville PC. The influence of formulation components on the aerosolisation properties of spray-dried powders. J Control Release. 2005;110(1):130–40. doi: 10.1016/j.jconrel.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Chew NY, Shekunov BY, Tong HH, Chow AH, Savage C, Wu J, et al. Effect of amino acids on the dispersion of disodium cromoglycate powders. J Pharm Sci. 2005;94(10):2289–300. doi: 10.1002/jps.20426. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, et al. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm. 2016;102:132–41. doi: 10.1016/j.ejpb.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res. 2007;24(2):203–27. doi: 10.1007/s11095-006-9146-7. [DOI] [PubMed] [Google Scholar]
- 49.Holdich RG. Chapter 2 particle characterisation. Shepshed: Midland Information Technology and Publishing; 2002. Fundamentals of particle technology. [Google Scholar]
- 50.Wei G, Mangal S, Denman J, Gengenbach T, Lee Bonar K, Khan RI, et al. Effects of coating materials and processing conditions on flow enhancement of cohesive acetaminophen powders by high-shear processing with pharmaceutical lubricants. J Pharm Sci. 2017;106(10):3022–32. doi: 10.1016/j.xphs.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Q, Denman JA, Gengenbach T, Das S, Qu L, Zhang H, et al. Characterization of the surface properties of a model pharmaceutical fine powder modified with a pharmaceutical lubricant to improve flow via a mechanical dry coating approach. J Pharm Sci. 2011;100(8):3421–30. doi: 10.1002/jps.22547. [DOI] [PubMed] [Google Scholar]
- 52.Nie H, Su Y, Zhang M, Song Y, Leone A, Taylor LS, et al. Solid-state spectroscopic investigation of molecular interactions between Clofazimine and Hypromellose phthalate in amorphous solid dispersions. Mol Pharm. 2016;13(11):3964–75. doi: 10.1021/acs.molpharmaceut.6b00740. [DOI] [PubMed] [Google Scholar]
- 53.Zhou QT, Loh ZH, Yu J, Sun SP, Gengenbach T, Denman JA, et al. How much surface coating of hydrophobic azithromycin is sufficient to prevent moisture-induced decrease in aerosolisation of hygroscopic amorphous colistin powder? AAPS J. 2016;18(5):1213–24. doi: 10.1208/s12248-016-9934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilcer G, Vanderbist F, Amighi K. Spray-dried carrier-free dry powder tobramycin formulations with improved dispersion properties. J Pharm Sci. 2009;98(4):1463–75. doi: 10.1002/jps.21545. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, Zhou QT, Sun S-P, Denman JA, Gengenbach TR, Barraud N, et al. Effects of surface composition on the aerosolisation and dissolution of inhaled antibiotic combination powders consisting of colistin and rifampicin. AAPS J. 2016;18(2):372–84. doi: 10.1208/s12248-015-9848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangal S, Meiser F, Tan G, Gengenbach T, Denman J, Rowles MR, et al. Relationship between surface concentration of L-leucine and bulk powder properties in spray dried formulations. Eur J Pharm Biopharm. 2015;94:160–9. doi: 10.1016/j.ejpb.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 57.Heinz A, Strachan CJ, Gordon KC, Rades T. Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy. J Pharm Pharmacol. 2009;61(8):971–88. doi: 10.1211/jpp/61.08.0001. [DOI] [PubMed] [Google Scholar]
- 58.Gandhi R, Pillai O, Thilagavathi R, Gopalakrishnan B, Kaul CL, Panchagnula R. Characterization of azithromycin hydrates. Eur J Pharm Sci. 2002;16(3):175–84. doi: 10.1016/s0928-0987(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 59.Rajkumar BJ, Ramakrishnan V. Infrared and raman spectra of L-valine nitrate and L-leucine nitrate. J Raman Spectrosc. 2000;31(12):1107–12. [Google Scholar]
- 60.Nie H, Mo H, Zhang M, Song Y, Fang K, Taylor LS, et al. Investigating the interaction pattern and structural elements of a drug–polymer complex at the molecular level. Mol Pharm. 2015;12(7):2459–68. doi: 10.1021/acs.molpharmaceut.5b00162. [DOI] [PubMed] [Google Scholar]
- 61.Weiler C, Egen M, Trunk M, Langguth P. Force control and powder dispersibility of spray dried particles for inhalation. J Pharm Sci. 2010;99(1):303–16. doi: 10.1002/jps.21849. [DOI] [PubMed] [Google Scholar]
- 62.Li HY, Neill H, Innocent R, Seville P, Williamson I, Birchall JC. Enhanced dispersibility and deposition of spray-dried powders for pulmonary gene therapy. J Drug Target. 2003;11(7):425–32. doi: 10.1080/10611860410001659786. [DOI] [PubMed] [Google Scholar]
- 63.Cipolla D, Blanchard J, Gonda I. Development of liposomal ciprofloxacin to treat lung infections. Pharm. 2016;8(1):6. doi: 10.3390/pharmaceutics8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duret C, Wauthoz N, Sebti T, Vanderbist F, Amighi K. Solid dispersions of itraconazole for inhalation with enhanced dissolution, solubility and dispersion properties. Int J Pharm. 2012;428(1):103–13. doi: 10.1016/j.ijpharm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Duret C, Merlos R, Wauthoz N, Sebti T, Vanderbist F, Amighi K. Pharmacokinetic evaluation in mice of amorphous itraconazole-based dry powder formulations for inhalation with high bioavailability and extended lung retention. Eur J Pharm Biopharm. 2014;86(1):46–54. doi: 10.1016/j.ejpb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60(2):532–8. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 67.Feng AL, Boraey MA, Gwin MA, Finlay PR, Kuehl PJ, Vehring R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int J Pharm. 2011;409(1–2):156–63. doi: 10.1016/j.ijpharm.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 68.Sou T, Kaminskas LM, Nguyen T-H, Carlberg R, McIntosh MP, Morton DAV. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur J Pharm Biopharm. 2013;83(2):234–43. doi: 10.1016/j.ejpb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 69.Jong T, Li J, Morton DAV, Zhou Q, Larson I. investigation of the changes in aerosolization behavior between the jet-milled and spray-dried colistin powders through surface energy characterization. J Pharm Sci. 2016;105(3):1156–63. doi: 10.1016/S0022-3549(15)00189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nie H, Byrn SR, Zhou Q. Stability of pharmaceutical salts in solid oral dosage forms. Drug Dev Ind Pharm. 2017;43(8):1215–28. doi: 10.1080/03639045.2017.1304960. [DOI] [PubMed] [Google Scholar]
- 72.Löbmann K, Grohganz H, Laitinen R, Strachan C, Rades T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs–part 1: preparation, stability and dissolution enhancement. Eur J Pharm Biopharm. 2013;85(3):873–81. doi: 10.1016/j.ejpb.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 73.Holma B. Effects of inhaled acids on airway mucus and its consequences for health. Environ Health Perspect. 1989;79:109–13. doi: 10.1289/ehp.8979109. [DOI] [PMC free article] [PubMed] [Google Scholar]