Abstract
Phosphatidylglycerol (PG) is considered to play an important role in the ordered assembly and structural maintenance of the photosynthetic apparatus in thylakoid membranes. However, its function in photosynthesis remains poorly understood. In this study we have identified a pgsA gene of Synechocystis sp. PCC6803 that encodes a PG phosphate synthase involved in the biosynthesis of PG. A disruption of the pgsA gene allowed us to manipulate the content of PG in thylakoid membranes and to investigate the function of PG in photosynthesis. The obtained pgsA mutant could grow only in the medium containing PG, and the photosynthetic activity of the pgsA mutant dramatically decreased with a concomitant decrease of PG content in thylakoid membranes when the cells grown in the presence of PG were transferred to the medium without PG. This decrease of photosynthetic activity was attributed to the decrease of photosystem (PS)II activity, but not to the decrease in PSI activity. These findings demonstrate that PG is essential for growth of Synechocystis sp. PCC6803 and provide the first direct evidence that PG plays an important role in PSII.
Thylakoid membranes in chloroplasts of eukaryotic plants and cyanobacterial cells are the sites of the primary processes of oxygenic photosynthesis. They are mainly composed of glycerolipids forming lipid bilayers and of protein complexes involved in photosynthetic electron transport and energy conversion (Siegenthaler, 1998). The lipid composition of thylakoid membranes is highly conserved among eukaryotic plants and cyanobacterial strains, and is distinct from that of other membranes, which contain phospholipids as the major glycerolipids (Block et al., 1983; Murata and Nishida, 1987; Wada and Murata, 1998). The most abundant glycerolipids of thylakoid membranes are glycolipids, monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), and sulfoquinovosyldiacylglycerol (SQDG). Phosphatidylglycerol (PG) is the only phospholipid in thylakoid membranes.
The role of glycerolipids in the function of thylakoid membranes has been studied in vitro and the important studies were summarized in a review (Siegenthaler, 1998). However, the findings obtained in those studies do not provide direct evidence for a role of glycerolipids in photosynthesis and cannot be directly applied to the function of glycerolipids in vivo. The function of glycerolipids has also been studied in vivo using mutants having different lipid composition of thylakoid membranes. Güler et al. (1996) made a null mutant of Synechococcus sp. PCC7942 deficient in production of SQDG by disruption of the sqdB gene involved in the biosynthesis of SQDG. Extensive analysis of the mutant revealed that the complete lack of SQDG did not affect the growth and photosynthesis of the cells grown under optimal conditions. These results demonstrate that SQDG is not essential for growth of the cyanobacterium and for photosynthesis.
A DGDG-deficient mutant was isolated from Arabidopsis by Dörmann et al. (1995). The content of DGDG in the mutant was about 8% of that of DGDG in the wild type. The mutant showed stunted growth, pale green leaves, reduced photosynthetic activity, and altered ultrastructure of thylakoid membranes. These results led to the conclusion that DGDG is important for photosynthesis and for maintenance of the structure of thylakoid membranes. Although the function of SQDG and DGDG has been studied using mutants as described above, the function of PG and MGDG has not been clearly studied in vivo, since mutants lacking MGDG and PG have not been available. Härtel et al. (1998) have recently investigated the function of PG in photosynthesis using the pho1 mutant of Arabidopsis. This mutant is thought to be defective in phosphate loading of the xylem, which results in a strongly restricted accumulation of phosphate in stems and leaves (Poirier et al., 1991). The mutant responds to phosphate deficiency in the leaves by decreasing the amount of phospholipids, especially PG. The content of PG in leaves of the mutant was shown to be 35% to 45% lower than that in the wild-type leaves. Despite this reduction in PG, there were no significant differences in growth and photosynthetic activities between the mutant and wild type. The results obtained with the pho1 mutant suggested that PG is not essential for growth and for photosynthesis. However, we cannot rule out the possibility that the reduced amount of PG is still sufficient to achieve the essential function.
In Escherichia coli, PG is synthesized from phosphatidic acid (PA), which is synthesized by acylations of glycerol-3-P. The PA is converted to CDP-diacylglycerol (CDP-DG) by CDP-DG synthase and then to PG phosphate (PGP) by PGP synthase. The last step of the biosynthesis of PG is dephosphorylation of PGP catalyzed by PGP phosphatase. The genes for all enzymes other than PGP phosphatase have been cloned from E. coli. It is known that PG is synthesized by the same pathway in chloroplasts and cyanobacterial cells (Joyard et al., 1998; Wada and Murata, 1998). However, genes for enzymes involved in the biosynthesis of PG have not been cloned from higher plants and cyanobacteria, with the exception of the cloning of the gene for CDP-DG synthase from potato and Arabidopsis (Kopka et al., 1997). To understand the function of PG in photosynthesis, it is important to clone the genes involved in the biosynthesis of PG and to manipulate the PG content in thylakoid membranes. The entire nucleotide sequence of the genome of Synechocystis sp. was recently determined (Kaneko et al., 1996). By identifying those genes in the genomic sequence that encode polypeptides homologous to the enzymes involved in the biosynthesis of PG in E. coli, it should be possible to manipulate the PG content in this cyanobacterial strain by inactivation of the identified gene.
In this study we identified a Synechocystis sp. gene, pgsA, that encodes a PGP synthase, and inactivated this gene to create a mutant that could not synthesize PG. The characterization of the mutant allowed us to understand the function of PG in photosynthesis.
RESULTS
Identification of the pgsA Gene from Synechocystis sp.
PGP synthase involved in the biosynthesis of PG catalyzes the reaction that forms PGP from CDP-DG and glycerol-3-P. The genes encoding this enzyme have already been cloned from some bacteria such as E. coli (Ohta et al., 1981) and Bacillus subtilis (Kontinen and Tokuda, 1995). To identify a gene for PGP synthase from Synechocystis sp. we searched the database (Cyanobase, Kazusa DNA Research Institute, Kisarazu, Japan) of the genomic sequence of Synechocystis sp. with an amino acid sequence of PGP synthase of E. coli, and found a gene encoding a polypeptide homologous to PGP synthase of E. coli. Comparison of the deduced amino acid sequence of the polypeptide encoded by the gene to that of PGP synthase of E. coli is shown in Figure 1. The Synechocystis sp. gene, designated pgsA, encodes an open reading frame of 537 nucleotides, which corresponds to 179 amino acid residues with an approximate molecular mass of 20 kD. The two sequences were 38% identical. This finding suggests that the gene of Synechocystis sp. encodes a PGP synthase. To confirm that the pgsA gene of Synechocystis sp. encodes a functional PGP synthase we first expressed the gene in the E. coli mutant YA5512, which is deficient in PGP synthase (Asai et al., 1989), and then checked to determine whether or not the gene complemented the mutant. This E. coli mutant has a single-base replacement in the coding region of the pgsA gene. This mutation changes the amino acid residue Thr-60 to Pro-60 in PGP synthase (Usui et al., 1994) and causes a very low activity of PGP synthase and a very low content of PG in the mutant. The content of cardiolipin (CL) in the mutant is also very low because it is synthesized from PG. The YA5512 mutant grows in Luria-Bertani (LB) medium supplemented with a high concentration of NaCl, but not in nutrient broth (NB) medium (Inoue et al., 1998). This mutant was transformed with pKK233–2 and pKK-pgsA and the growth on LB and NB agar plates was checked. Like the mutant YA5512, the transformant of YA5512 with pKK233–2 (control) could grow on LB agar plates, but not on NB agar plates. By contrast, the transformant of YA5512 with pKK-pgsA could grow on both types of agar plates. This finding demonstrates that the mutant was complemented by the pgsA gene of Synechocystis sp. with respect to the growth on the NB agar plate.
Figure 1.
Comparison between the deduced amino acid sequence of PGP synthase of Synechocystis sp. encoded by the pgsA gene and the deduced amino acid sequence of PGP synthase of E. coli. The amino acid residues conserved in both sequences are indicated by asterisks. Hyphens indicate gaps that were added to maximize the alignment of the sequences.
Total lipids extracted from the transformants of YA5512 with pKK233–2 and pKK-pgsA were analyzed by thin layer chromatography (TLC) to demonstrate that the activity of PGP synthase was also complemented by the transformation with the pgsA gene of Synechocystis sp. In YA5512 and the transformant with pKK233–2, low amounts of PG and CL were detected (data not shown). However, in the transformant with pKK-pgsA, high amounts of PG and CL were detected (data not shown). Table I shows the lipid composition of YA5512 and the transformants with pKK233–2 and pKK-pgsA. Lipids separated on the TLC plate were analyzed by gas chromatography. The relative content of PG was 2 mol% in each of YA5512 and the transformant with pKK233–2, whereas that of PG in the transformant with pKK-pgsA was markedly increased to 41 mol%. These results clearly demonstrate that the pgsA gene of Synechocystis sp. is a structural gene for PGP synthase.
Table I.
Lipid composition of YA5512 and transformants of YA5512 made with the expression vectors pKK233-2 and pKK-pgsA
| Strain | Lipid
|
||
|---|---|---|---|
| PE | PG | CL | |
| mol% | |||
| YA5512 | 96 ± 0.2 | 2 ± 0.5 | 2 ± 0.5 |
| YA5512/pKK233-2 | 96 ± 1.0 | 2 ± 0.5 | 2 ± 1.0 |
| YA5512/pKK-pgsA | 50 ± 2.0 | 41 ± 3.0 | 9 ± 1.0 |
Lipid classes separated on TLC plates were subjected to methanolysis, and the resultant fatty acid methylesters were analyzed by gas chromatography. Each value represents the average and sd from three independent experiments.
Inactivation of the pgsA Gene in Synechocystis sp.
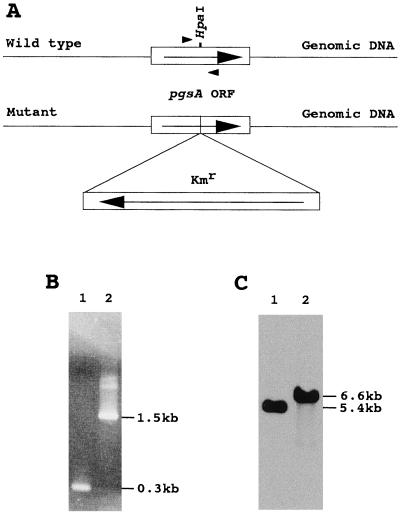
To understand the function of PG in photosynthesis we inactivated the pgsA gene of Synechocystis sp. by inserting a cassette of kanamycin resistant gene (Kmr) into the coding region of the pgsA gene, as shown in Figure 2A. Since cyanobacterial cells normally contain many copies of chromosomal DNA (Herdman et al., 1979), PCR and Southern hybridization analyses were made to confirm whether all of the copies of the pgsA gene had been inactivated in the mutant strain. Figure 2B shows the results of the PCR analysis. In the case of the wild type, a 0.3-kb DNA fragment corresponding to a part of the native pgsA gene was amplified. In the case of the mutant strain, a 1.5-kb DNA fragment corresponding to the inactivated pgsA gene was amplified, but a 0.3-kb DNA fragment corresponding to the native pgsA gene was not amplified. These results suggest that all copies of the pgsA gene were inactivated in the mutant strain. The inactivation of the pgsA gene in the mutant strain was also confirmed by genomic Southern hybridization analysis using the native pgsA gene as a DNA probe (Fig. 2C). In the wild type, a single hybridizing signal was detected at the 5.4-kb position, whereas in the case of the mutant strain a hybridizing signal was detected at the 6.6-kb position, but no signal was detected at the 5.4-kb position. These results further demonstrate that all copies of the pgsA gene were completely inactivated in the mutant strain.
Figure 2.
Insertional mutagenesis of the pgsA gene of Synechocystis sp. A, Structure of the pgsA gene in the wild type and the insertional mutant of Synechocystis sp. The directions of transcriptions of Kmr and the pgsA gene are indicated by arrows. B, PCR analysis of the pgsA gene in the wild type (lane 1) and the pgsA mutant (lane 2). The positions of DNA size markers are indicated on the right. The positions of the primers used for PCR reactions were shown by arrowheads in Figure 2A. C, Southern hybridization analysis of the pgsA gene in the wild type (lane 1) and the pgsA mutant (lane 2). Genomic DNAs extracted from the wild type and the pgsA mutant cells were digested with XbaI. One microgram of DNA was applied to each lane. The membrane was hybridized at 42°C using the pgsA gene of Synechocystis sp. as a DNA probe.
Growth of the pgsA Mutant
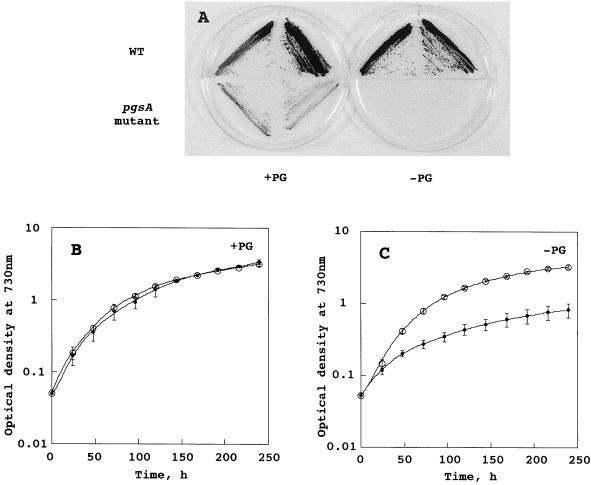
The growth of the wild type and the pgsA mutant under photoautotrophic growth condition was investigated to understand the function of PG for growth. Figure 3A shows the growth of the wild type and the pgsA mutant on agar plates. Although the wild-type cells grew regardless of the presence or absence of PG, the pgsA mutant could grow only on an agar plate containing PG.
Figure 3.
Growth profile of the wild type and the pgsA mutant of Synechocystis sp. A, Growth of the wild type and the pgsA mutant on agar plates. The cells grown in the medium containing 60 μm PG were streaked onto plates containing 20 μg mL−1 kanamycin and 60 μm PG (+PG) or no PG (−PG), and the plates were incubated at 30°C for 3 weeks. B, Wild type (○) and mutant cells (●) were grown in the medium containing 60 μm PG. The values are the averages and sd from three independent experiments. C, Growth profile of the wild type (○) and the pgsA mutant (●) in the medium without PG. The cells grown to stationary phase in the medium containing PG were transferred to the medium not containing PG. The values are the averages and sd from three independent experiments.
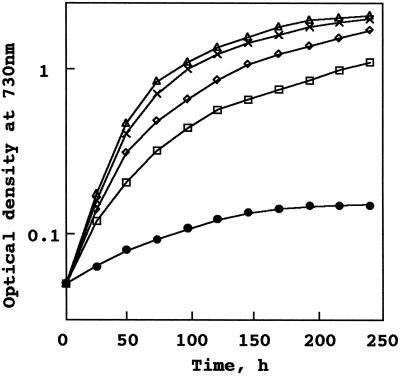
The growth profiles of the wild type and the pgsA mutant in liquid growth medium were also checked. In the growth medium containing PG, identical curves were obtained for the wild type and the pgsA mutant (Fig. 3B). However, when the cells grown in the growth medium containing PG were transferred to medium without PG (Fig. 3C), a clear difference between the wild type and the mutant was observed. The wild type grew much as in the medium containing PG, whereas the pgsA mutant strain stopped growing at an earlier stage. Figure 4 shows the effect of PG concentration on the growth of the pgsA mutant. The pgsA mutant cells grown in the BG-11 medium containing PG were transferred to the medium without PG and incubated for 3 d, then transferred again to new growth media containing various concentrations of PG. The growth of the pgsA mutant was dependent on the concentration of PG in the growth medium. Although the pgsA mutant stopped growing gradually following the inoculation into the medium that did not contain PG, the growth of the mutant was accelerated according to the increased concentration of PG. Addition of 20 μm PG to the growth medium fully supported the growth of the mutant. These results demonstrate that PG is essential for growth of Synechocystis sp.
Figure 4.
Effect of PG concentration on the growth of the pgsA mutant of Synechocystis sp. The pgsA mutant cells grown to logarithmic growth phase in the medium containing PG were transferred to media containing various concentrations of PG. ●, 0 μm; □, 2 μm; ◊, 5 μm; x, 20 μm; ▵, 60 μm. The values are averages from two independent experiments.
To examine whether other phospholipids can support the growth of the pgsA mutant, we further checked the effects of phosphatidyl-Ser, phosphatidylethanolamine, phosphatidylcholine, PA, and CL on the growth of this mutant. However, none of these phospholipids was able to support the growth of the pgsA mutant, either in liquid growth medium or on agar plates (data not shown). These results demonstrate that PG has a specific function required for growth of Synechocystis sp.
Changes in PG Content after a Deprivation of PG
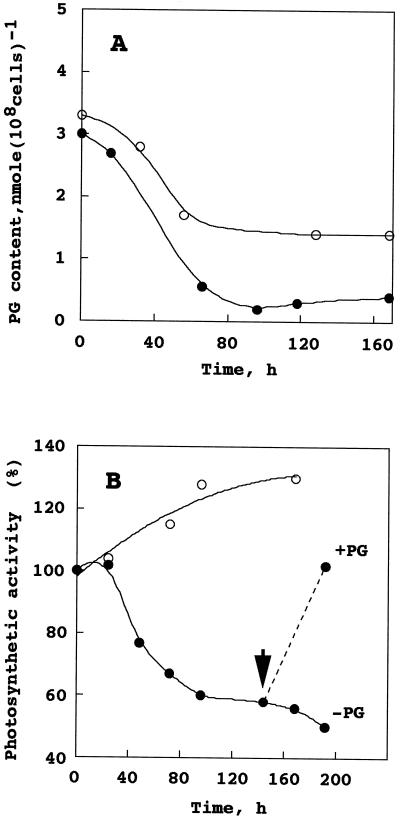
To check the changes in the content of PG in the pgsA mutant cells after a deprivation of PG from the growth medium, lipids were extracted from intact cells and analyzed. Figure 5A shows changes in the content of PG in intact cells of the wild type and the pgsA mutant. Although the contents of PG in the wild type and the pgsA mutant were essentially the same when the cells were grown in the presence of PG, a clear difference between the two strains was observed in the content of PG after a deprivation of PG from the growth medium. The content of PG in the wild type cells decreased from 3.3 to 1.4 nmol (108 cells)−1 after a deprivation of PG for 3 d. By contrast, the content of PG in the pgsA mutant cells markedly decreased from 3.0 to 0.3 nmol (108 cells)−1. These results suggest that the pgsA mutant is not capable of synthesizing PG, and that the pgsA gene is involved in the biosynthesis of PG.
Figure 5.
Changes in PG content and photosynthetic activity of intact cells of the wild type and the pgsA mutant of Synechocystis sp. after a deprivation of PG from the growth medium. A, Changes in PG content. Wild type (○) and mutant (●) cells grown in the medium containing 20 μm PG were transferred to the medium without PG and incubated for the designated times. The values are averages from two independent experiments. B, Changes in photosynthetic activity. Wild type (●) and mutant (○) cells grown in the medium containing 20 μm PG were transferred to the medium without PG and incubated for the designated times. In the case of the mutant, the culture was divided into two flasks after a deprivation of PG for 6 d. The culture in one flask was further incubated without PG, whereas that in the other was supplemented with 20 μm PG. The arrow indicates the time of addition of PG. The values are averages from two independent experiments.
To clarify the effect of PG deficiency on the composition of lipids in thylakoid membranes, thylakoid membranes were analyzed after a deprivation of PG from the growth medium. Table II shows the changes in the lipid composition of thylakoid membranes isolated from the wild type and the pgsA mutant. In the wild type the content of PG decreased from 34% to 19%, whereas in the pgsA mutant it decreased more dramatically, from 15% to 4%. The contents of SQDG and MGDG increased slightly in both strains concomitantly with the decrease in PG content. Similar changes in lipid composition were also observed in intact cells of both the wild type and the pgsA mutant (data not shown).
Table II.
Changes in lipid composition of the thylakoid membranes of the wild type and pgsA mutant of Synechocystis sp. PCC6803 after a deprivation of PG from the growth medium
| Strain | PG | Lipid
|
|||
|---|---|---|---|---|---|
| aMGDG | DGDG | SQDG | PG | ||
| mol% | |||||
| Wild type | +b | 28 | 19 | 19 | 34 |
| −c | 35 | 23 | 23 | 19 | |
| Mutant | + | 42 | 20 | 23 | 15 |
| − | 52 | 15 | 29 | 4 | |
MGDG contains a minor amount of monoglucosyldiacylglycerol. The values are the averages from two independent experiments.
Thylakoid membranes were isolated from the cells grown in the presence of 20 μm PG.
Cells grown in the presence of PG were transferred to medium without PG, incubated for 5 d, and then thylakoid membranes were isolated.
Changes in Photosynthetic Activities after a Deprivation of PG
To elucidate the effect of reduced content of PG on photosynthesis, the photosynthetic oxygen evolving activity (net photosynthesis, water to carbon dioxide) of intact cells of the wild type and the pgsA mutant was measured, as shown in Figure 5B. The photosynthetic activity of intact cells of the wild type increased slightly, and these cells grew normally when transferred to medium without PG. By contrast, the activity of intact cells of the pgsA mutant decreased dramatically after these cells were transferred to medium without PG. After incubating the cells for 3 d, the activity decreased to 60% of the intact cells grown in the medium containing PG, and the cells had stopped growing. This reduced activity was fully recovered to the original level of the activity of the cells following addition of 20 μm PG to the growth medium. This result suggests that the exogenously added PG was taken up into the cells and incorporated into the thylakoid membranes. The incorporation of PG into thylakoid membranes was confirmed by the analysis of the lipid composition of thylakoid membranes as described previously (see Table II). This PG-induced recovery of activity was maintained even after incubation for 6 d. Upon the recovery of the photosynthetic activity the growth of cells recommenced, suggesting that the cells continued to survive even after incubation for 6 d. These results strongly support the idea that PG plays an important role in the photosynthesis and growth of Synechocystis sp.
We also investigated the effect of the reduced content of PG on PSII activity. The PSII activity in intact cells of the wild type and the pgsA mutant was essentially the same when the cells were grown in the presence of PG. It was interesting that we found that 1,4-benzoquinone (pBQ) used as an electron acceptor from PSII inhibited the activity of the pgsA mutant after the deprivation of PG for 5 d, but not the activity in intact cells of the wild type. As shown in Table III, the PSII activity in intact cells of the wild type increased by addition of pBQ, whereas the activity in intact cells of the pgsA mutant was completely inhibited after the deprivation of PG. Other quinones, 2,6-dichloro-p-benzoquinone (DCBQ) and 2,6-dimethyl-p-benzoquinone (DMBQ), also inhibited the activity of the pgsA mutant cells grown in the absence of PG, but with a different concentration dependency (data not shown). It is known that each of these quinones shows a different affinity for accepting electrons from the QA site of the D2 protein or the QB site of the D1 protein in the PSII reaction center (Nakane et al., 1991; Satoh et al., 1995). These data suggest that the changes in the conformation of the PSII reaction center are induced by the decrease of the PG content in the thylakoid membranes, and that the affinity of quinones to the QA or QB site is thereby changed.
Table III.
Inhibition of PSII activity by quinones in intact cells of the wild type and the pgsA mutant of Synechocystis sp. PCC6803
| Strain | Quinone | PG | Photosynthetic Activity |
|---|---|---|---|
| μmol O2 mg Chl−1 h−1 | |||
| Wild type | None | +a | 430 ± 15 |
| −b | 460 ± 20 | ||
| pBQ | + | 510 ± 35 | |
| − | 490 ± 30 | ||
| DMBQ | + | 470 ± 30 | |
| − | 450 ± 25 | ||
| DCBQ | + | 110 ± 15 | |
| − | 130 ± 20 | ||
| Mutant | None | + | 420 ± 20 |
| − | 250 ± 10 | ||
| pBQ | + | 410 ± 30 | |
| − | 0 | ||
| DMBQ | + | 440 ± 25 | |
| − | 0 | ||
| DCBQ | + | 100 ± 10 | |
| − | 0 |
Intact cells were treated for 5 min in darkness and oxygen evolution was measured at saturating light intensity in the presence of 0.5 mm K3Fe(CN)6 with or without 1 mm quinone. The temperature of measurements was 30°C. Each value represents the average and sd from three independent measurements.
Cells were grown in the presence of 20 μm.
Cells grown in the presence of PG were transferred to medium without PG and grown for 5 d.
To further clarify the function of PG in photosynthesis, thylakoid membranes were isolated from the wild type and the pgsA mutant cells before or after a deprivation of PG from the growth medium, and the photosynthetic activities were measured. PSII and PSI activities of the thylakoid membranes isolated from the wild type and the pgsA mutant cells are compared in Table IV. The PSI activity of the thylakoid membranes, as measured using reduced diaminodurene (DADH2) and methylviologen (MV) as electron donor and acceptor, respectively, was not significantly affected in either the wild type or the pgsA mutant by a deprivation of PG from the growth medium. The photosynthetic activity of the thylakoid membranes, which was measured as oxygen uptake representing an electron transport activity from water to MV, also did not change in the wild type by a deprivation of PG from the growth medium. By contrast, the photosynthetic activity from water to MV of thylakoid membranes of the pgsA mutant cells grown for 6 d without PG decreased to about 50% of that of thylakoid membranes isolated from the cells grown in the presence of PG. PSII activity of the thylakoid membranes was measured using pBQ as an electron acceptor. The activity of thylakoid membranes of the wild type was high and did not change by a deprivation of PG. In the pgsA mutant the activity of the thylakoid membranes isolated from the cells grown in the presence of PG was similar to that of the thylakoid membranes of the wild type, but the activity of the thylakoid membranes isolated after a deprivation of PG was inhibited, as it was in intact cells and could not be measured by adding artificial quinone acceptors. Although we could not measure the PSII activity of the thylakoid membranes isolated from the pgsA mutant cells after a deprivation of PG, the simultaneously measured activity of electron transport from water to MV, but not the PSI activity, decreased. These results demonstrate that the decrease of the content of PG in the thylakoid membranes following deprivation of PG led to the decrease in PSII activity.
Table IV.
Three parameters of photosynthetic activities were measured in thylakoid membranes isolated from the wild type and the pgsA mutant of Synechocystis sp. PCC6803, which were grown in the presence or in the absence of PG
| Strain | PG | Photosynthetic Activity
|
||
|---|---|---|---|---|
| H2O → MV | H2O → pBQ | DADH2 → MV | ||
| μmol O2 mg Chl−1 h−1 | ||||
| Wild type | +a | 210 ± 15 | 250 ± 20 | 810 ± 40 |
| −b | 220 ± 20 | 240 ± 20 | 830 ± 45 | |
| Mutant | + | 190 ± 10 | 235 ± 20 | 845 ± 45 |
| − | 105 ± 10 | 0 | 770 ± 40 | |
Thylakoid membranes were treated for 5 min in darkness and photosynthetic activities were measured. The temperature of measurements was 30°C. Each value represents the average and sd from three independent measurements.
Thylakoid membranes were isolated from the cells grown in the presence of 20 μm PG.
Cells grown in the presence of PG were transferred to medium without PG, incubated for 6 d, and then thylakoid membranes were isolated.
DISCUSSION
In this study we have identified the pgsA gene of Synechocystis sp. and demonstrated that it encodes a PGP synthase based on the sequence similarity to PGP synthase of E. coli and on its functional complementation of an E. coli pgsA mutant, YA5512, defective in PGP synthase. The identification of the pgsA gene allowed us to generate a mutant of Synechocystis sp. defective in the biosynthesis of PG by inactivation of the pgsA gene and to study the function of PG in photosynthesis.
The function of PG in photosynthesis has previously been studied in vitro. Jordan et al. (1983) and Siegenthaler et al. (1987) reported that 70% to 80% depletion of PG from thylakoid membranes by digestion of the membranes with phospholipase A2 inhibit PSII activity and whole-chain electron transport activity by more than 50%. PG is bound to the D1 protein in the PSII reaction center, and it might be a functional effector and membrane anchor of the D1 protein in the PSII core complex (Kruse and Schmid, 1995). Recent study also suggested that PG is involved in the dimerization of heterodimer proteins in PSII complex (Kruse et al., 2000). It is well known that the turnover rate of D1 protein is very high compared with those of other proteins (Aro et al., 1990, 1993). These findings suggested that PG plays an important role in maintaining the structure of the PSII reaction center in thylakoid membranes. However, these studies could not provide direct evidence that PG plays such a role in vivo. In this study we created a pgsA mutant of Synechocystis sp. that was incapable of synthesizing PG. Using this mutant, we manipulated the content of PG in thylakoid membranes and showed that PG was required for growth and that PSII activity, but not PSI activity, decreased in the pgsA mutant after a deprivation of PG from thylakoid membranes. These results indicate that PG is essential for the growth of Synechocystis sp. and plays an important role in PSII.
As investigated in the present study we have also disrupted the cdsA gene of Synechocystis sp. that might encode CDP-DG synthase involved in the biosynthesis of PG and we investigated the function of PG. The obtained experimental data led us to the similar conclusion that PG is essential for growth and important for PSII activity of photosynthesis in Synechocystis sp. (N. Sato, M. Hagio, H. Wada, and M. Tsuzuki, data submitted for publication).
In the pgsA mutant of Synechocystis sp., the activity of photosynthesis decreased and the cells stopped growing when they were transferred to the medium without PG. It is likely that this cessation of growth was due to more than simply the reduction in activity of photosynthesis, since the cells still showed about 60% of the original activity of photosynthesis even after the cells stopped growing. This finding suggests that PG has an important function not only in photosynthesis, but also in non-photosynthetic processes. For this reason we next investigated the growth of the pgsA mutant under a growth condition in which the growth of the cells was independent of photosynthesis. It is known that Synechocystis sp. can grow under a light-activated heterotrophic growth (LAHG) condition in which cultures are incubated in darkness except once a day for 5 min, when they are illuminated (Anderson and McIntosh, 1991). The growth of the cells under LAHG is dependent on Glc in the growth medium, but not on photosynthesis. In the present study we investigated the growth of the pgsA mutant under the LAHG condition to clarify the function of PG in non-photosynthetic processes. The mutant could grow under the condition in the presence of PG, but not in the absence of PG (data not shown). This finding clearly demonstrates that PG is required even for photosynthesis-independent growth. Further experiments with the pgsA mutant may allow us to determine why PG is necessary for the photosynthesis-independent growth of Synechocystis sp.
MATERIALS AND METHODS
Organisms and Culture Conditions
The wild type of Synechocystis sp. PCC6803 was grown photoautotrophically at 28°C in BG-11 medium (Allen, 1968) supplemented with 4 mm HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid)-NaOH, pH 7.5. The pgsA mutant was grown in the same BG-11 medium, but in the presence of 20 μg mL−1 kanamycin and 20 or 60 μm dioleoyl-PG (P9664, Sigma, St. Louis). For growth of the cells on agar plates, 1.2% (w/v) agar was added to the growth medium. Light was provided by fluorescent lamps at a photon flux density of approximately 30 μmol photons m−2 s−1. The culture was aerated on a rotational shaker (NR-3, TAITEC, Saitama, Japan) at 120 strokes min−1. Growth of the cultures was monitored by determination of the optical density at 730 nm (OD730).
Complementation of Escherichia coli Mutant YA5512 with the pgsA Gene of Synechocystis sp.
E. coli mutant YA5512 defective in PGP synthase was provided by K. Matsumoto (Saitama University). A coding region of the pgsA gene of Synechocystis sp. was amplified by PCR with the two primers, 5′-CGCCATGGATATACCCAACTGGGTA-3′ and 5′-CGCTGCAGTTAGCCAGCCTCGACTT-3′. The eight-nucleotide sequences 5′-CGCCATGG-3′ including a NcoI site and 5′-CGCTGCAG-3′ including a PstI site were added to the 5′-ends of the forward primer and the reverse primer, respectively. The amplified DNA fragment was digested with NcoI and PstI, and ligated into an expression vector, pKK233–2 (CLONTECH, Palo Alto, CA). The resultant plasmid, designated pKK-pgsA, was used for transformation of E. coli YA5512. YA5512 was also transformed with pKK233–2, and the obtained transformant was used as a control. The transformants of YA5512 created using the expression vectors pKK-pgsA and pKK233–2 were grown at 37°C in LB medium supplemented with 100 μg mL−1 ampicillin. When OD600 of the E. coli cultures reached 0.5, isopropyl-1-thio-β-galactoside was added to a final concentration of 0.4 mm and the cultures were further incubated for 3 h at 37°C. After the incubation the cells were collected by centrifugation and used for lipid analysis.
Disruption of the pgsA Gene in Synechocystis sp.
The pgsA gene of Synechocystis sp. was amplified by PCR with the primers 5′-CCAAGCTTTGAACGTGGCCTTAATT-3′ and 5′-TACCGAGCTATCTTGGCATGATTAC-3′. The amplified DNA fragment was ligated into the pCRII vector (Original TA Cloning Kit, Invitrogen, San Diego). The obtained plasmid was digested with EcoRI to prepare a 2.5-kb DNA fragment including the pgsA gene. The 2.5-kb DNA fragment was ligated into an EcoRI site of pBluescript II KS (+) (Stratagene, La Jolla, CA) to construct the plasmid pBlue-pgsA. Disruption of the pgsA gene was performed by inserting the Kmr cartridge into the blunt-ended HpaI site in the coding region of the pgsA gene in pBlue-pgsA. The Kmr cartridge was prepared from pUC4KIXX (Amersham, Buckinghamshire, UK) as a 1.2-kb fragment by digestion with SmaI. The obtained plasmid including the disrupted pgsA gene (pBlue-pgsA::Kmr) was used for transformation of the wild type of Synechocystis sp. The transformation was carried out as described by Golden et al. (1987). Kanamycin-resistant transformants were selected and streaked several times to segregate the mutants in which all copies of the pgsA gene in the chromosome were disrupted. Disruption of the pgsA gene in the pgsA mutant was confirmed by genomic Southern hybridization analysis and PCR using the two primers 5′-CCAGCACTGACTGGTTGGATGGTTA-3′ and 5′-AACGGTGCTAACAACAAGGCGATCG-3′.
Genomic Southern Hybridization Analysis
Genomic DNAs isolated from the cells of the wild type and pgsA mutant of Synechocystis sp. were digested with the appropriate restriction enzymes, separated by electrophoresis on a 1% (w/v) agarose gel, and transferred to a nylon membrane (Hybond-N+, Amersham). The membrane was used for hybridization using a DNA labeling and detection system (ECL kit, Amersham). The coding region of the pgsA gene was used as a probe.
Preparation of Thylakoid Membranes
Thylakoid membranes were isolated according to Nishiyama et al. (1993) with some modifications. Cells were collected from a 600 mL culture with a cell density at 5 μg chlorophyll (Chl) mL−1 by centrifugation at 5,000g for 10 min. The sedimented cells were washed once with 100 mL of medium containing 50 mm HEPES-NaOH, pH 7.5 and 30 mm CaCl2 (medium A). These procedures were performed at 20°C, and the following procedures were performed at 0°C to 4°C. The cells were resuspended in 13 mL of medium A containing 0.8 m sorbitol, 1 m glycinebetaine, and 1 mm 6-aminohexanoic acid (medium B). The suspension was mixed with an equal volume of glass beads with a diameter of 0.1 mm and the mixture was placed in a homogenizer (Beadbeater, Biospec Products, Bartlesville, OK). Cell breakage was performed twice for 30 s, resulting in breakage of more than 80% of the cells. After the precipitation of glass beads, the supernatant was centrifuged at 2,000g for 5 min to remove unbroken cells. The obtained supernatant was transferred into two tubes and then centrifuged at 40,000g for 20 min. The pelleted thylakoid membranes in one of the two tubes were suspended in water and used for lipid analysis. The membranes in the other tube were suspended in medium B and used for measurement of photosynthetic activities. The concentration of Chl was determined by the method of Arnon et al. (1974).
Lipid Analysis
Lipids were extracted from intact cells and thylakoid membranes by the method of Bligh and Dyer (1959). Lipids and fatty acids were analyzed as described previously (Wada and Murata, 1989).
Measurement of Photosynthetic Activities
Photosynthetic activities were measured by means of oxygen exchange with a Clark-type oxygen electrode as described by Gombos et al. (1991) and Nishiyama et al. (1993) with some modifications. Photosynthetic oxygen evolution (net photosynthesis) was monitored in intact cells with no exogenously added donor or acceptor of electrons. Intact cells were washed twice with BG-11 medium and were suspended in the same medium for measurements. Photosynthetic electron transport activity of thylakoid membranes from water to MV was measured in 50 mm HEPES-NaOH, pH 7.5, 1 m glycinebetaine, 0.8 m sorbitol, 0.3 mm MV, and 1 mm NaN3. PSII activity of intact cells and thylakoid membranes was measured by the transport of electrons from water to pBQ. Intact cells were washed twice with BG-11 medium and suspended in BG-11 medium supplemented with 1.0 mm pBQ and 1.0 mm K3Fe(CN)6 for measurements (Ono and Murata, 1981). The activity of thylakoid membranes was measured in 50 mm HEPES-NaOH, pH 7.5, but in the presence of 1 m glycinebetaine and 0.8 m sorbitol. PSI activity in the thylakoid membranes was measured by the transport of electrons from DADH2 to MV. Thylakoid membranes were suspended in 50 mm HEPES-NaOH, pH 7.5, 1.0 mm diaminodurene, 1.0 mm Na-ascorbate, 1.5 mm MV, 0.01 mm 3-(3, 4-dichlorophenyl)-1,1-dimethylurea, and 2 mm KCN (Pakrasi et al., 1988). Light from an incandescent lamp combined with a red optical filter (2–61, Corning, Corning, NY) was provided for all measurements of photosynthetic activities. Light intensity of the illumination was 500 μmol photons m−2 s−1. The concentrations of intact cells and the membranes were adjusted to give about 2 and 10 μg Chl mL−, respectively.
ACKNOWLEDGMENT
We thank Professor Kouji Matsumoto (Saitama University) for providing the E. coli pgsA mutant (YA5512).
Footnotes
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture (grant no. 12640635 to H.W.). M.H. and Z.G. were supported by fellowships from the Japan Society for the Promotion of Science.
LITERATURE CITED
- Allen MM. Simple conditions for growth of unicellular blue-green algae on plates. J Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Anderson SL, McIntosh L. Light-activated heterotrophic growth of the cyanobacterium Synechocystis sp. strain PCC6803: a blue-light-requiring process. J Bacteriol. 1991;173:2761–2767. doi: 10.1128/jb.173.9.2761-2767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. Photochemical activity and components of membrane preparations from blue-green algae: I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Aro E-M, Hundal T, Carlberg I, Andersson B. In vitro studies on light-induced inhibition of photosystem II and D1-protein degradation at low temperature. Biochim Biophys Acta. 1990;1019:269–275. [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Asai Y, Katayose Y, Hikita C, Ohta A, Shibuya I. Suppression of the lethal effect of acidic-phospholipid deficiency by defective formation of the major outer membrane lipoprotein in Escherichia coli. J Bacteriol. 1989;171:6867–6869. doi: 10.1128/jb.171.12.6867-6869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Block MA, Dorne A-J, Joyard J, Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts: II. Biochemical characterization. J Biol Chem. 1983;258:13281–13286. [PubMed] [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C. Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell. 1995;7:1801–1810. doi: 10.1105/tpc.7.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SS, Brusslan J, Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Gombos Z, Wada H, Murata N. Direct evaluation of effects of fatty-acid unsaturation on the thermal properties of photosynthetic activities, as studied by mutation and transformation of Synechocystis PCC6803. Plant Cell Physiol. 1991;32:205–211. [Google Scholar]
- Güler S, Seeliger A, Härtel H, Renger G, Benning C. A null mutant of Synechococcus sp. PCC7942 deficient in the sulfolipid sulfoquinovosyl diacylglycerol. J Biol Chem. 1996;271:7501–7507. doi: 10.1074/jbc.271.13.7501. [DOI] [PubMed] [Google Scholar]
- Härtel H, Essigmann B, Lokstein H, Hoffmann-Benning S, Peters-Kottig M, Benning C. The phospholipid-deficient pho1 mutant of Arabidopsis thaliana is affected in the organization, but not in the light acclimation, of the thylakoid membrane. Biochim Biophys Acta. 1998;1415:205–218. doi: 10.1016/s0005-2736(98)00197-7. [DOI] [PubMed] [Google Scholar]
- Herdman M, Janvier M, Rippka R, Stanier RY. Genome size of cyanobacteria. J Gen Microbiol. 1979;111:73–85. [Google Scholar]
- Inoue K, Kishimoto A, Suzuki M, Matsuzaki H, Matsumoto K, Shibuya I. Suppression of the lethal effect of acidic-phospholipid deficiency in Escherichia coli by Bacillus subtilis chromosomal locus ypoP. Biosci Biotechnol Biochem. 1998;62:540–545. doi: 10.1271/bbb.62.540. [DOI] [PubMed] [Google Scholar]
- Jordan BR, Chow WS, Baker AJ. The role of phospholipids in the molecular organization of pea chloroplast membranes: effect of phospholipid depletion on photosynthetic activities. Biochim Biophys Acta. 1983;725:77–86. [Google Scholar]
- Joyard J, Maréchal E, Miège C, Block MA, Dorne A-J, Douce R. Structure, distribution and biosynthesis of glycerolipids from higher plant chloroplasts. In: Siegenthaler P-A, Murata N, editors. Lipids in Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 65–81. [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. PCC6803: II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- Kontinen VP, Tokuda H. Overexpression of phosphatidylglycerophosphate synthase restores protein translocation in a secG deletion mutant of Escherichia coli at low temperature. FEBS Lett. 1995;364:157–160. doi: 10.1016/0014-5793(95)00378-m. [DOI] [PubMed] [Google Scholar]
- Kopka J, Ludewig M, Müller-Röber B. Complementary DNAs encoding eukaryotic-type cytidine-5′-diphosphate-diacylglycerol synthases of two plant species. Plant Physiol. 1997;113:997–1002. doi: 10.1104/pp.113.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse O, Hankamer B, Konczak C, Gerle C, Morris E, Radunz A, Schmid GH, Barber J. Phosphatidylglycerol is involved in the dimerization of photosystem II. J Biol Chem. 2000;275:6509–6514. doi: 10.1074/jbc.275.9.6509. [DOI] [PubMed] [Google Scholar]
- Kruse O, Schmid GH. The role of phosphatidylglycerol as a functional effector and membrane anchor of the D1-core peptide from photosystem II-particles of the cyanobacterium Oscillatoria chalybea. Z Naturforsch. 1995;50c:380–390. [PubMed] [Google Scholar]
- Murata N, Nishida I. Lipids of blue-green algae (cyanobacteria) In: Stumpf PK, editor. The Biochemistry of Plants. San Diego: Academic Press; 1987. pp. 315–347. [Google Scholar]
- Nakane H, Iwaki M, Satoh K, Ito S. Artificial quinones replace the function of quinone electron acceptor (QA) in the isolated D1–D2-cytochrome b559 photosystem II reaction center complex. Plant Cell Physiol. 1991;32:1165–1171. [Google Scholar]
- Nishiyama Y, Kovács E, Lee CB, Hayashi H, Watanabe T, Murata N. Photosynthetic adaptation to high temperature associated with thylakoid membranes of Synechococcus PCC7002. Plant Cell Physiol. 1993;34:337–343. [Google Scholar]
- Ohta A, Waggoner K, Radominska-Pyrek A, Dowhan W. Cloning of genes involved in membrane lipid synthesis: effects of amplification of phosphatidylglycerophosphate synthase in Escherichia coli. J Bacteriol. 1981;147:552–562. doi: 10.1128/jb.147.2.552-562.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Murata N. Chilling susceptibility of the blue-green alga Anacystis nidulans: I. Effect of growth temperature. Plant Physiol. 1981;67:176–181. doi: 10.1104/pp.67.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi HB, Williams JGK, Arntzen CJ. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988;7:325–332. doi: 10.1002/j.1460-2075.1988.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J. A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 1991;97:1087–1093. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Oh-hashi M, Kashino Y, Koike H. Mechanism of electron flow through the QB site in photosystem II: I. Kinetics of the reduction of electron acceptors at the QB and plastoquinone sites in photosystem II particles from the cyanobacterium Synechococcus vulcanus. Plant Cell Physiol. 1995;36:597–605. [Google Scholar]
- Siegenthaler P-A. Molecular organization of acyl lipids in photosynthetic membranes of higher plants. In: Siegenthaler P-A, Murata N, editors. Lipids in Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 119–144. [Google Scholar]
- Siegenthaler P-A, Smutny J, Rawler A. Involvement of distinct populations of phosphatidylglycerol and phosphatidylcholine molecules in photosynthetic electron-flow activities. Biochim Biophys Acta. 1987;891:85–93. [Google Scholar]
- Usui M, Sembongi H, Matsuzaki H, Matsumoto K, Shibuya I. Primary structures of the wild-type and mutant alleles encoding the phosphatidylglycerophosphate synthase of Escherichia coli. J Bacteriol. 1994;176:3389–3392. doi: 10.1128/jb.176.11.3389-3392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Murata N. Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol. 1989;30:971–978. [Google Scholar]
- Wada H, Murata N. Membrane lipids in cyanobacteria. In: Siegenthaler P-A, Murata N, editors. Lipids in Photosynthesis. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 65–81. [Google Scholar]